Effects of Maternal Nutrition and One-Carbon Metabolite Supplementation on Fetal Jejunal Morphology and Hexose Transporter Expression in Beef Cattle
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Experimental Design and Animal Management
2.3. Dietary Treatments and Feeding Management
2.4. Sample Collection and Tissue Preparation
2.5. Immunofluorescence Staining
2.6. Image Analysis
2.7. Statistical Analysis
3. Results
3.1. Morphology of the Small Intestine
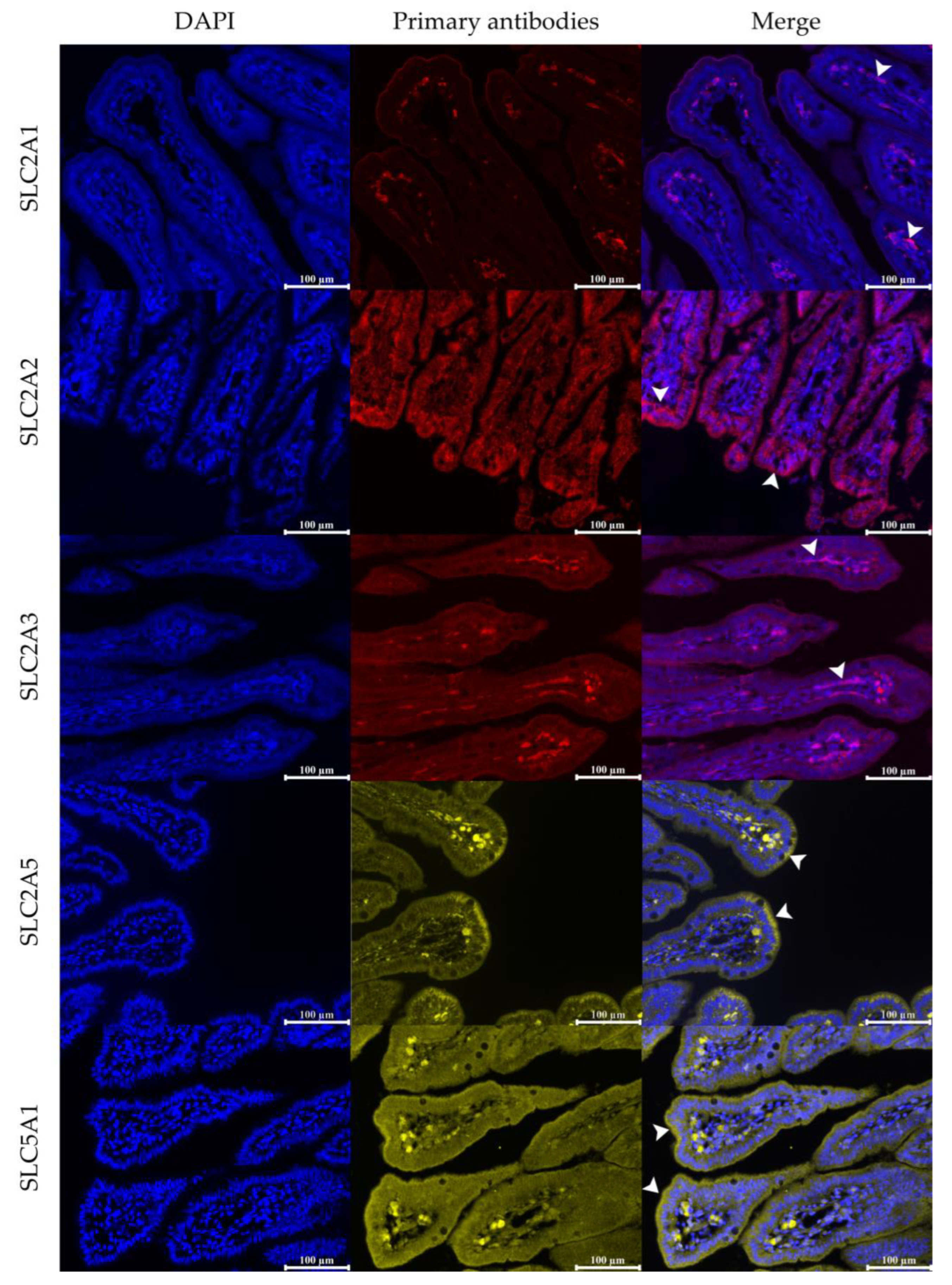
3.2. Relative Protein Abundance of SLC2A1
3.3. Relative Protein Abundance of SLC2A2
3.4. Relative Protein Abundance of SLC2A3
3.5. Relative Protein Abundance of SLC2A5
3.6. Relative Protein Abundance of SLC5A1
4. Discussion
4.1. Morphological Adaptations of the Fetal Small Intestine
4.2. Hexose Transporters of the Small Intestine
4.2.1. The Abundance of SLC2A1 and SLC2A3 Proteins
4.2.2. The Abundance of SLC2A2, SLC2A5, and SLC5A1 Proteins
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harmon, D.L.; Swanson, K.C. Review: Nutritional regulation of intestinal starch and protein assimilation in ruminants. Animal 2020, 14, s17–s28. [Google Scholar] [CrossRef]
- Overton, T.R.; Cameron, M.R.; Elliott, J.P.; Clark, J.H.; Nelson, D.R. Ruminal fermentation and passage of nutrients to the duodenum of lactating cows fed mixtures of corn and barley. J. Dairy Sci. 1995, 78, 1981–1998. [Google Scholar] [CrossRef]
- Mills, J.; France, J.; Dijkstra, J. A review of starch digestion in the lactating dairy cow and proposals for a mechanistic model: 1. Dietary starch characterisation and ruminal starch digestion. J. Anim. Feed. Sci. 1999, 8, 219–340. [Google Scholar] [CrossRef]
- Noziere, P.; Ortigues-Marty, I.; Loncke, C.; Sauvant, D. Carbohydrate quantitative digestion and absorption in ruminants: From feed starch and fibre to nutrients available for tissues. Animal 2010, 4, 1057–1074. [Google Scholar] [CrossRef] [PubMed]
- Navale, A.M.; Paranjape, A.N. Glucose transporters: Physiological and pathological roles. Biophys. Rev. 2016, 8, 5–9. [Google Scholar] [CrossRef]
- Ma, J.; Shah, A.; Wang, Z.; Hu, R.; Zou, H.; Wang, X.; Zhao, S.; Kong, X. Dietary supplementation with glutamine improves gastrointestinal barrier function and promotes compensatory growth of growth-retarded yaks. Animal 2021, 15, 100108. [Google Scholar] [CrossRef]
- Lohrenz, A.K.; Duske, K.; Schonhusen, U.; Losand, B.; Seyfert, H.M.; Metges, C.C.; Hammon, H.M. Glucose transporters and enzymes related to glucose synthesis in small intestinal mucosa of mid-lactation dairy cows fed 2 levels of starch. J. Dairy Sci. 2011, 94, 4546–4555. [Google Scholar] [CrossRef]
- Liao, S.F.; Harmon, D.L.; Vanzant, E.S.; McLeod, K.R.; Boling, J.A.; Matthews, J.C. The small intestinal epithelia of beef steers differentially express sugar transporter messenger ribonucleic acid in response to abomasal versus ruminal infusion of starch hydrolysate. J. Anim. Sci. 2010, 88, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Inoue, R.; Matsumoto, M.; Yajima, T.; Ushida, K.; Iwanaga, T. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem. Cell Biol. 2011, 135, 183–194. [Google Scholar] [CrossRef]
