A Survey of the Reproductive Lesions in Captive Female Non-Human Primates in Italy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
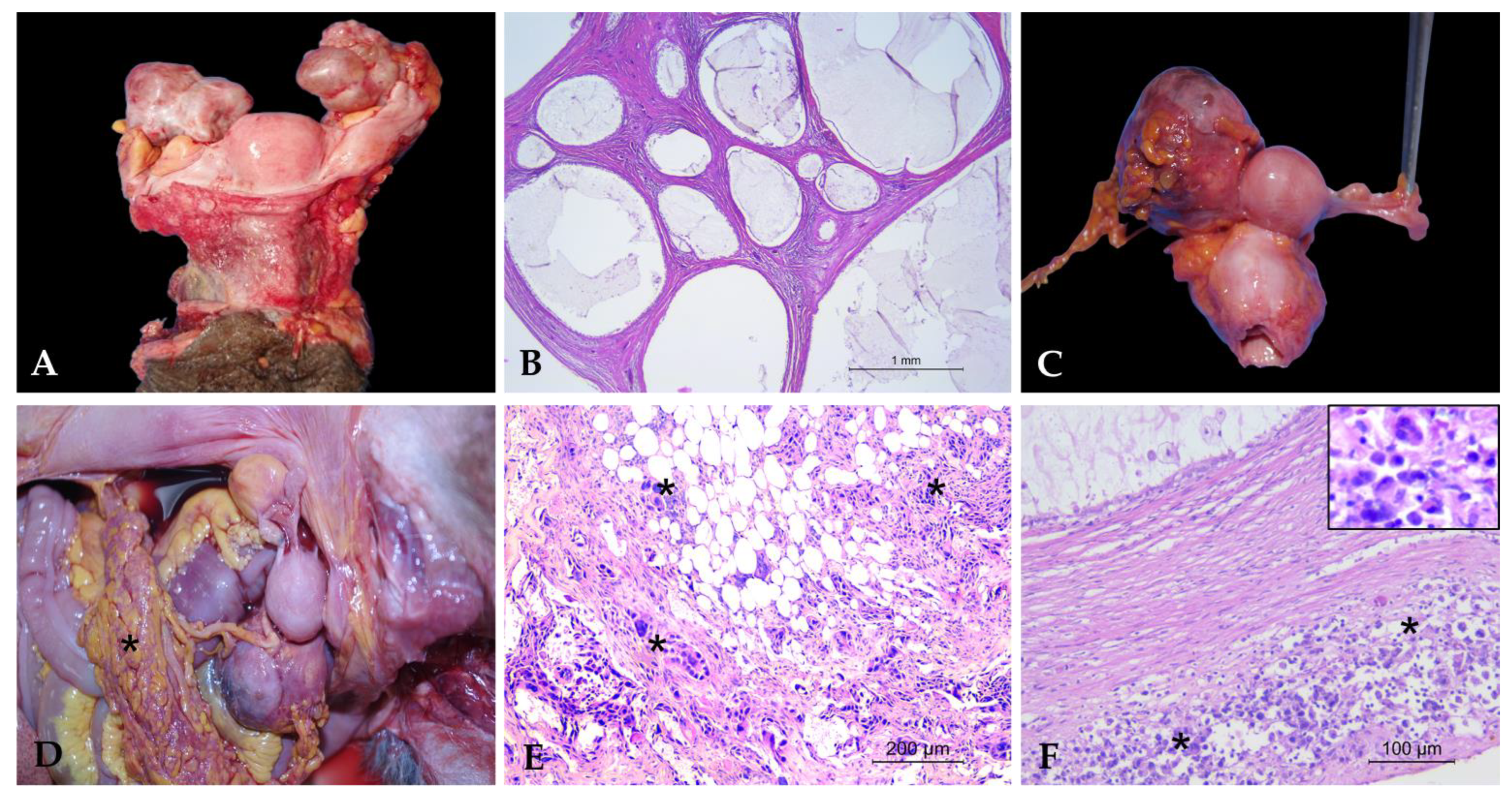
3.1. Uterine Pathologies
3.1.1. Non-Neoplastic Uterine Lesions
3.1.2. Neoplastic Uterine Lesions
3.2. Ovarian Pathologies
3.2.1. Non-Neoplastic Ovarian Lesions
3.2.2. Neoplastic Ovarian Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bauer, C. The baboon (Papio sp.) as a model for female reproduction studies. Contraception 2015, 92, 120–123. [Google Scholar] [CrossRef]
- Moresco, A.; Feltrer-Rambaud, Y.; Wolfman, D.; Agnew, D.W. Reproductive one health in primates. Am. J. Primatol. 2022, 84, e23325. [Google Scholar] [CrossRef]
- Cline, J.M.; Wood, C.E.; Vidal, J.D.; Tarara, R.P.; Buse, E.; Weinbauer, G.F.; de Rijk, E.P.; van Esch, E. Selected Background Findings and Interpretation of Common Lesions in the Female Reproductive System in Macaques. Toxicol. Pathol. 2008, 36, 142s–163s. [Google Scholar] [CrossRef] [PubMed]
- Chaffee, B.K.; Beck, A.P.; Owston, M.A.; Kumar, S.; Baze, W.B.; Magden, E.R.; Dick, E.J., Jr.; Lammey, M.; Abee, C.R. Spontaneous Reproductive Tract Lesions in Aged Captive Chimpanzees. Vet. Pathol. 2016, 53, 425–435. [Google Scholar] [CrossRef]
- Didier, E.S.; MacLean, A.G.; Mohan, M.; Didier, P.J.; Lackner, A.A.; Kuroda, M.J. Contributions of Nonhuman Primates to Research on Aging. Vet. Pathol. 2016, 53, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; KwaK, J.; Kang, B.C.; Ku, S.Y. Non-human primate: The new frontier model of female reproductive engineering. Front. Bioeng. Biotechnol. 2025, 13, 1536750. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.H.; Rogers, J.; Dumesic, D.A.; Levine, J.E. Naturally Occurring and Experimentally Induced Rhesus Macaque Models for Polycystic Ovary Syndrome: Translational Gateways to Clinical Application. Med. Sci. 2019, 7, 107. [Google Scholar] [CrossRef]
- Kirejczyk, S.; Pinelli, C.; Gonzalez, O.; Kumar, S.; Dick, E., Jr.; Gumber, S. Urogenital Lesions in Nonhuman Primates at 2 National Primate Research Centers. Vet. Pathol. 2021, 58, 147–160. [Google Scholar] [CrossRef]
- Green, S.L.; Tolwani, R.J.; Waggie, K.S.; Otto, G.M. Endometriosis and a paraovarian cyst in a rhesus macaque. Vet. Radiol. Ultrasound 1999, 40, 271–274. [Google Scholar] [CrossRef]
- Wilkinson, M.; Walters, S.; Smith, T.; Wilkinson, A. Reproductive abnormalities in aged female Macaca fascicularis. J. Med. Primatol. 2008, 37 (Suppl. S1), 88–93. [Google Scholar] [CrossRef]
- Gall, A.J.; Olds, J.E.; Wunschmann, A.; Selmic, L.E.; Rasmussen, J.; Lewis, A.D. Lesions of the female reproductive tract in Japanese macaque (Macaca fuscata) from two captive colonies. J. Zoo Wildl. Med. 2018, 49, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kondova, I.; Braskamp, G.; Heidt, P.J.; Collignon, W.; Haaksma, T.; de Groot, N.; Otting, N.; Doxiadis, G.; Westmoreland, S.V.; Vallender, E.J.; et al. Spontaneous endometriosis in rhesus macaques: Evidence for a genetic association with specific Mamu-A1 alleles. Primate Biol. 2017, 4, 117–125. [Google Scholar] [CrossRef][Green Version]
