GUCY2D-Associated Retinopathy: A Comparative Study Between Humans and German Spitz Dogs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Patients
2.3. Clinical Examination and Tests
3. Results
3.1. Humans
3.2. German Spitz Dogs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LCA | Leber’s congenital amaurosis |
| CRD | Cone-rod dystrophy |
| OCT | optical coherence tomography |
| ERG | electroretinogram |
| AR | autosomal recessive |
| AD | autosomal dominant |
| UFPR | Federal University of Paraná |
| BCVA | best corrected visual acuity |
| FAF | autofluorescence |
| VF | visual field |
| RD | retinal dystrophy |
| NRD | neurosensory retinal detachment |
| LP | light perception |
| HM | hand movements |
| RPE | retinal pigment epithelium |
| ONL | outer nuclear layer |
References
- Koch, K.-W.; Stryer, L. Highly cooperative feedback contol of retinal rod guanylate cyclase by calcium ions. Nature 1998, 334, 64–66. [Google Scholar] [CrossRef]
- Duda, T.; Koch, K.W. Retinal diseases linked with photoreceptor guanylate cyclase. Mol. Cell Biochem. 2002, 230, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Motta, F.L.; Martin, R.P.; Filippelli-Silva, R.; Salles, M.V.; Sallum, J.M.F. Relative frequency of inherited retinal dystrophies in Brazil. Sci. Rep. 2018, 8, 15939. [Google Scholar] [CrossRef]
- Hamel, C.P. Cone rod dystrophies. Orphanet J. Rare Dis. 2007, 2, 7. [Google Scholar] [CrossRef]
- Jiang, F.; Xu, K.; Zhang, X.; Xie, Y.; Bai, F.; Li, Y. GUCY2D mutations in a Chinese cohort with autosomal dominant cone or cone–rod dystrophies. Doc. Ophthalmol. 2015, 131, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bortolini, M.; Winkler, P.A.; Moreno, J.C.D.; Sato, M.T.; Guareschi, B.L.V.; Petersen-Jones, S.M.; Montiani-Ferreira, F. Preliminary characterization of a novel form of progressive retinal atrophy in the German Spitz dog associated with a frameshift mutation in GUCY2D. Vet. Ophthalmol. 2023, 26, 532–547. [Google Scholar] [CrossRef]
- Occelli, L.M.; Pasmanter, N.; Ayoub, E.E.; Petersen-Jones, S.M. Changes in retinal layer thickness with maturation in the dog: An in vivo spectral domain—Optical coherence tomography imaging study. BMC Vet. Res. 2020, 16, 225. [Google Scholar] [CrossRef]
- Mowat, F.M.; Petersen-Jones, S.M.; Williamson, H.; Williams, D.L.; Luthert, P.J.; Ali, R.R.; Bainbridge, J.W. Topographical characterization of cone photoreceptors and the area centralis of the canine retina. Mol. Vis. 2008, 14, 2518–2527. [Google Scholar] [PubMed] [PubMed Central]
- Ofri, R.; Ekesten, B. Baseline retinal OCT measurements in normal female beagle: The effects of eccentricity, meridian, and age on retinal layer thickess. Vet. Ophthalmol. 2020, 23, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tuntivanich, N.; Mentzer, A.L.; Eifler, D.M.; Montiani-Ferreira, F.; Forcier, J.Q.; Johnson, C.A.; Petersen-Jones, S.M. Assessment of the dark-adaptation time required for recovery of electroretinographic responses in dogs after fundus photography and indirect ophthalmoscopy. Am. J. Vet. Res. 2005, 10, 1798–1804. [Google Scholar] [CrossRef]
- Dekomien, G.; Runte, M.; Gödde, R.; Epplen, J.T. Generalized progressive retinal atrophy of Sloughi dogs is due to an 8-bp insertion in exon 21 of the PDE6B gene. Cytogenet. Cell Genet. 2000, 90, 261–267. [Google Scholar] [CrossRef]
- Sharon, D.; Wimberg, H.; Kinarty, Y.; Koch, K.W. Genotype-functional-phenotype correlations in photoreceptor guanylate cyclase (GC-E) encoded by GUCY2D. Prog. Retin. Eye Res. 2018, 63, 69–91. [Google Scholar] [CrossRef]
- ClinVar. ClinVar [Internet]. Nih.gov. 2019. Available online: https://www.ncbi.nlm.nih.gov/clinvar/ (accessed on 2 July 2024).