- Kayano, T.; Fukumoto, H.; Eddy, R.; Fan, Y.S.; Byers, M.; Shows, T.B.; Bell, G. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J. Biol. Chem. 1988, 263, 15245–15248. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Glimm, D.R.; Kennelly, J.J. Distribution of mammalian facilitative glucose transporter messenger RNA in bovine tissues. Int. J. Biochem. 1993, 25, 1897–1903. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in brain in health and disease. Pflug. Arch. 2020, 472, 1299–1343. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.C.; Fujikura, K.; Suzuki, T.; Tanaka, S.; Takata, K. Glucose transporter GLUT3 in the rat placental barrier: A possible machinery for the transplacental transfer of glucose. Endocrinology 1997, 138, 3997–4004. [Google Scholar] [CrossRef]
- Mamun, A.A.; Hayashi, H.; Yamamura, A.; Nayeem, M.J.; Sato, M. Hypoxia induces the translocation of glucose transporter 1 to the plasma membrane in vascular endothelial cells. J. Physiol. Sci. 2020, 70, 44. [Google Scholar] [CrossRef]
- Augustin, R. The protein family of glucose transport facilitators: It’s not only about glucose after all. IUBMB Life 2010, 62, 315–333. [Google Scholar] [CrossRef]
- Wang, M.; Yang, C.; Wang, Q.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Yang, H.; Yin, Y. The relationship between villous height and growth performance, small intestinal mucosal enzymes activities and nutrient transporters expression in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 606–615. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Zhou, T.; Feng, Y.; Li, L.-a. Common factors and nutrients affecting intestinal villus height-A review. Anim. Biosci. 2025, 38, 1557. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.A.; Mendiondo, C.; Raghavan, S. Tissue engineering of the gastrointestinal tract: The historic path to translation. J. Biol. Eng. 2022, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.S.; Burns, A.J. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 2005, 319, 367–382. [Google Scholar] [CrossRef]
- Meyer, A.M.; Caton, J.S. Role of the Small Intestine in Developmental Programming: Impact of Maternal Nutrition on the Dam and Offspring. Adv. Nutr. 2016, 7, 169–178. [Google Scholar] [CrossRef]
- Trahair, J.F.; DeBarro, T.M.; Robinson, J.S.; Owens, J.A. Restriction of nutrition in utero selectively inhibits gastrointestinal growth in fetal sheep. J. Nutr. 1997, 127, 637–641. [Google Scholar] [CrossRef]
- Howie, G.; Sloboda, D.; Vickers, M. Maternal undernutrition during critical windows of development results in differential and sex-specific effects on postnatal adiposity and related metabolic profiles in adult rat offspring. Br. J. Nutr. 2012, 108, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C.E.; Ozanne, S.E. Transgenerational developmental programming. Hum. Reprod. Update 2014, 20, 63–75. [Google Scholar] [CrossRef]
- Long, N.M.; Vonnahme, K.A.; Hess, B.W.; Nathanielsz, P.W.; Ford, S.P. Effects of early gestational undernutrition on fetal growth, organ development, and placentomal composition in the bovine. J. Anim. Sci. 2009, 87, 1950–1959. [Google Scholar] [CrossRef]
- Phomvisith, O.; Muroya, S.; Otomaru, K.; Oshima, K.; Oshima, I.; Nishino, D.; Haginouchi, T.; Gotoh, T. Maternal Undernutrition Affects Fetal Thymus DNA Methylation, Gene Expression, and, Thereby, Metabolism and Immunopoiesis in Wagyu (Japanese Black) Cattle. Int. J. Mol. Sci. 2024, 25, 9242. [Google Scholar] [CrossRef]
- Chadio, S.; Katsafadou, A.; Kotsampasi, B.; Michailidis, G.; Mountzouris, K.C.; Kalogiannis, D.; Christodoulou, V. Effects of maternal undernutrition during late gestation and/or lactation on colostrum synthesis and immunological parameters in the offspring. Reprod. Fertil. Dev. 2016, 28, 384–393. [Google Scholar] [CrossRef]
- Duarte, M.; Gionbelli, M.; Paulino, P.; Serão, N.; Martins, T.; Tótaro, P.; Neves, C.; Valadares Filho, S.; Dodson, M.; Zhu, M. Effects of maternal nutrition on development of gastrointestinal tract of bovine fetus at different stages of gestation. Livest. Sci. 2013, 153, 60–65. [Google Scholar] [CrossRef]
