Evaluation of the Dual Antiviral and Immunomodulatory Effects of Phallus indusiatus in a Feline Infectious Peritonitis Model Using PBMCs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Protein Expression and Purification
2.3. Inhibition of FIPV Mpro Protease
2.4. Animals and Experimental Design
2.5. The Preparation of the Mushroom Extraction Procedure
2.6. Preparation of PBMCs
2.7. In Vitro Optimize Toxicity of P. indusiatus, LPS, and DMSO
2.8. Anti-Inflammatory Assay Using Nitrite Scavenging Activity
2.9. Statistical Analysis
3. Results
3.1. Expression and Purification of FIPV Mpro
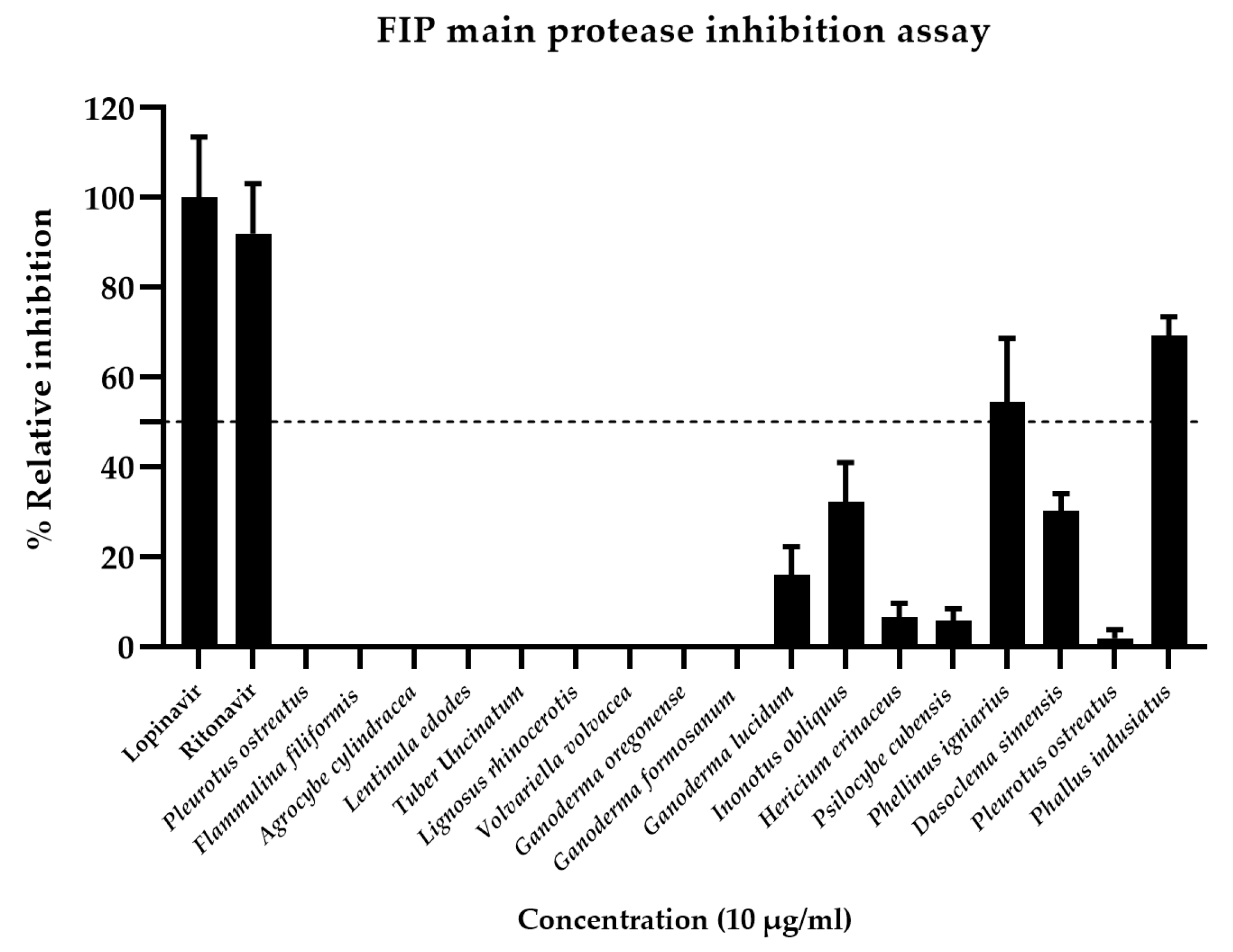
3.2. Inhibition of FIPV Mpro Activity by Antiviral Drugs and Crude Mushroom Extract
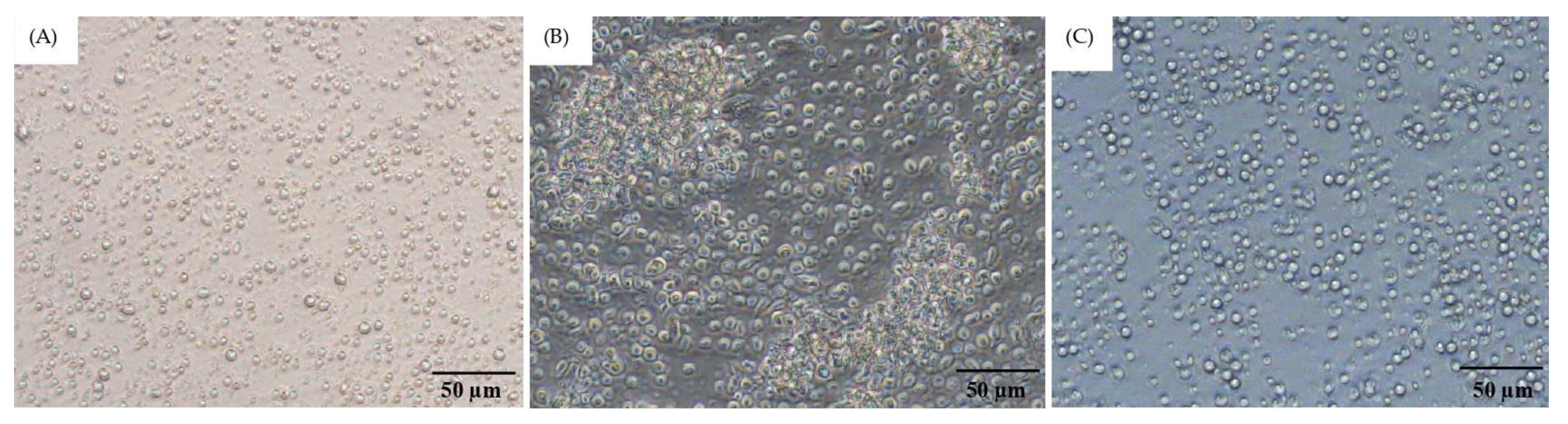
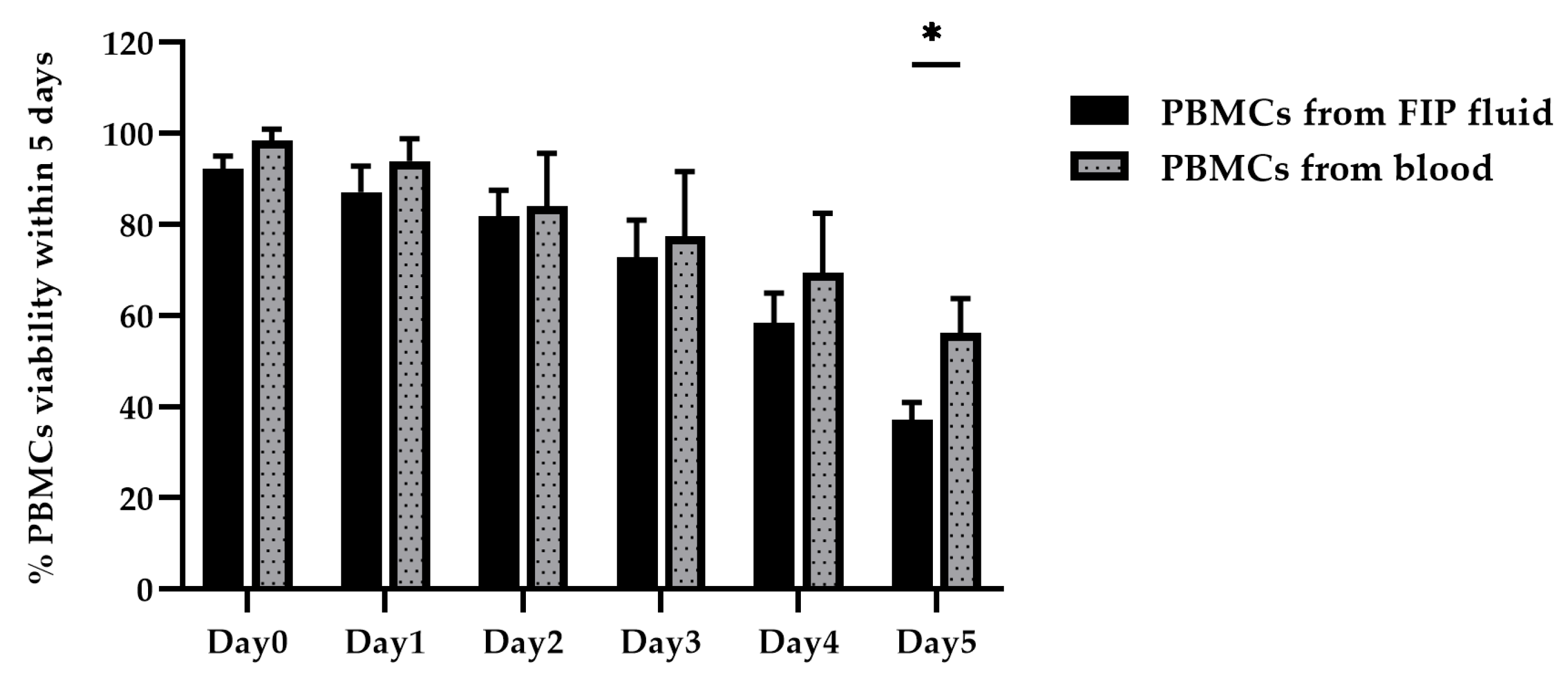
3.3. Optimization of PBMC Culture Conditions Using Whole Blood Samples and FIP Fluid
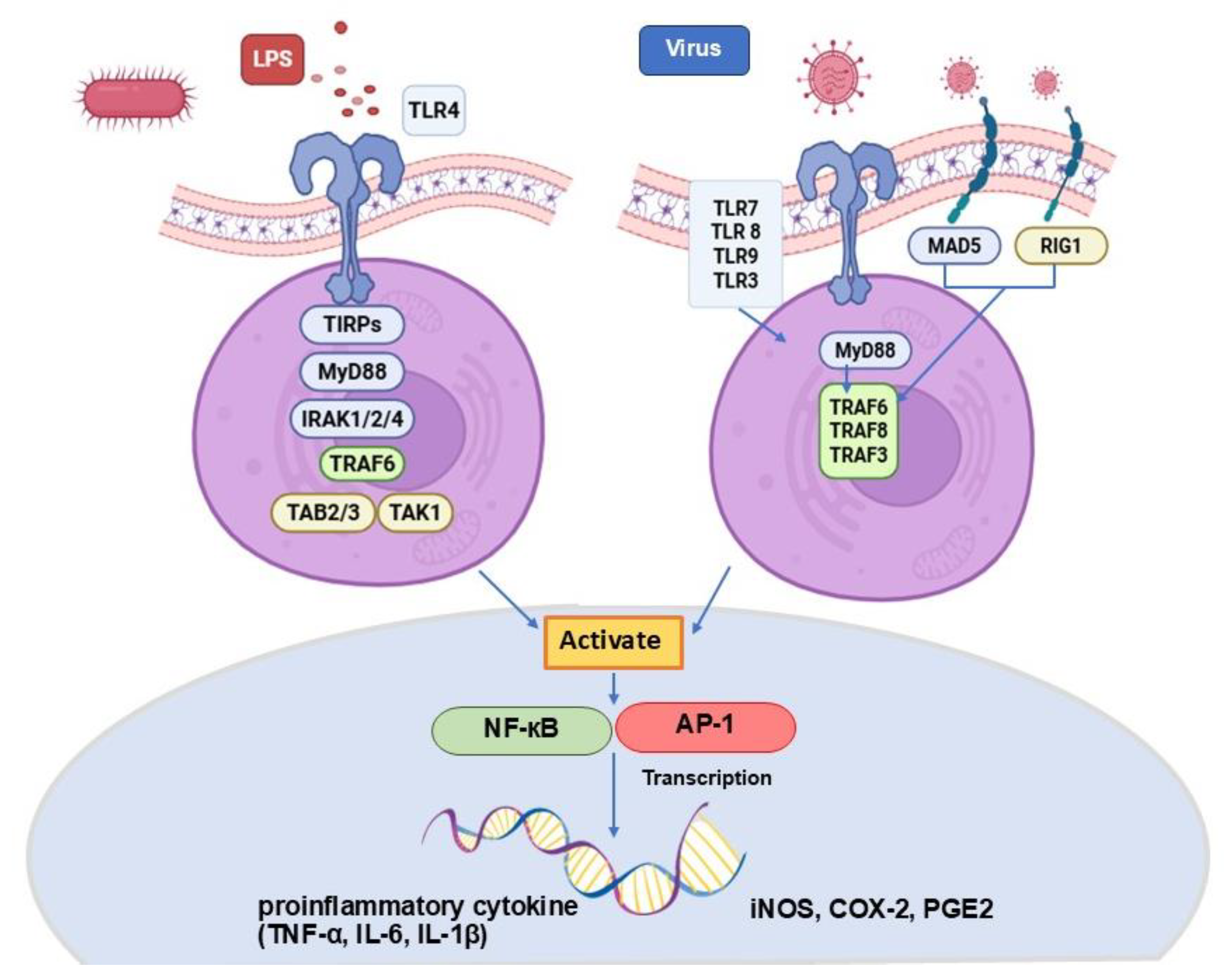
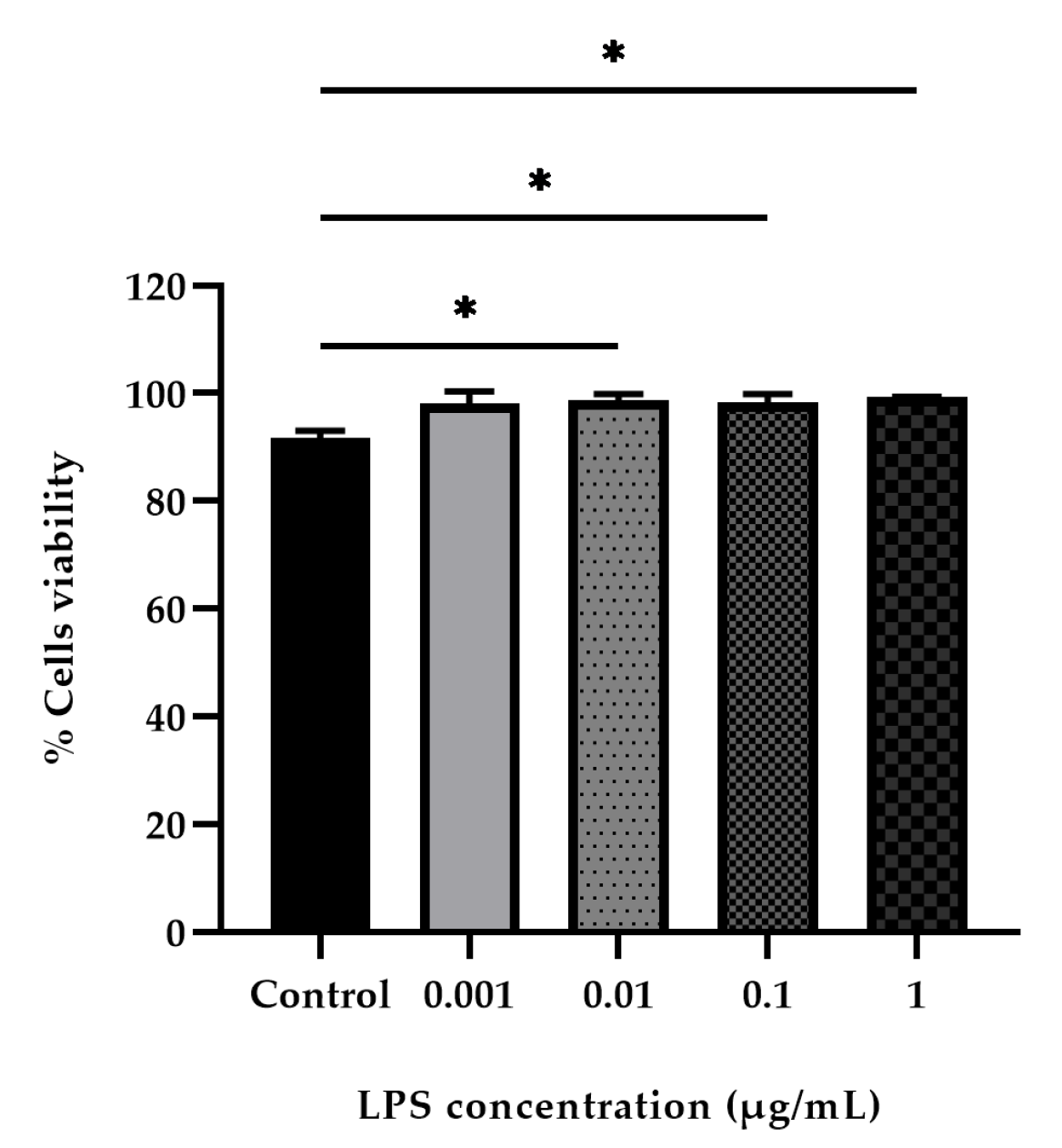
3.4. LPS-Stimulated PBMCs
3.5. Effect of P. indusiatus on the Cell Viability of PBMCs
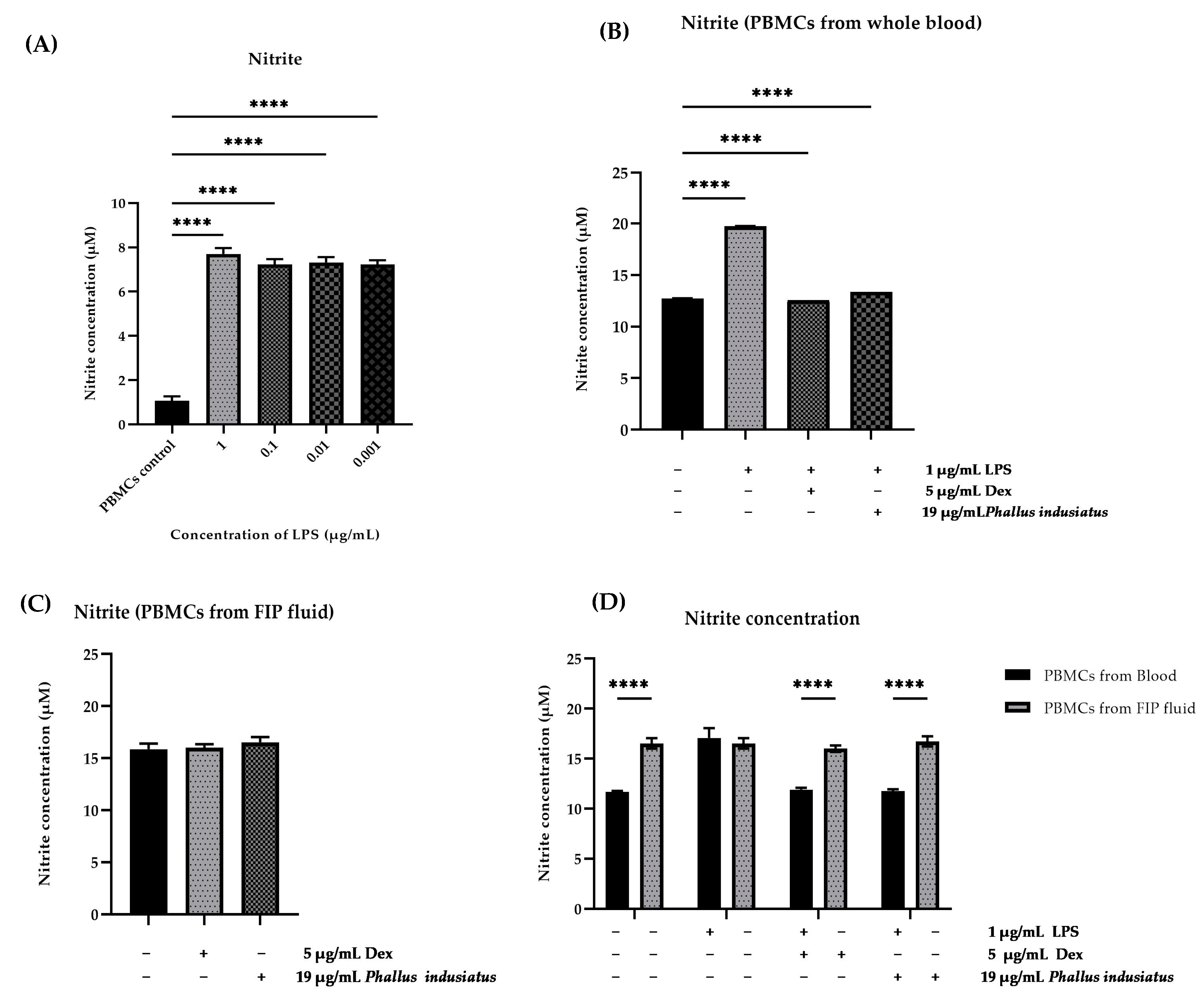
3.6. Evaluation of Nitrite Scavenging Activity of P. indusiatus Extract in LPS-Stimulated PBMCs
3.7. Phytochemical Composition and Literature Support for Anti-Inflammatory Activity
| Preparation | Bioactive Compound | Experimental Model | Key Findings | References |
|---|---|---|---|---|
| Crude extract, hot water, and ethanol. | Phenolics, flavonoids, and tannins. | Murine macrophages used to study the anti-inflammatory effects of LPS stimulation. | The anti-inflammatory activity was demonstrated by a reduction in the level of released nitric oxide (NO). | Theeraraksakul, K. et al., 2023 [21] |
| Crude extract, peel and green mixture, core, and whole mushroom. | Catechins, flavonoids, polysaccharides, monosaccharides, mucopolysaccharides, allantoin, alkaloids, polyphenolic compounds, epicatechin, gallic acid, and caffeic acid. | The experiment was conducted using LPS-induced RAW 264.7 macrophages. | The anti-inflammatory activity was demonstrated by the inhibition of NO, IL-1β, IL-6, and TNF-α secretion. | Ruksiriwanich, W. et al., 2022., Nazir, Y., et al. 2021., Sedtananun, S. et al., 2025 [24,25,26] |
| Hot water extract of crude polysaccharide from mushroom fruiting body. | Polysaccharides. | Colitis was induced in mice using dextran sulfate sodium (DSS). | Reduced the expression of inducible iNOS | Kanwal, S. et al., 2020 [27] |