- Zafar, S.; Ahmad, K.; Ali, A.; Baig, R. Retinitis pigmentosa genes implicated in South Asian populations: A systematic review. J. Pak. Med. Assoc. 2017, 67, 1734–1739. [Google Scholar] [PubMed]
- Gul, H.; Shah, A.H.; Harripaul, R.; Abbasi, S.W.; Faheem, M.; Zubair, M.; Muzammal, M.; Khan, S.; Vincent, J.B.; Khan, M.A. Homozygosity mapping coupled with whole-exome sequencing and protein modelling identified a novel missense mutation in GUCY2D in a consanguineous Pakistani family with Leber congenital amaurosis. J. Genet. 2021, 100, 57. [Google Scholar] [CrossRef] [PubMed]
- Hahn, L.C.; Georgiou, M.; Almushattat, H.; van Schooneveld, M.J.; de Carvalho, E.R.; Wesseling, N.L.; Brink, J.B.T.; Florijn, R.J.; Lissenberg-Witte, B.I.; Strubbe, I.; et al. The Natural History of Leber Congenital Amaurosis and Cone–Rod Dystrophy Associated with Variants in the GUCY2D Gene. Ophthalmol. Retin. 2022, 6, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.G.; Cideciyan, A.V.; Peshenko, I.V.; Sumaroka, A.; Olshevskaya, E.V.; Cao, L.; Schwartz, S.B.; Roman, A.J.; Olivares, M.B.; Sadigh, S.; et al. Determining consequences of retinal membrane guanylyl cyclase (RetGC1) deficiency in human leber congenital amaurosis en route to therapy: Residual cone-photoreceptor vision correlates with biochemical properties of the mutants. Hum. Mol. Genet. 2013, 22, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Bouzia, Z.; Georgiou, M.; Hull, S.; Robson, A.G.; Fujinami, K.; Rotsos, T.; Pontikos, N.; Arno, G.; Webster, A.R.; Hardcastle, A.J.; et al. GUCY2D-Associated Leber Congenital Amaurosis: A Retrospective Natural History Study in Preparation for Trials of Novel Therapies. Am. J. Ophthalmol. 