- Abi Salloum, B.; Veiga-Lopez, A.; Abbott, D.H.; Burant, C.F.; Padmanabhan, V. Developmental programming: Exposure to testosterone excess disrupts steroidal and metabolic environment in pregnant sheep. Endocrinology 2015, 156, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
- Daneshi, M.; Borowicz, P.P.; Entzie, Y.L.; Syring, J.G.; King, L.E.; Safain, K.S.; Anas, M.; Reynolds, L.P.; Ward, A.K.; Dahlen, C.R.; et al. Influence of Maternal Nutrition and One-Carbon Metabolites Supplementation during Early Pregnancy on Bovine Fetal Small Intestine Vascularity and Cell Proliferation. Vet. Sci. 2024, 11, 146. [Google Scholar] [CrossRef]
- Chmurzynska, A. Fetal programming: Link between early nutrition, DNA methylation, and complex diseases. Nutr. Rev. 2010, 68, 87–98. [Google Scholar] [CrossRef]
- Wang, X.; Lan, X.; Radunz, A.E.; Khatib, H. Maternal nutrition during pregnancy is associated with differential expression of imprinted genes and DNA methyltranfereases in muscle of beef cattle offspring. J. Anim. Sci. 2015, 93, 35–40. [Google Scholar] [CrossRef]
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef]
- van Vliet, M.M.; Schoenmakers, S.; Gribnau, J.; Steegers-Theunissen, R.P. The one-carbon metabolism as an underlying pathway for placental DNA methylation–a systematic review. Epigenetics 2024, 19, 2318516. [Google Scholar] [CrossRef]
- Mentch, S.J.; Locasale, J.W. One-carbon metabolism and epigenetics: Understanding the specificity. Ann. N. Y. Acad. Sci. 2016, 1363, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, S.C.; Marczewski, S.E. Methionine, homocysteine, one carbon metabolism and fetal growth. Rev. Endocr. Metab. Disord. 2012, 13, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Daneshi, M.; Borowicz, P.P.; Hirchert, M.R.; Entzie, Y.L.; Syring, J.G.; King, L.E.; Safain, K.S.; Anas, M.; Reynolds, L.P.; Ward, A.K.; et al. Influence of maternal nutrition and one-carbon metabolites supplementation on bovine antimicrobial peptides in fetal and maternal tissues. Front. Vet. Sci. 2024, 11, 1505427. [Google Scholar] [CrossRef] [PubMed]
- Whittier, W.D.; Currin, J.F.; Schramm, H.; Holland, S.; Kasimanickam, R.K. Fertility in Angus cross beef cows following 5-day CO-Synch+ CIDR or 7-day CO-Synch+ CIDR estrus synchronization and timed artificial insemination. Theriogenology 2013, 80, 963–969. [Google Scholar] [CrossRef]
- Schneider, J.E.; Brozek, J.M.; Keen-Rhinehart, E. Our stolen figures: The interface of sexual differentiation, endocrine disruptors, maternal programming, and energy balance. Horm. Behav. 2014, 66, 104–119. [Google Scholar] [CrossRef]
- Gionbelli, T.R.S.; Rotta, P.P.; Veloso, C.M.; Valadares Filho, S.C.; Carvalho, B.C.; Marcondes, M.I.; Ferreira, M.F.L.; Souza, J.V.F.; Santos, J.; Lacerda, L.C.; et al. Intestinal development of bovine foetuses during gestation is affected by foetal sex and maternal nutrition. J. Anim. Physiol. Anim. Nutr. 2017, 101, 493–501. [Google Scholar] [CrossRef]
- Curran, S. Fetal sex determination in cattle and horses by ultrasonography. Theriogenology 1992, 37, 17–21. [Google Scholar] [CrossRef]
- NASEM. Nutrient Requirements of Beef Cattle, 8th ed.; National Academies Press: Washington, DC, USA, 2016; pp. xvii, 475 p. Ill. [Google Scholar]