| This study utilized an ethanolic extract derived from the whole mushroom. | Phenolics, flavonoids, and tannins. [21] | PBMCs from healthy cats were stimulated with LPS and PBMCs from FIP fluid | The anti-inflammatory activity was demonstrated by the inhibition of NO in PBMCs isolated from healthy cats. | Hlaoperm, C. et al. |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartmann, K. Feline infectious peritonitis. Vet. Clin. N. Am. Small Anim. Pract. 2005, 35, 39–79. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Mari, V.; Lanave, G.; Lorusso, E.; Lucente, M.S.; Desario, C.; Colaianni, M.L.; Elia, G.; Ferringo, F.; Alfano, F.; et al. Mutation analysis of the spike protein in Italian feline infectious peritonitis virus and feline enteric coronavirus sequences. Res. Vet. Sci. 2021, 135, 15–19. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Wang, Q.; Liang, X.Y.; Zhang, S.; Bao, D.; Zhao, H.; Li, S.B.; Wang, K.; Hu, G.X.; Gao, F.S. An updated review of feline coronavirus: Mind the two biotypes. Virus Res. 2023, 326, 199059. [Google Scholar] [CrossRef]
- Xing, L.; Remick, D.G. Relative cytokine and cytokine inhibitor production by mononuclear cells and neutrophils. Shock 2003, 20, 10–16. [Google Scholar] [CrossRef]
- Addie, D.; Belák, S.; Boucraut, B.C.; Egberink, H.; Frymus, T.; Gruffydd, J.T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; Lutz, H.; et al. Feline infectious peritonitis: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 594–604. [Google Scholar] [CrossRef]
- Saylor, S.S.; Coggins, S.; Barker, E.N.; Gunn-Moore, D.; Jeevaratnam, K.; Norris, J.M.; Hughes, D.; Stacey, E.; MacFarlane, L.; O’Brien, C.; et al. Retrospective study and outcome of 307 cats with feline infectious peritonitis treated with legally sourced veterinary compounded preparations of remdesivir and GS-441524 (2020–2022). J. Feline Med. Surg. 2023, 25, 1098612X231194460. [Google Scholar] [CrossRef]
- Habtemariam, S. The chemistry, pharmacology and therapeutic potential of the edible mushroom Dictyophora indusiata (Vent ex. Pers.) Fischer (syn. Phallus indusiatus). Biomedicines 2019, 7, 98. [Google Scholar] [CrossRef]
- Suleiman, W.B.; Shehata, R.M.; Younis, A.M. In vitro assessment of multipotential therapeutic importance of Hericium erinaceus mushroom extracts using different solvents. Bioresour. Bioprocess. 2022, 9, 1–13. [Google Scholar] [CrossRef]
- Luo, R.; Zhang, Z.; Li, Y. An examination of the LPS–TLR4 immune response through the analysis of molecular structures and protein–protein interactions. Cell Commun. Signal. 2025, 23, 142. [Google Scholar] [CrossRef]
- Hennessy, C.; McKernan, D.P. Anti-viral pattern recognition receptors as therapeutic targets. Cells 2021, 10, 2258. [Google Scholar] [CrossRef]
- Yu, L.; Gao, F.; Li, Y.; Su, D.; Han, L.; Li, Y.; Zhang, X.; Feng, Z. Role of pattern recognition receptors in the development of MASLD and potential therapeutic applications. Biomed. Pharmacother. 2024, 175, 116724. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, Y.J. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 2007, 76, 447–480. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-inflammatory effect of sulforaphane on LPS-activated microglia potentially through JNK/AP-1/NF-κB inhibition and Nrf2/HO-1 activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Vuong, W.; Khan, M.B.; Fischer, C.; Arutyunova, E.; Lamer, T.; Shields, J.; Saffran, H.A.; McKay, R.T.; van Belkum, M.J.; Joyce, M.A.; et al. Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat. Commun. 2020, 11, 4282. [Google Scholar] [CrossRef] [PubMed]