- Brown, S.L.; Anderson, D.C.; Dick, E.J., Jr.; Guardado-Mendoza, R.; Garcia, A.P.; Hubbard, G.B. Neoplasia in the chimpanzee (Pan spp.). J. Med. Primatol. 2009, 38, 137–144. [Google Scholar] [CrossRef]
- Stringer, E.M.; De Voe, R.S.; Valea, F.; Toma, S.; Mulvaney, G.; Pruitt, A.; Troan, B.; Loomis, M.R. Medical and surgical management of reproductive neoplasia in two western lowland gorillas (Gorilla gorilla gorilla). J. Med. Primatol. 2010, 39, 328–335. [Google Scholar] [CrossRef]
- Videan, E.N.; Satterfield, W.C.; Buchl, S.; Lammey, M.L. Diagnosis and prevalence of uterine leiomyomata in female chimpanzees (Pan troglodytes). Am. J. Primatol. 2011, 73, 665–670. [Google Scholar] [CrossRef]
- Pereira, A.H.B.; Rocha, F.C.; Carvalho, M.P.S.; Barbosa, B.E.P.; Balthazar, D.A.; Moreira, S.B.; Pissinatti, A.; Ubiali, D.G. Female Reproductive System Neoplasms in Neotropical Primates. J. Med. Primatol. 2025, 54, e70027. [Google Scholar] [CrossRef] [PubMed]
- Barrier, B.F.; Allison, J.; Hubbard, G.B.; Dick, E.J., Jr.; Brasky, K.M.; Schust, D.J. Spontaneous adenomyosis in the chimpanzee (Pan troglodytes): A first report and review of the primate literature: Case report. Hum. Reprod. 2007, 22, 1714–1717. [Google Scholar] [CrossRef][Green Version]
- Hanley, P.W.; Barnhart, K.F.; Satterfield, W.C.; McArthur, M.J.; Buchl, S.J.; Baze, W.B. Obstructive uropathy secondary to uterine leiomyoma in a chimpanzee (Pan troglodytes). Comp. Med. 2012, 62, 543–545. [Google Scholar][Green Version]
- DiGiacomo, R.F. Gynecologic pathology in the rhesus monkey (Macaca mulatta). II. Findings in laboratory and free-ranging monkeys. Vet. Pathol. 1977, 14, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Forslund, O.; Antonsson, A.; Nordin, P.; Stenquist, B.; Göran Hansson, B. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J. Gen. Virol. 1999, 80, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Nespeca, G.; Grest, P.; Rosenkrantz, W.S.; Ackermann, M.; Favrot, C. Detection of novel papillomaviruslike sequences in paraffin-embedded specimens of invasive and in situ squamous cell carcinomas from cats. Am. J. Vet. Res. 2006, 67, 2036–2041. [Google Scholar] [CrossRef]
- Galietta, V.; Fonti, N.; Cocumelli, C.; Raso, C.; Di Cerbo, P.; Parisi, F.; Bovi, E.; Parmigiani, R.; Pietrella, G.; Cersini, A.; et al. Histiocytic Sarcoma in a Captive Hybrid Orangutan (Pongo sp.): Morphological and Immunohistochemical Features. Animals 2024, 14, 852. [Google Scholar] [CrossRef]