- Syring, J.G.; Crouse, M.S.; Entzie, Y.L.; King, L.E.; Hirchert, M.R.; Ward, A.K.; Reynolds, L.P.; Borowicz, P.P.; Dahlen, C.R.; Caton, J.S. One-carbon metabolite supplementation increases vitamin B12, folate, and methionine cycle metabolites in beef heifers and fetuses in an energy dependent manner at day 63 of gestation. J. Anim. Sci. 2024, 102, skae202. [Google Scholar] [CrossRef] [PubMed]
- Jacometo, C.B.; Zhou, Z.; Luchini, D.; Correa, M.N.; Loor, J.J. Maternal supplementation with rumen-protected methionine increases prepartal plasma methionine concentration and alters hepatic mRNA abundance of 1-carbon, methionine, and transsulfuration pathways in neonatal Holstein calves. J. Dairy Sci. 2017, 100, 3209–3219. [Google Scholar] [CrossRef] [PubMed]
- Crouse, M.S.; Freetly, H.C.; Lindholm-Perry, A.K.; Neville, B.W.; Oliver, W.T.; Lee, R.T.; Syring, J.G.; King, L.E.; Reynolds, L.P.; Dahlen, C.R.; et al. One-carbon metabolite supplementation to heifers for the first 14 d of the estrous cycle alters the plasma and hepatic one-carbon metabolite pool and methionine-folate cycle enzyme transcript abundance in a dose-dependent manner. J. Anim. Sci. 2023, 101, skac419. [Google Scholar] [CrossRef]
- Gagnon, A.; Khan, D.R.; Sirard, M.A.; Girard, C.L.; Laforest, J.P.; Richard, F.J. Effects of intramuscular administration of folic acid and vitamin B12 on granulosa cells gene expression in postpartum dairy cows. J. Dairy Sci. 2015, 98, 7797–7809. [Google Scholar] [CrossRef]
- Meyer, A.M.; Reed, J.J.; Vonnahme, K.A.; Soto-Navarro, S.A.; Reynolds, L.P.; Ford, S.P.; Hess, B.W.; Caton, J.S. Effects of stage of gestation and nutrient restriction during early to mid-gestation on maternal and fetal visceral organ mass and indices of jejunal growth and vascularity in beef cows. J. Anim. Sci. 2010, 88, 2410–2424. [Google Scholar] [CrossRef]
- Hiżewska, L.; Osiak-Wicha, C.; Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Andres, K.; Schwarz, T.; Arciszewski, M.B. Morphometric analysis of developmental alterations in the small intestine of goose. Animals 2023, 13, 3292. [Google Scholar] [CrossRef]
- Short, K.; Derrickson, E.M. Compensatory changes in villus morphology of lactating Mus musculus in response to insufficient dietary protein. J. Exp. Biol. 2020, 223, jeb210823. [Google Scholar] [CrossRef]
- Bressenot, A.; Pooya, S.; Bossenmeyer-Pourie, C.; Gauchotte, G.; Germain, A.; Chevaux, J.B.; Coste, F.; Vignaud, J.M.; Gueant, J.L.; Peyrin-Biroulet, L. Methyl donor deficiency affects small-intestinal differentiation and barrier function in rats. Br. J. Nutr. 2013, 109, 667–677. [Google Scholar] [CrossRef]
- Crouse, M.S.; Greseth, N.P.; McLean, K.J.; Crosswhite, M.R.; Pereira, N.N.; Ward, A.K.; Reynolds, L.P.; Dahlen, C.R.; Neville, B.W.; Borowicz, P.P.; et al. Maternal nutrition and stage of early pregnancy in beef heifers: Impacts on hexose and AA concentrations in maternal and fetal fluids1. J. Anim. Sci. 2019, 97, 1296–1316. [Google Scholar] [CrossRef]
- Syring, J.G.; Crouse, M.S.; Neville, T.L.; Ward, A.K.; Dahlen, C.R.; Reynolds, L.P.; Borowicz, P.P.; McLean, K.J.; Neville, B.W.; Caton, J.S. Concentrations of vitamin B12 and folate in maternal serum and fetal fluids, metabolite interrelationships, and hepatic transcript abundance of key folate and methionine cycle genes: The impacts of maternal nutrition during the first 50 d of gestation. J. Anim. Sci. 2023, 101, skad139. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.P.; Wang, W.J.; Degen, A.A.; Guo, Y.M.; Kang, J.P.; Liu, P.P.; Ding, L.M.; Shang, Z.H.; Zhou, J.W.; Long, R.J. Small intestinal morphology and sugar transporters expression when consuming diets of different energy levels: Comparison between Tibetan and small-tailed Han sheep. Animal 2022, 16, 100463. [Google Scholar] [CrossRef]