2020, 210, 59–70. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Cideciyan, A.V.; Sumaroka, A.; Roman, A.J.; Wu, V.; Swider, M.; Sheplock, R.; Krishnan, A.K.; Garafalo, A.V. Leber congenital amaurosis due to GUCY2D mutations: Longitudinal analysis of retinal structure and visual function. Int. J. Mol. Sci. 2021, 22, 2031. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.G.; Cideciyan, A.V.; Sumaroka, A.; Roman, A.J.; Charng, J.; Lu, M.; Choi, W.; Sheplock, R.; Swider, M.; Kosyk, M.S.; et al. Outcome measures for clinical trials of leber congenital amaurosis caused by the intronic mutation in the CEP290 gene. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2609–2622. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Sun, W.; Xiao, X.; Li, S.; Jia, X.; Li, X.; Yu, B.; Wang, P.; Zhang, Q. Novel variants in GUCY2D causing retinopathy and the genotype-phenotype correlation. Exp. Eye Res. 2021, 208, 108637. [Google Scholar] [CrossRef]
- Annear, M.J.; Mowat, F.M.; Occelli, L.M.; Smith, A.J.; Curran, P.G.; Bainbridge, J.W.; Ali, R.R.; Petersen-Jones, S.M. A Comprehensive Study of the Retinal Phenotype of Rpe65-Deficient Dogs. Cells 2021, 10, 115. [Google Scholar] [CrossRef]
- Graham, K.L.; McCowan, C.I.; Caruso, K.; Billson, F.M.; Whittaker, C.J.G.; White, A. Optical coherence tomography of the retina, nerve fiber layer, and optic nerve head in dogs with glaucoma. Vet. Ophthalmol. 2019, 23, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Dufour, V.L.; Yu, Y.; Pan, W.; Ying, G.-S.; Aguirre, G.D.; Beltran, W.A. In-vivo longitudinal changes in thickness of the canine postnatal retina. Exp. Eye Res. 2020, 192, 107926. [Google Scholar] [CrossRef]