- Citarella, A.; Scala, A.; Piperno, A.; Micale, N. SARS-CoV-2 Mpro: A potential target for peptidomimetics and small-molecule inhibitors. Biomolecules 2021, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Piao, L.; Ren, C.; Zhang, W.; Zhu, Y.; Kong, R. Identifying natural products as feline coronavirus Mpro inhibitors by structure-based virtual screening and enzyme-based assays. ACS Omega 2025, 10, 2092–2101. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Tan, Y.; Chen, X.; Zhao, Q.; Fu, S.; Li, S.; Chen, C.; Yang, H. Crystallization and preliminary crystallographic study of feline infectious peritonitis virus main protease in complex with an inhibitor. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2014, 70, 1612–1615. [Google Scholar] [CrossRef]
- Moyadee, W.; Roytrakul, S.; Jaresitthikunchai, J.; Phaonakrop, N.; Choowongkomon, K.; Ploypetch, S.; Tansakul, N.; Rattanasrisomporn, A.; Rattanasrisomporn, J. Serum proteomic approach to identifying differentially expressed proteins in effusive feline infectious peritonitis. Sci. Rep. 2025, 15, 18899. [Google Scholar] [CrossRef]
- Khumtong, K.; Rapichai, W.; Saejung, W.; Khamsingnok, P.; Meecharoen, N.; Ratanabunyong, S.; Dong, H.V.; Tuanthap, S.; Rattanasrisomporn, A.; Choowongkomon, K.; et al. Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification with Xylenol Orange Targeting Nucleocapsid Gene for Detection of Feline Coronavirus Infection. Viruses 2025, 17, 418. [Google Scholar] [CrossRef]
- Kosukwatthana, P.; Rungsuriyawiboon, O.; Rattanasrisomporn, J.; Kimram, K.; Tansakul, N. Cytotoxicity and Immunomodulatory Effects of Cannabidiol on Canine PBMCs: A Study in LPS-Stimulated and Epileptic Dogs. Animals 2024, 14, 3683. [Google Scholar] [CrossRef]
- Theeraraksakul, K.; Jaengwang, K.; Choowongkomon, K.; Tabtimmai, L. Exploring the biological functions and anti-melanogenesis of Phallus indusiatus for mushroom-based cosmetic applications. Cosmetics 2023, 10, 121. [Google Scholar] [CrossRef]
- Bull, M.; Lee, D.; Stucky, J.; Chiu, Y.L.; Rubin, A.; Horton, H.; McElrath, M.J. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J. Immunol. Methods 2007, 322, 57–69. [Google Scholar] [CrossRef]
- Bull, M.; Lee, D.; Stucky, J.; Chiu, Y.L.; Rubin, A.; Horton, H.; McElrath, M.J. Study of Macrophage Activity in Cats with FIP and Naturally FCoV-Shedding Healthy Cats. Pathogens 2024, 13, 437. [Google Scholar]