- Nishimoto-Kakiuchi, A.; Netsu, S.; Okabayashi, S.; Taniguchi, K.; Tanimura, H.; Kato, A.; Suzuki, M.; Sankai, T.; Konno, R. Spontaneous endometriosis in cynomolgus monkeys as a clinically relevant experimental model. Hum. Reprod. 2018, 33, 1228–1236. [Google Scholar] [CrossRef]
- Cooper, T.K.; Gabrielson, K.L. Spontaneous lesions in the reproductive tract and mammary gland of female non-human primates. Birth Defects Res. B Dev. Reprod. Toxicol. 2007, 80, 149–170. [Google Scholar] [CrossRef]
- Saunder, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef] [PubMed]
- Crump, J.; Suker, A.; White, L. Endometriosis: A review of recent evidence and guidelines. Aust. J. Gen. Pract. 2024, 53, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Schrager, S.; Yogendran, L.; Marquez, C.M.; Sadowski, E.A. Adenomyosis: Diagnosis and Management. Am. Fam. Physician 2022, 105, 33–38. [Google Scholar] [PubMed]
- Ami, Y.; Suzaki, Y.; Goto, N. Endometriosis in cynomolgus monkeys retired from breeding. J. Vet. Med. Sci. 1993, 55, 7–11. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Petraglia, F.; Catherino, W.H.; Donnez, J. Pathogenesis of uterine fibroids: Current understanding and future directions. Fertil. Steril. 2024, 122, 6–11. [Google Scholar] [CrossRef]
- Stewart, E.A.; Cookson, C.L.; Gandolfo, R.A.; Schulze-Rath, R. Epidemiology of uterine fibroids: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1501–1512. [Google Scholar] [CrossRef]
- Dagur, G.; Suh, Y.; Warren, K.; Singh, N.; Fitzgerald, J.; Khan, S.A. Urological complications of uterine leiomyoma: A review of literature. Int. Urol. Nephrol. 2016, 48, 941–948. [Google Scholar] [CrossRef]
- Giuliani, E.; As-Sanie, S.; Marsh, E.E. Epidemiology and management of uterine fibroids. Int. J. Gynaecol. Obstet. 2020, 149, 3–9. [Google Scholar] [CrossRef]
- Hertig, A.T.; MacKey, J.J.; Feeley, G.; Kampschmidt, K. Dysplasia of the lower genital tract in the female monkey, Macaca fascicularis, the crab-eating macaque from Southeast Asia. Am. J. Obstet. Gynecol. 1983, 145, 968–977. [Google Scholar] [CrossRef]
- Wood, C.E.; Borgerink, H.; Register, T.C.; Scott, L.; Cline, J.M. Cervical and vaginal epithelial neoplasms in cynomolgus monkeys. Vet. Pathol. 2004, 41, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Yang, C.; Tan, Q.; Song, W.; Lu, M.; Zhao, W.; Lou, G.; Li, Z.; Li, K.; Hou, Y. Sites of distant metastases and overall survival in ovarian cancer: A study of 1481 patients. Gynecol. Oncol. 2018, 150, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Hubbard, G.B.; Leland, M.M.; Dunn, B.G.; Best, R.G. Spontaneous ovarian tumors in twelve baboons: A review of ovarian neoplasms in non-human primates. J. Med. Primatol. 2003, 32, 48–56. [Google Scholar] [CrossRef]
- Lowenstine, L.J.; McManamon, R.; Terio, K.A. Comparative Pathology of Aging Great Apes: Bonobos, Chimpanzees, Gorillas, and Orangutans. Vet. Pathol. 2016, 53, 250–276. [Google Scholar] [CrossRef] [PubMed]