- Somma, A.T.; Moreno, J.C.D.; Sato, M.T.; Rodrigues, B.D.; Bacellar-Galdino, M.; Occelli, L.M.; Petersen-Jones, S.M.; Montiani-Ferreira, F. Characterization of a novel form of progressive retinal atrophy in Whippet dogs: A clinical, electroretinographic, and breeding study. Vet. Ophthalmol. 2017, 20, 450–459. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, E.H.C.; Boon, C.J.F. Serous business: Delineating the broad spectrum of diseases with subretinal fluid in the macula. Prog. Retin. Eye Res. 2021, 84, 100955. [Google Scholar] [CrossRef]
- Kelawala, D.N.; Patil, D.B.; Parikh, P.V.; Sheth, M.J.; Joshi, C.G.; Reddy, B. Clinical studies on progressive retinal atrophy in 31 dogs. Iran. J. Vet. Res. 2017, 18, 119–123. [Google Scholar] [PubMed Central]
- Urkasemsin, G.; Pongpanich, M.; Sariya, L.; Kongcharoen, A.; Buddhirongawatr, R.; Rungarunlert, S.; Ferreira, J.N.; Chetruengchai, W.; Phokaew, C.; Srichomthong, C.; et al. Whole genome sequencing identifies a homozygous nonsense mutation in the JPH2 gene in Shih Tzu dogs with progressive retinal atrophy. Anim. Genet. 2021, 52, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Pardon, L.P.; Ho, A.C.; Lauer, A.K.; Yoon, D.; Boye, S.E.; Boye, S.L.; Roman, A.J.; Wu, V.; Garafalo, A.V.; et al. Safety and efficacy of ATSN-101 in patients with Leber congenital amaurosis caused by biallelic mutations in GUCY2D: A phase 