- Rubin, D.C.; Levin, M.S. Mechanisms of intestinal adaptation. Best. Pr. Res. Clin. Gastroenterol. 2016, 30, 237–248. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Li, H.; Jian, L.; Luo, H.; Wang, B.; Zhang, C.; Zhao, X.; Xue, Y.; Peng, S.; et al. Maternal Folic Acid Supplementation Differently Affects the Small Intestinal Phenotype and Gene Expression of Newborn Lambs from Differing Litter Sizes. Animals 2020, 10, 2183. [Google Scholar] [CrossRef]
- King, L.; Syring, J.; Entzie, Y.; Hirchert, M.R.; Crouse, M.S.; Caton, J.; Dahlen, C.R.; Ward, A.K. 180 Supplementation of One-Carbon Metabolites to Beef Heifers During Early Gestation Increases Fetal Organ Weight at Day 63 of Gestation. J. Anim. Sci. 2022, 100, 87. [Google Scholar] [CrossRef]
- Safain, K.S.; Crouse, M.S.; Syring, J.G.; Entzie, Y.L.; King, L.E.; Hirchert, M.R.; Ward, A.K.; Reynolds, L.P.; Borowicz, P.P.; Dahlen, C.R.; et al. One-carbon metabolites supplementation and nutrient restriction alter the fetal liver metabolomic profile during early gestation in beef heifers. J. Anim. Sci. 2024, 102, skae258. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. The supply of choline is important for fetal progenitor cells. Semin. Cell Dev. Biol. 2011, 22, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Prezotto, L.D.; Camacho, L.E.; Lemley, C.O.; Keomanivong, F.E.; Caton, J.S.; Vonnahme, K.A.; Swanson, K.C. Nutrient restriction and realimentation in beef cows during early and mid-gestation and maternal and fetal hepatic and small intestinal in vitro oxygen consumption. Animal 2016, 10, 829–837. [Google Scholar] [CrossRef]
- Santos, L.R.d.; Costa, T.C.; Souza, R.O.; Gionbelli, T.R.S.; Oliveira Junior, I.M.d.; Ramírez-Zamudio, G.D.; Nascimento, K.B.; Duarte, M.d.S.; Gionbelli, M.P. Development of the gastrointestinal tract of newborn goats under maternal feed restriction at different stages of gestation. Rev. Bras. Zootec. 2023, 52, e20230051. [Google Scholar] [CrossRef]
- Srugo, S.A.; Bloise, E.; Nguyen, T.T.-T.N.; Connor, K.L. Impact of maternal malnutrition on gut barrier defense: Implications for pregnancy health and fetal development. Nutrients 2019, 11, 1375. [Google Scholar] [CrossRef]
- Ostrowska, M.; Jarczak, J.; Zwierzchowski, L. Glucose transporters in cattle-a review. Anim. Sci. Pap. Rep. 2015, 33, 191–212. [Google Scholar]
- Zhao, F.Q.; Keating, A.F. Expression and regulation of glucose transporters in the bovine mammary gland. J. Dairy Sci. 2007, 90 (Suppl. 1), E76–E86. [Google Scholar] [CrossRef]
- Pantaleon, M.; Harvey, M.B.; Pascoe, W.S.; James, D.E.; Kaye, P.L. Glucose transporter GLUT3: Ontogeny, targeting, and role in the mouse blastocyst. Proc. Natl. Acad. Sci. USA 1997, 94, 3795–3800. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Sheikhahmadi, A.; Wang, Y.; Jiao, H.; Lin, H.; Song, Z. Effects of heat stress on the gene expression of nutrient transporters in the jejunum of broiler chickens (Gallus gallus domesticus). Int. J. Biometeorol. 2015, 59, 127–135. [Google Scholar] [CrossRef]
- Entzie, Y.L.; King, L.E.; Syring, J.G.; Hirchert, M.R.; Caton, J.S.; Crouse, M.S.; Dahlen, C.R.; Ward, A.K. The effect of one- carbon metabolite supplementation in combination with plane of nutrition during early gestation on maternal serum and fetal fluids. Aspen Perinat. Bio. Sym. Abstr. I-XVI (Sci. Program Bookl. p. 28) 2022. Page 28. Available online: https://www.asas.org/docs/default-source/perinatal/2022-_perinatal_program_fnl_fnl.pdf?sfvrsn=4c5956d1_1 (accessed on 1 September 2025).