| Category of NHP | Family | Common Name | Scientific Name | Code | Age | Uterus | Ovary |
|---|---|---|---|---|---|---|---|
| Old World NHP | Cercopithecidae | Assam macaque | Macaca assamensis | Ma | 33 | Cystic endometrial hyperplasia | |
| Adenomyosis | |||||||
| Cercopithecidae | Cynomolgus macaque | Macaca fascicularis | Mfa 1 | 17 | Leiomyoma | ||
| Cercopithecidae | Cynomolgus macaque | Macaca fascicularis | Mfa 2 | adult | Ovarian Adenocarcinoma with peritoneal carcinomatosis | ||
| Cercopithecidae | Japanese macaque | Macaca fuscata | Mfu 1 | 24 | Leiomyoma | ||
| Cercopithecidae | Japanese macaque | Macaca fuscata | Mfu 2 | 24 | Leiomyoma | ||
| Cercopithecidae | Japanese macaque | Macaca fuscata | Mfu 3 | 29 | Leiomyoma | ||
| Cercopithecidae | Rhesus macaque | Macaca mulatta | Mm | 30 | Leiomyoma | ||
| Cercopithecidae | Pig-tailed macaque | Macaca nemestrina | Mn | 20 | Endometriosis | ||
| Adenomyosis | |||||||
| Hylobatidae | Lar gibbon | Hylobates Iar | HI | 31 | Cystic endometrial hyperplasia | ||
| Adenomyosis | |||||||
| Hominidae | Chimpanzee | Pan troglodytes | Pt | 27 | Leiomyoma | ||
| Hominidae | Orangutan | Pongo pygmaeus | Pp 1 | 45 | Leiomyoma | Follicular cysts | |
| Metastatic histiocytic sarcoma | |||||||
| Hominidae | Orangutan | Pongo pygmaeus | Pp 2 | 37 | Follicular cysts | ||
| New World NHP | Cebidae | Tufted capuchin | Sapajus apella | Sa1 | 19 | Cystic endometrial hyperplasia | |
| Cebidae | Tufted capuchin | Sapajus apella | Sa2 | 27 | Adenocarcinoma of the cervix | ||
| Prosimian | Lemuridae | Ring-tailed lemur | Lemur catta | Lc | 18 | Metastatic mammary carcinoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galietta, V.; Cocumelli, C.; Parmigiani, R.; Bovi, E.; Palmerini, T.; Acri, C.; Di Cerbo, P.; Aloisi, M.; Cersini, A.; Eleni, C. A Survey of the Reproductive Lesions in Captive Female Non-Human Primates in Italy. Vet. Sci. 2025, 12, 856. https://doi.org/10.3390/vetsci12090856
Galietta V, Cocumelli C, Parmigiani R, Bovi E, Palmerini T, Acri C, Di Cerbo P, Aloisi M, Cersini A, Eleni C. A Survey of the Reproductive Lesions in Captive Female Non-Human Primates in Italy. Veterinary Sciences. 2025; 12(9):856. https://doi.org/10.3390/vetsci12090856
Chicago/Turabian StyleGalietta, Valentina, Cristiano Cocumelli, Raffaella Parmigiani, Emanuela Bovi, Tiziana Palmerini, Chiara Acri, Pilar Di Cerbo, Marco Aloisi, Antonella Cersini, and Claudia Eleni. 2025. "A Survey of the Reproductive Lesions in Captive Female Non-Human Primates in Italy" Veterinary Sciences 12, no. 9: 856. https://doi.org/10.3390/vetsci12090856
APA StyleGalietta, V., Cocumelli, C., Parmigiani, R., Bovi, E., Palmerini, T., Acri, C., Di Cerbo, P., Aloisi, M., Cersini, A., & Eleni, C. (2025). A Survey of the Reproductive Lesions in Captive Female Non-Human Primates in Italy. Veterinary Sciences, 12(9), 856. https://doi.org/10.3390/vetsci12090856