1/2, multicentre, open-label, unilateral dose escalation study. Lancet 2024, 404, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Boye, S.E.; Hauswirth, W.W.; Boye, S.L.; University of Florida Research Foundation Inc. Raav-Guanylate Cyclase Compositions and Methods for Treating Leber’s Congenital Amaurosis-1 (lca1). U.S. Patent US2022/0186260A1, 16 June 2022. [Google Scholar]
- Beckwith-Cohen, B.; Winkler, P.A.; Azarkevich, A.J.; Sun, K.; Carrion, G.M.; Bortolini, M.; Ferreira, F.M.; Boye, S.E.; Boye, S.L.; Petersen-Jones, S.M. Gene augmentation therapy imparts partial visual recovery in a large animal model of GUCY2D Leber congenital amaurosis (LCA1). Investig. Ophthalmol. Vis. Sci. 2025, 66, 2699. [Google Scholar]
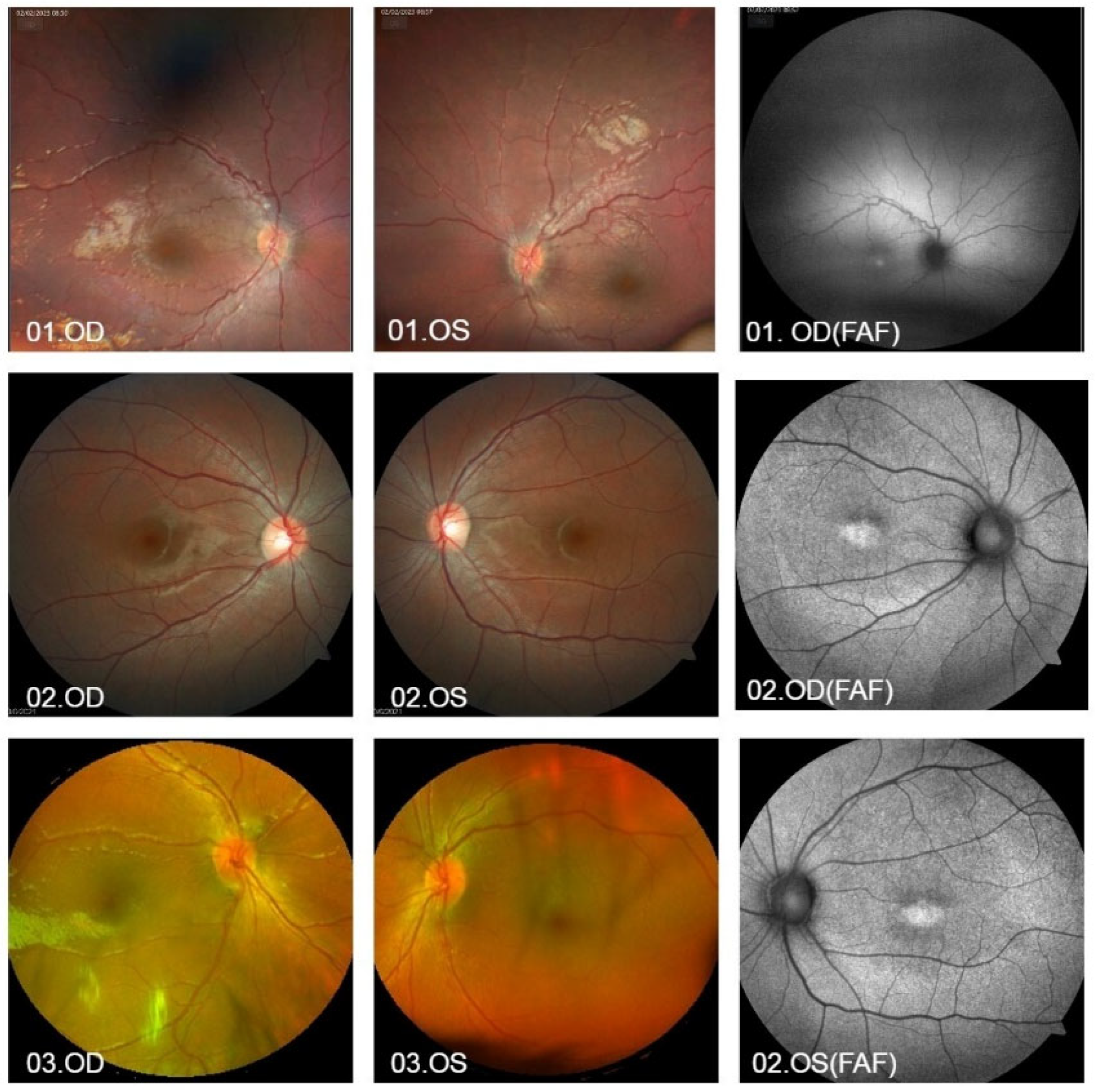
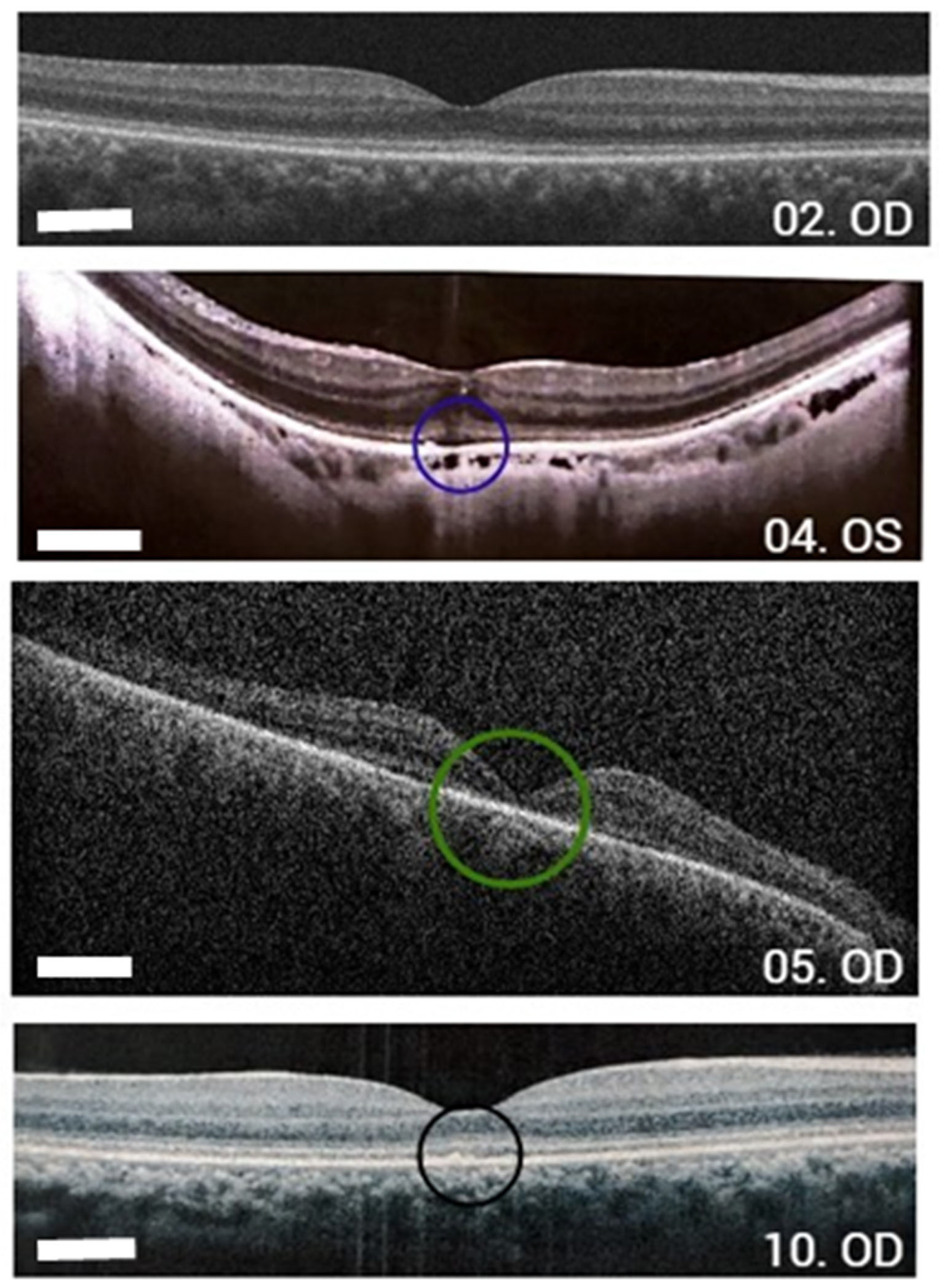
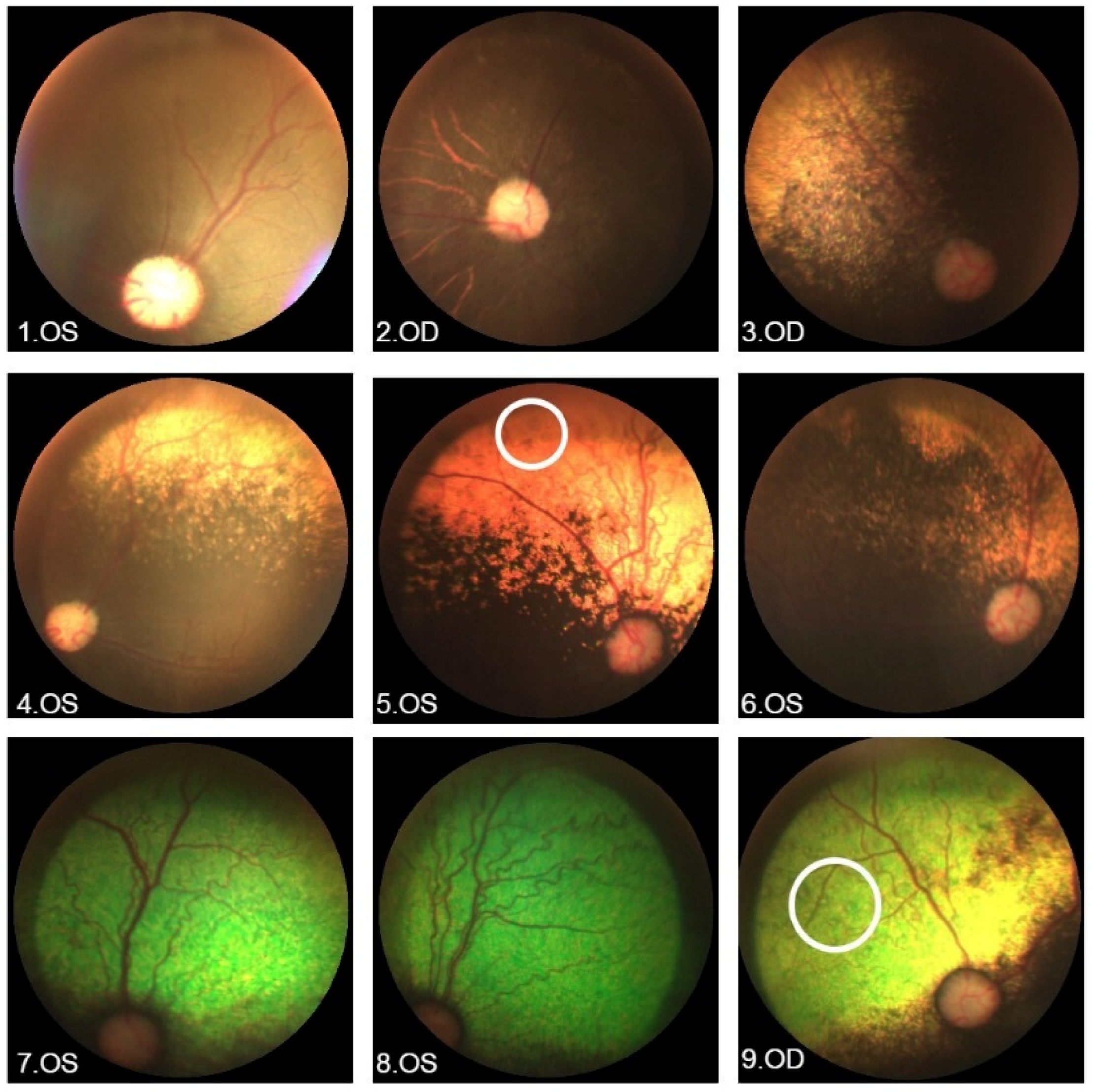

| ID | Allele 1 | Allele 2 | Sex | Age (Years) | BCVA (Snellen Table) | History and Eye Exam | Macular Alteration |
|---|---|---|---|---|---|---|---|
| 01 | c.1956+1G>A (paternal) | c.1245delT (maternal) | M | 10 | No LP OU | 3-month-old nystagmus (sibling of G08) | Foveal hyper-autofluorescence |
| 08 | c.1956+1G>A (paternal) | c.1245delT (maternal) | F | 8 | LP OU | Nystagmus, strabismus (sibling of G01) | Foveal hyper-autofluorescence |
| 02 | c.2620G>A (p.Glu874Lys) | c.1A>G (p.Met1?) | F | 17 | 20/60 OU | Phenotype of cone dystrophy | Foveal hyper-autofluorescence |
| 03 | c.1245delT (p.Phe415LeufsX73) | c.2598G>C (p.Lys866Asn) | M | 4 | HM OU | Nystagmus and ET | No |