- Reynolds, L.P.; Vonnahme, K.A.; Lemley, C.O.; Redmer, D.A.; Grazul-Bilska, A.T.; Borowicz, P.P.; Caton, J.S. Maternal stress and placental vascular function and remodeling. Curr. Vasc. Pharmacol. 2013, 11, 564–593. [Google Scholar] [CrossRef]
- Takagi, H.; King, G.L.; Aiello, L.P. Hypoxia upregulates glucose transport activity through an adenosine-mediated increase of GLUT1 expression in retinal capillary endothelial cells. Diabetes 1998, 47, 1480–1488. [Google Scholar] [CrossRef]
- Vannucci, S.J.; Seaman, L.B.; Vannucci, R.C. Effects of hypoxia-ischemia on GLUT1 and GLUT3 glucose transporters in immature rat brain. J. Cereb. Blood Flow Metab. 1996, 16, 77–81. [Google Scholar] [CrossRef]
- Kanjanaruch, C.; Bochantin, K.A.; Davila Ruiz, B.J.; Syring, J.; Entzie, Y.; King, L.; Borowicz, P.P.; Crouse, M.S.; Caton, J.S.; Dahlen, C.R.; et al. One-carbon metabolite supplementation to nutrient-restricted beef heifers affects placental vascularity during early pregnancy. J. Anim. Sci. 2024, 102, skae044. [Google Scholar] [CrossRef]
- Ghosh, C.; Westcott, R.; Skvasik, D.; Khurana, I.; Khoury, J.; Blumcke, I.; El-Osta, A.; Najm, I.M. GLUT1 and cerebral glucose hypometabolism in human focal cortical dysplasia is associated with hypermethylation of key glucose regulatory genes. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Simpson, I.A.; Appel, N.M.; Hokari, M.; Oki, J.; Holman, G.D.; Maher, F.; Koehler-Stec, E.M.; Vannucci, S.J.; Smith, Q.R. Blood-brain barrier glucose transporter: Effects of hypo- and hyperglycemia revisited. J. Neurochem. 1999, 72, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Fladeby, C.; Skar, R.; Serck-Hanssen, G. Distinct regulation of glucose transport and GLUT1/GLUT3 transporters by glucose deprivation and IGF-I in chromaffin cells. Biochim. Biophys. Acta 2003, 1593, 201–208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fatima, J.; Iqbal, C.W.; Houghton, S.G.; Kasparek, M.S.; Duenes, J.A.; Zheng, Y.; Sarr, M.G. Hexose transporter expression and function in mouse small intestine: Role of diurnal rhythm. J. Gastrointest. Surg. 2009, 13, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Corpe, C.P.; Burant, C.F. Hexose transporter expression in rat small intestine: Effect of diet on diurnal variations. Am. J. Physiol. 1996, 271, G211–G216. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflug. Arch. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Kellett, G.L. The facilitated component of intestinal glucose absorption. J. Physiol. 2001, 531, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Seong, J.K.; Ryu, D.Y. Tissue-specific and de novo promoter methylation of the mouse glucose transporter 2. Biol. Pharm. Bull. 2005, 28, 2054–2057. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Hodjat, M.; Rahimifard, M.; Nigjeh, M.N.; Azizi, M.; Baeeri, M.; Bayrami, Z.; Gholami, M.; Hassani, S.; Abdollahi, M. Assessment of arsenic-induced modifications in the DNA methylation of insulin-related genes in rat pancreatic islets. Ecotoxicol. Environ. Saf. 2020, 201, 110802. [Google Scholar] [CrossRef]
- Bison, A.; Marchal-Bressenot, A.; Li, Z.; Elamouri, I.; Feigerlova, E.; Peng, L.; Houlgatte, R.; Beck, B.; Pourie, G.; Alberto, J.M.; et al. Foetal programming by methyl donor deficiency produces steato-hepatitis in rats exposed to high fat diet. Sci. Rep. 2016, 6, 37207. [Google Scholar] [CrossRef]
- Said, H.M.; Chatterjee, N.; Haq, R.U.; Subramanian, V.S.; Ortiz, A.; Matherly, L.H.; Sirotnak, F.; Halsted, C.; Rubin, S.A. Adaptive regulation of intestinal folate uptake: Effect of dietary folate deficiency. Am. J. Physiol. Cell Physiol. 2000, 279, C1889–C1895. [Google Scholar] [CrossRef]
- Song, A.; Mao, Y.; Wei, H. GLUT5: Structure, functions, diseases and potential applications. Acta Biochim. Biophys. Sin. 2023, 55, 1519–1538. [Google Scholar] [CrossRef]
- Kim, J.; Song, G.; Wu, G.; Bazer, F.W. Functional roles of fructose. Proc. Natl. Acad. Sci. USA 2012, 109, E1619–E1628. [Google Scholar] [CrossRef]