- Nazir, Y.; Linsaenkart, P.; Khantham, C.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; et al. High efficiency in vitro wound healing of Dictyophora indusiata extracts via anti-Inflammatory and collagen stimulating (MMP-2 inhibition) mechanisms. J. Fungi. 2021, 7, 1100. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Khantham, C.; Linsaenkart, P.; Chaitep, T.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Režek Jambrak, A.; Nazir, Y.; Yooin, W.; et al. Anti-inflammation of bioactive compounds from ethanolic extracts of edible bamboo mushroom (Dictyophora indusiata) as functional health promoting food ingredients. Int. J. Food Sci. Technol. 2022, 57, 110–122. [Google Scholar] [CrossRef]
- Sedtananun, S.; Sujiwattanarat, P.; Hoang Tra, M.H.; Suphakun, P.; Seetaha, S.; Auetragul, A.; Choowongkomon, K.; Tabtimmai, L. Phallus indusiatus Extracts Promoted MCF-7 Apoptosis Under TNFα-induced Tumor Microenvironment by Attenuating NF-kappaB and Akt Activation. Asian Pac. J. Cancer Prev. 2025, 26, 479. [Google Scholar] [CrossRef]
- Kanwal, S.; Joseph, T.P.; Aliya, S.; Song, S.; Saleem, M.Z.; Nisar, M.A.; Wang, Y.; Meyiah, A.; Ma, Y.; Xin, Y. Attenuation of DSS induced colitis by Dictyophora indusiata polysaccharide (DIP) via modulation of gut microbiota and inflammatory related signaling pathways. J. Funct. Foods 2020, 64, 103641. [Google Scholar] [CrossRef]
- Rong, Y.; Zhang, C.; Gao, W.C.; Zhao, C. Optimization of the expression of the main protease from SARS-CoV-2. Protein Expr. Purif. 2023, 203, 106208. [Google Scholar] [CrossRef]
- Ortiz, R.; Dejesus, E.; Khanlou, H.; Voronin, E.; van Lunzen, J.; Andrade-Villanueva, J.; Fourie, J.; De Meyer, S.; De Pauw, M.; Lefebvre, E.; et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS 2008, 22, 1389–1397. [Google Scholar] [CrossRef]
- Schoergenhofer, C.; Jilma, B.; Stimpfl, T.; Karolyi, M.; Zoufaly, A. Pharmacokinetics of lopinavir and ritonavir in patients hospitalized with coronavirus disease 2019 (COVID-19). Ann. Intern. Med. 2020, 173, 670–672. [Google Scholar] [CrossRef]
- Nutho, B.; Mahalapbutr, P.; Hengphasatporn, K.; Pattaranggoon, N.C.; Simanon, N.; Shigeta, Y.; Hannongbua, S.; Rungrotmongkol, T. Why are lopinavir and ritonavir effective against the newly emerged coronavirus 2019? Atomistic insights into the inhibitory mechanisms. Biochemistry 2020, 59, 1769–1779. [Google Scholar]
- Tsika, A.C.; Gallo, A.; Fourkiotis, N.K.; Argyriou, A.I.; Sreeramulu, S.; Löhr, F.; Rogov, V.V.; Richter, C.; Linhard, V.; Gande, S.L.; et al. Binding adaptation of GS-441524 diversifies Macro domains and downregulates SARS-CoV-2 de-MARylation capacity. J. Mol. Biol. 2022, 434, 167720. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Song, X.Q. Oral GS-441524 derivatives: Next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase. Front. Immunol. 2022, 13, 1015355. [Google Scholar] [CrossRef]