| 04 | c.1052A>G (p.Tyr351Cys) | c.1052A>G (p.Tyr351Cys) | F | 55 | 20/80 OD–20/70 OS | Night blindness since childhood, myopia | Yes (hypertrophy of RPE foveal) OD, foveal hyper-autofluorescence OS |
| 05 | c.1972C>T (p.His658Tyr) | c.1972C>T (p.His658Tyr) | M | 37 | HM OU | Consanguineous parents Keratoconus, corneal leucoma in OS | Bull’s eye OU, ring hypo-autofluorescence around the fovea. |
| 06 | c.1957-2A>G | c.1957-2A>G | M | 14 | HM OD-LP OS | Consanguineous parents | No |
| 07 | c.1343C>A (p.Ser448X) | c.1343C>A (p.Ser448X) | M | 10 | No LP OU | Consanguineous parents Low VA, nystagmus from birth Hyperopia | No |
| 09 | c.1343C>A (p.Ser448X) | c.1957-2A>G | F | 4 | LP OU | Nystagmus, strabismus, low VA since 2 months | - |
| 10 | c.2302C>T (p.Arg768Trp) | c.767A>C (p.Gln256Pro) | F | 23 | 20/150 OU | Change in macular brightness | Foveal RPE atrophy, foveal hyper-autofluorescence with hipo-autofluorescence areas OU |
| ID | Gender | Age at Diagnosis (Months) | ERG Age (Months) | OCT Age (Months) |
|---|---|---|---|---|
| 1. | M | 3 | 3 | |
| 2. | M | 3 | 3 | 32 |
| 3. | M | 3 | 3 | 36 |
| 4. | F | 5 | 5 | |
| 5. | F | 22 | 9 | |
| 6. | M | 9 | 9 | |
| 7. | M | 3 | 3 | 15 |
| 8. | M | 3 | 4 | 8 |
| 9. | F | 3 | 4 | 8 |
| 10. | M | 3 | 4 | 13 |
| 11. | M | 4 | 4 | |
| 12. | M | 15 | 15 | |
| 13. | F | 2 | 2 | 4 |
| 14. | F | 2 | 2 | |
| 15. | F | 2 | 2 | |
| 16. | M | 1.5 | 3 |
| Human Group | Dog Group | |
|---|---|---|
| Age | 4 to 55 years | 2 to 22 months |
| Autosomal inheritance | 100% AR | 100% AR |
| Extremely low BCVA | 70% | 100% |
| Nystagmus | 50% | 81.3% |