- Mochizuki, K.; Takabe, S.; Goda, T. Changes on histone H3 modifications on the GLUT5 gene and its expression in Caco-2 cells co-treated with a p44/42 MAPK inhibitor and glucocorticoid hormone. Biochem. Biophys. Res. Commun. 2008, 371, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Douard, V.; Mochizuki, K.; Goda, T.; Ferraris, R.P. Diet-induced epigenetic regulation in vivo of the intestinal fructose transporter Glut5 during development of rat small intestine. Biochem. J. 2011, 435, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, M.; Budak, M.; Gülmez, Z.D.; Bulut, E. Methylation of GLUT5 and Electromotile Responses During Chronic and Acute Sodium Salicylate Administration in the Cochlear Outer Hair Cells. Kafkas J. Med. Sci. 2024, 14, 72–79. [Google Scholar] [CrossRef]
- Castello, A.; Guma, A.; Sevilla, L.; Furriols, M.; Testar, X.; Palacin, M.; Zorzano, A. Regulation of GLUT5 gene expression in rat intestinal mucosa: Regional distribution, circadian rhythm, perinatal development and effect of diabetes. Biochem. J. 1995, 309 Pt 1, 271–277. [Google Scholar] [CrossRef]
- Davidson, N.O.; Hausman, A.M.; Ifkovits, C.A.; Buse, J.B.; Gould, G.W.; Burant, C.F.; Bell, G.I. Human intestinal glucose transporter expression and localization of GLUT5. Am. J. Physiol. 1992, 262, C795–C800. [Google Scholar] [CrossRef]
- Rand, E.B.; Depaoli, A.M.; Davidson, N.O.; Bell, G.I.; Burant, C.F. Sequence, tissue distribution, and functional characterization of the rat fructose transporter GLUT5. Am. J. Physiol. 1993, 264, G1169–G1176. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Sauerwein, H.; Higashiyama, Y.; Picard, B.; Abe, H. Prenatal developmental changes in glucose transporters, intermediary metabolism and hormonal receptors related to the IGF/insulin-glucose axis in the heart and adipose tissue of bovines. Reprod. Nutr. Dev. 2006, 46, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Shirazi-Beechey, S.P.; Hirayama, B.A.; Wang, Y.; Scott, D.; Smith, M.W.; Wright, E.M. Ontogenic development of lamb intestinal sodium-glucose co-transporter is regulated by diet. J. Physiol. 1991, 437, 699–708. [Google Scholar] [CrossRef] [PubMed]
| Antigen 1 | Type | Dilution | Code | Supplier |
|---|---|---|---|---|
| SLC2A1 | Rabbit, Monoclonal | 1:1000 | ab115730 | Abcam, Boston, MA, USA |
| SLC2A2 | Rabbit, Polyclonal | 1:150 | ab54460 | Abcam, Boston, MA, USA |
| SLC2A3 | Rabbit, Polyclonal | 1:500 | ab15311 | Abcam, Boston, MA. USA |
| SLC2A5 | Mouse, Monoclonal | 1:400 | Sc271055 | Santa Cruz, CA, USA |
| SLC5A1 | Rabbit, Polyclonal | 1:100 | PA5-28240 | Invitrogen, Mt Prospect, IL, USA |
| Supplementation | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | Nut | −OCM | +OCM | SEM 3 | Nut 4 | SEM 5 | Nut | OCM | Nut × OCM | |
| Villus height, μm | CON | 354.69 | 365.39 | 10.10 | 360.04 | 7.08 | 0.005 | 0.27 | 0.97 | |
| RES | 383.00 | 394.02 | 388.68 | |||||||
| OCM 6 | 368.84 | 379.88 | ||||||||
| Crypt depth, μm | CON | 100.2 ab | 94.61 a | 3.60 | 97.40 | 2.46 | 0.36 | 0.51 | 0.02 | |
| RES | 95.49 a | 105.69 b | 100.59 | |||||||
| OCM | 97.85 | 100.15 | ||||||||
| Villus height/Crypt depth | CON | 3.62 | 3.91 | 0.21 | 3.76 | 0.14 | 0.28 | 0.95 | 0.15 | |
| RES | 4.14 | 3.83 | 3.98 | |||||||
| OCM | 3.88 | 3.87 | ||||||||
| Inner circular muscle thickness, μm | CON | 54.40 A | 52.78 aAB | 2.05 | 53.59 | 1.40 | 0.09 | 0.35 | 0.08 | |
| RES | 54.28 A | 59.59 bB | 56.94 | |||||||
| OCM | 54.34 | 56.18 | ||||||||
| Outer longitudinal muscle thickness, μm | CON | 31.57 A | 30.60 aAB | 1.16 | 31.09 | 0.80 | 0.12 | 0.31 | 0.06 | |
| RES | 31.25 A | 34.48 bB | 32.85 | |||||||
| OCM | 31.40 | 32.54 | ||||||||
| Total muscle layer thickness, μm 7 | CON | 85.33 a | 83.39 a | 2.75 | 84.36 | 1.90 | 0.03 | 0.26 | 0.06 | |
| RES | 86.07 a | 94.07 b | 90.07 | |||||||
| OCM | 85.70 | 88.73 | ||||||||
| Supplementation | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Area | Nut | −OCM | +OCM | SEM 3 | Nut 4 | SEM 5 | Nut | OCM | Nut × OCM | |
| Villus | CON | 15.34 | 10.44 | 1.40 | 12.89 | 0.97 | 0.83 | 0.003 | 0.58 | |
| RES | 14.88 | 11.49 | 13.18 | |||||||
| OCM 6 | 15.11 | 10.96 | ||||||||
| Crypt | CON | 1.96 a | 1.28 b | 0.14 | 1.62 | 0.09 | 0.03 | 0.002 | 0.09 | |
| RES | 2.03 a | 1.83 a | 1.93 | |||||||
| OCM | 1.99 | 1.55 | ||||||||