- Perera, K.D.; Rathnayake, A.D.; Liu, H.; Pedersen, N.C.; Groutas, W.C.; Chang, K.O.; Kim, Y. Characterization of amino acid substitutions in feline coronavirus 3C-like protease from a cat with feline infectious peritonitis treated with a protease inhibitor. Vet. Microbiol. 2019, 237, 108398. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C.; Kim, Y.; Liu, H.; Galasiti Kankanamalage, A.C.; Eckstrand, C.; Groutas, W.C.; Bannasch, M.; Meadows, J.M.; Chang, K.O. Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J. Feline Med. Surg. 2018, 20, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Su, H.; Zhao, W.; Xie, H.; Shao, Q.; Xu, Y. What coronavirus 3C-like protease tells us: From structure, substrate selectivity, to inhibitor design. Med. Res. Rev. 2021, 41, 1965–1998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Shyr, Z.; Lo, D.C.; Zheng, W. Viral proteases as targets for coronavirus disease 2019 drug development. J. Pharmacol. Exp. Ther. 2021, 378, 166–172. [Google Scholar] [CrossRef]
- Abian, O.; Ortega-Alarcon, D.; Jimenez-Alesanco, A.; Ceballos-Laita, L.; Vega, S.; Reyburn, H.T.; Rizzuti, B.; Velazquez-Campoy, A. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int. J. Biol. Macromol. 2020, 164, 1693–1703. [Google Scholar] [CrossRef]
- Majerová, T.; Konvalinka, J. Viral proteases as therapeutic targets. Mol. Aspects Med. 2022, 88, 101159. [Google Scholar] [CrossRef]
- Varghese, R.; Efferth, T.; Ramamoorthy, S. Exploring the anti-COVID-19 potential of mushroom metabolites: Current status and perspectives. In Traditional Medicines and Natural Products as Preventive and Therapeutic Agents Against COVID-19; Academic Press: Cambridge, MA, USA, 2025; pp. 317–337. [Google Scholar]
- Chun, S.; Gopal, J.; Muthu, M. Antioxidant activity of mushroom extracts/polysaccharides—Their antiviral properties and plausible anti-COVID-19 properties. Antioxidants 2021, 10, 1899. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Ling, J. Medicinal fungi with antiviral effect. Molecules 2022, 27, 4457. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K. Cordycepin: A bioactive metabolite of Cordyceps militaris and polyadenylation inhibitor with therapeutic potential against Covid-19. J. Biomol. Struct. Dyn. 2020, 40, 3745–3752. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Gao, L.; Wang, C.; Liu, B.; Jin, Y.; Xing, Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydr Polym. 2016, 144, 382–389. [Google Scholar] [CrossRef] [PubMed]
- El-Mekkawy, S.; Meselhy, M.R.; Nakamura, N.; Tezuka, Y.; Hattori, M.; Kakiuchi, N.; Shimotohno, K.; Kawahata, T.; Otake, T. Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum. Phytochemistry 1998, 49, 1651–1657. [Google Scholar] [CrossRef]
- Bienzle, D.; Reggeti, F.; Clark, M.E.; Chow, C. Immunophenotype and functional properties of feline dendritic cells derived from blood and bone marrow. Vet. Immunol. Immunopathol. 2003, 96, 19–30. [Google Scholar] [CrossRef]
- Parihar, A.; Eubank, T.D.; Doseff, A.I. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J. Innate Immun. 2010, 2, 204–215. [Google Scholar] [CrossRef]