| ERG | Absence of responses from the cones and rods | Absence of responses from the cones and rods |
| OCT | Reduced/absence reflectivity of the RPE and the ellipsoid zone | Reduced/absence reflectivity of the RPE and the ellipsoid zone; Presence NRD |
| Fundus Photography |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guareschi, B.L.V.; Sallum, J.M.F.; Salles, M.V.; de Moraes, J.G.O.; Bortolini, M.; Cray, C.; Moore, B.A.; da Rosa, C.C.; Montiani-Ferreira, F. GUCY2D-Associated Retinopathy: A Comparative Study Between Humans and German Spitz Dogs. Vet. Sci. 2025, 12, 879. https://doi.org/10.3390/vetsci12090879
Guareschi BLV, Sallum JMF, Salles MV, de Moraes JGO, Bortolini M, Cray C, Moore BA, da Rosa CC, Montiani-Ferreira F. GUCY2D-Associated Retinopathy: A Comparative Study Between Humans and German Spitz Dogs. Veterinary Sciences. 2025; 12(9):879. https://doi.org/10.3390/vetsci12090879
Chicago/Turabian StyleGuareschi, Bianca L. V., Juliana M. F. Sallum, Mariana V. Salles, João G. O. de Moraes, Mariza Bortolini, Carolyn Cray, Bret A. Moore, Carolina C. da Rosa, and Fabiano Montiani-Ferreira. 2025. "GUCY2D-Associated Retinopathy: A Comparative Study Between Humans and German Spitz Dogs" Veterinary Sciences 12, no. 9: 879. https://doi.org/10.3390/vetsci12090879
APA StyleGuareschi, B. L. V., Sallum, J. M. F., Salles, M. V., de Moraes, J. G. O., Bortolini, M., Cray, C., Moore, B. A., da Rosa, C. C., & Montiani-Ferreira, F. (2025). GUCY2D-Associated Retinopathy: A Comparative Study Between Humans and German Spitz Dogs. Veterinary Sciences, 12(9), 879. https://doi.org/10.3390/vetsci12090879