| Total | CON | 17.30 | 11.72 | 1.44 | 14.51 | 1.02 | 0.67 | 0.001 | 0.48 | |
| RES | 16.91 | 13.32 | 15.11 | |||||||
| OCM | 17.11 | 12.52 | ||||||||
| Supplementation | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Area | Nut | −OCM | +OCM | SEM 3 | Nut 4 | SEM 5 | Nut | OCM | Nut × OCM | |
| Villus | CON | 11.25 a | 10.29 ab | 0.62 | 10.77 | 0.44 | 0.06 | 0.66 | 0.04 | |
| RES | 8.88 b | 10.36 ab | 9.62 | |||||||
| OCM 6 | 10.06 | 10.33 | ||||||||
| Crypt | CON | 0.19 | 0.17 | 0.02 | 0.18 | 0.01 | 0.54 | 0.48 | 0.68 | |
| RES | 0.17 | 0.16 | 0.17 | |||||||
| OCM | 0.18 | 0.16 | ||||||||
| Total | CON | 11.64 a | 10.51 ab | 0.63 | 11.07 | 0.45 | 0.04 | 0.76 | 0.04 | |
| RES | 9.03 b | 10.53 ab | 9.78 | |||||||
| OCM | 10.33 | 10.52 | ||||||||
| Supplementation | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Area | Nut | −OCM | +OCM | SEM 3 | Nut 4 | SEM 5 | Nut | OCM | Nut × OCM | |
| Villus | CON | 13.08 | 13.79 | 1.81 | 13.44 | 1.28 | 0.08 | 0.42 | 0.23 | |
| RES | 18.36 | 14.78 | 16.57 | |||||||
| OCM 6 | 15.72 | 14.28 | ||||||||
| Crypt | CON | 1.69 | 1.04 | 0.15 | 1.37 | 0.10 | 0.97 | <0.0001 | 0.84 | |
| RES | 1.72 | 1.02 | 1.36 | |||||||
| OCM | 1.71 | 1.03 | ||||||||
| Total | CON | 14.77 | 14.84 | 1.84 | 14.81 | 1.30 | 0.08 | 0.24 | 0.23 | |
| RES | 20.09 | 15.80 | 17.95 | |||||||
| OCM | 17.43 | 15.32 | ||||||||
| Supplementation | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Area | Nut | −OCM | +OCM | SEM 3 | Nut 4 | SEM 5 | Nut | OCM | Nut × OCM | |
| Villus | CON | 2.55 a | 2.56 a | 0.36 | 2.56 | 0.25 | 0.19 | 0.04 | 0.05 | |
| RES | 2.30 a | 3.79 b | 3.04 | |||||||
| OCM 6 | 2.42 | 3.18 | ||||||||
| Crypt | CON | 2.46 | 2.29 | 0.23 | 2.38 | 0.15 | 0.22 | 0.03 | 0.17 | |
| RES | 2.49 | 1.71 | 2.10 | |||||||
| OCM | 2.48 | 2.00 | ||||||||
| Total | CON | 4.94 | 4.86 | 0.39 | 4.90 | 0.25 | 0.46 | 0.39 | 0.28 | |
| RES | 4.80 | 5.51 | 5.15 | |||||||
| OCM | 4.87 | 5.18 | ||||||||
| Supplementation | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Area | Nut | −OCM | +OCM | SEM 3 | Nut 4 | SEM 5 | Nut | OCM | Nut × OCM | |
| Villus | CON | 4.44 b | 8.24 a | 0.87 | 6.34 | 0.61 | <0.0001 | 0.05 | 0.01 | |
| RES | 10.21 a | 9.80 a | 10.00 | |||||||
| OCM 6 | 7.32 | 9.02 | ||||||||
| Crypt | CON | 4.33 a | 4.66 a | 0.34 | 4.50 | 0.24 | 0.005 | 0.20 | 0.02 | |
| RES | 4.14 a | 2.96 b | 3.55 | |||||||
| OCM | 4.24 | 3.81 | ||||||||
| Total | CON | 8.77 b | 12.91 a | 0.95 | 10.84 | 0.67 | 0.004 | 0.17 | 0.003 | |
| RES | 14.35 a | 12.76 a | 13.56 | |||||||
| OCM | 11.56 | 12.83 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daneshi, M.; Borowicz, P.P.; Montgomery, V.; Entzie, Y.L.; Syring, J.G.; King, L.E.; Safain, K.S.; Anas, M.; Reynolds, L.P.; Ward, A.K.; et al. Effects of Maternal Nutrition and One-Carbon Metabolite Supplementation on Fetal Jejunal Morphology and Hexose Transporter Expression in Beef Cattle. Vet. Sci. 2025, 12, 884. https://doi.org/10.3390/vetsci12090884
Daneshi M, Borowicz PP, Montgomery V, Entzie YL, Syring JG, King LE, Safain KS, Anas M, Reynolds LP, Ward AK, et al. Effects of Maternal Nutrition and One-Carbon Metabolite Supplementation on Fetal Jejunal Morphology and Hexose Transporter Expression in Beef Cattle. Veterinary Sciences. 2025; 12(9):884. https://doi.org/10.3390/vetsci12090884
Chicago/Turabian StyleDaneshi, Mojtaba, Pawel P. Borowicz, Virginia Montgomery, Yssi L. Entzie, Jessica G. Syring, Layla E. King, Kazi Sarjana Safain, Muhammad Anas, Lawrence P. Reynolds, Alison K. Ward, and et al. 2025. "Effects of Maternal Nutrition and One-Carbon Metabolite Supplementation on Fetal Jejunal Morphology and Hexose Transporter Expression in Beef Cattle" Veterinary Sciences 12, no. 9: 884. https://doi.org/10.3390/vetsci12090884
APA StyleDaneshi, M., Borowicz, P. P., Montgomery, V., Entzie, Y. L., Syring, J. G., King, L. E., Safain, K. S., Anas, M., Reynolds, L. P., Ward, A. K., Dahlen, C. R., Crouse, M. S., & Caton, J. S. (2025). Effects of Maternal Nutrition and One-Carbon Metabolite Supplementation on Fetal Jejunal Morphology and Hexose Transporter Expression in Beef Cattle. Veterinary Sciences, 12(9), 884. https://doi.org/10.3390/vetsci12090884