- Lopes, R.; Sampaio, F.; Carvalho, H.L.; Garcês, A.; Fernandes, C.; Neves, C.V.; Brito, A.S.; Marques, T.; Sousa, C.; Silva, A.R.; et al. Feline Infectious Peritonitis Effusion Index: A Novel Diagnostic Method and Validation of Flow Cytometry-Based Delta Total Nucleated Cells Analysis on the Sysmex XN-1000V®. Vet. Sci. 2024, 11, 563. [Google Scholar] [CrossRef]
- Browne, D.J.; Miller, C.M.; Doolan, D.L. Technical pitfalls when collecting, cryopreserving, thawing, and stimulating human T-cells. Front. Immunol. 2024, 15, 1382192. [Google Scholar] [CrossRef]
- Xu, Y.; Jagannath, C.; Liu, X.D.; Sharafkhaneh, A.; Kolodziejska, K.E.; Eissa, N.T. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity 2007, 27, 135–144. [Google Scholar] [CrossRef]
- Oshio, T.; Kawashima, R.; Kawamura, Y.I.; Hagiwara, T.; Mizutani, N.; Okada, T.; Otsubo, T.; Inagaki-Ohara, K.; Matsukawa, A.; Haga, T.; et al. Chemokine receptor CCR8 is required for lipopolysaccharide-triggered cytokine production in mouse peritoneal macrophages. PLoS ONE 2014, 9, e94445. [Google Scholar] [CrossRef]
- Tucureanu, M.M.; Rebleanu, D.; Constantinescu, C.A.; Deleanu, M.; Voicu, G.; Butoi, E.; Calin, M.; Manduteanu, I. Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of Gi-protein inhibitor. Int. J. Nanomed. 2018, 13, 63–76. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.; Mao, H.; Yan, X. Degraded λ-carrageenan activates NF-κB and AP-1 pathways in macrophages and enhances LPS-induced TNF-α secretion through AP-1. Biochim. Biophys. Acta 2014, 1840, 2162–2170. [Google Scholar] [CrossRef]
- Thompson, M.R.; Kaminski, J.J.; Kurt-Jones, E.A.; Fitzgerald, K.A. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011, 3, 920. [Google Scholar] [CrossRef]
- Kolli, D.; Velayutham, T.S.; Casola, A. Host-viral interactions: Role of pattern recognition receptors (PRRs) in human pneumovirus infections. Pathogens 2013, 2, 232–263. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Tsukamoto, H.; Kouwaki, T.; Seya, T.; Oshiumi, H. Recognition of viral RNA by pattern recognition receptors in the induction of innate immunity and excessive inflammation during respiratory viral infections. Viral Immunol. 2017, 30, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Ngkelo, A.; Meja, K.; Yeadon, M.; Adcock, I.; Kirkham, P.A. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and G i α dependent PI-3kinase signalling. J. Inflamm. 2012, 9, 1–7. [Google Scholar] [CrossRef]
- Jansky, L.; Reymanova, P.; Kopecky, J. Dynamics of cytokine production in human peripheral blood mononuclear cells stimulated by LPS, or infected by Borrelia. Physiol. Res. 2003, 52, 593–598. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, Y.; Jiang, H.; Zhang, D.; Yang, Y.; Geng, X.; Li, Y. Functional differences and similarities in activated peripheral blood mononuclear cells by lipopolysaccharide or phytohemagglutinin stimulation between human and cynomolgus monkeys. Ann. Transl. Med. 2021, 9, 257. [Google Scholar] [CrossRef]
- Kubin, M.; Kamoun, M.; Trinchieri, G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J. Exp. Med. 1994, 180, 211–222. [Google Scholar] [CrossRef]
- Ulmer, A.J.; Flad, H.D.; Rietschel, T.; Mattern, T. Induction of proliferation and cytokine production in human T lymphocytes by lipopolysaccharide (LPS). Toxicology 2000, 152, 37–45. [Google Scholar] [CrossRef]
- Malbon, A.J.; Michalopoulou, E.; Meli, M.L.; Barker, E.N.; Tasker, S.; Baptiste, K.; Kipar, A. Colony stimulating factors in early feline infectious peritonitis virus infection of monocytes and in end stage feline infectious peritonitis; a combined in vivo and in vitro approach. Pathogens 2020, 9, 893. [Google Scholar] [CrossRef]
- Cornelissen, E.; Dewerchin, H.L.; Van Hamme, E.; Nauwynck, H.J. Absence of surface expression of feline infectious peritonitis virus (FIPV) antigens on infected cells isolated from cats with FIP. Vet. Microbiol. 2007, 121, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Matsuda, T.; Turner, M.; Miyasaka, N.; Buchan, G.; Tang, B.; Sato, K.; Shimi, M.; Maid, R.; Feldmann, M.; et al. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur. J. Immunol. 1988, 18, 1797–1802. [Google Scholar] [CrossRef] [PubMed]
- Bergroth, V.; Zvaifler, N.J.; Firestein, G.S. Cytokines in chronic inflammatory arthritis. III. Rheumatoid arthritis monocytes are not unusually sensitive to γ-interferon, but have defective γ-interferon–mediated HLA–DQ and HLA–DR induction. Arthritis Rheum. 1989, 32, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S.; Alvaro-Gracia, J.M.; Maki, R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J. Immunol. 1990, 144, 3347–3353. [Google Scholar] [CrossRef]
- Sakurai, H.; Kohsaka, H.; Liu, M.F.; Higashiyama, H.; Hirata, Y.; Kanno, K.; Saito, I.; Miyasaka, N. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J. Clin. Investig. 1995, 96, 2357–2363. [Google Scholar] [CrossRef]
- Davies, C.A.; Rocks, S.A.; O’Shaughnessy, M.C.; Perrett, D.; Winyard, P.G. Analysis of nitrite and nitrate in the study of inflammation. In Inflammation Protocols, Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2003; pp. 305–320. [Google Scholar]
- Capozza, P.; Pratelli, A.; Camero, M.; Lanave, G.; Greco, G.; Pellegrini, F.; Tempesta, M. Feline coronavirus and alpha-herpesvirus infections: Innate immune response and immune escape mechanisms. Animals 2021, 11, 3548. [Google Scholar] [CrossRef]
- Garren, M.R.; Ashcraft, M.; Qian, Y.; Douglass, M.; Brisbois, E.J.; Handa, H. Nitric oxide and viral infection: Recent developments in antiviral therapies and platforms. Appl. Mater. Today 2021, 22, 100887. [Google Scholar] [CrossRef]
- Omar, M.; Abdelal, H.O. Nitric oxide in parasitic infections: A friend or foe? J. Parasit. Dis. 2022, 46, 1147–1163. [Google Scholar] [CrossRef]
- Olyslaegers, D.A.; Dedeurwaerder, A.; Desmarets, L.M.; Vermeulen, B.L.; Dewerchin, H.L.; Nauwynck, H.J. Altered expression of adhesion molecules on peripheral blood leukocytes in feline infectious peritonitis. Vet. Microbiol. 2013, 166, 438–449. [Google Scholar] [CrossRef]
- Özbek, M.; Özkan, C.; Kaya, A.; Yıldırım, S.; Kozat, S.; Akgül, Y. Clinicopathological and biochemical evaluation of Feline Infectious Peritonitis in Turkish Van cats. J. Hell. Vet. Med. Soc. 2022, 73, 4379–4388. [Google Scholar] [CrossRef]
- Waltz, P.; Escobar, D.; Botero, A.M.; Zuckerbraun, B.S. Nitrate/nitrite as critical mediators to limit oxidative injury and inflammation. Antioxid. Redox Signal. 2015, 23, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Q.; Fei, F.; Huo, H.H.; Huang, B.; Meng, X.S.; Zhang, T.; Liu, B.L. Impact of nitrite exposure on plasma biochemical parameters and immune-related responses in Takifugu rubripes. Aquat. Toxicol. 2020, 218, 105362. [Google Scholar] [CrossRef] [PubMed]
- Sherding, R.G. Feline infectious peritonitis (feline coronavirus). J. Small Anim. Pract. 2009, 50, 132–143. [Google Scholar]
- Malbon, A.J.; Fonfara, S.; Meli, M.L.; Hahn, S.; Egberink, H.; Kipar, A. Feline infectious peritonitis as a systemic inflammatory disease: Contribution of liver and heart to the pathogenesis. Viruses 2019, 11, 1144. [Google Scholar] [CrossRef]
- Zappia, C.D.; Torralba-Agu, V.; Echeverria, E.; Fitzsimons, C.P.; Fernández, N.; Monczor, F. Antihistamines potentiate dexamethasone anti-inflammatory effects. Impact on glucocorticoid receptor-mediated expression of inflammation-related genes. Cells 2021, 10, 3026. [Google Scholar]
- Jiang, Z.; Zhu, L. Update on molecular mechanisms of corticosteroid resistance in chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2016, 37, 1–8. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Role of mushroom polysaccharides in improving gut health and associated diseases. In Microbiome, Immunity, Digestive Health and Nutrition; Academic Press: London, UK; Elsevier: Amsterdam, The Netherlands, 2022; pp. 431–448. [Google Scholar]
- Thrikawala, S.U.; Anderson, M.H.; Rosowski, E.E. Glucocorticoids Suppress NF-κB–Mediated Neutrophil Control of Aspergillus fumigatus Hyphal Growth. J. Immunol. 2024, 213, 971–987. [Google Scholar] [CrossRef]
- Kumar, Y.; Xu, B. New Insights into Chemical Profiles and Health-Promoting Effects of Edible Mushroom Dictyophora indusiata (Vent ex. Pers.) Fischer: A Review. J. Fungi. 2025, 11, 75. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Fu, W.; Liang, Z.; Yu, Y.; Li, T.; Tong, J.; Liu, F.; Nie, J.; Lu, Q.; et al. Optimization and validation of a virus-like particle pseudotyped virus neutralization assay for SARS-CoV-2. MedComm 2024, 5, e615. [Google Scholar] [CrossRef]
- Luo, H.; Lv, L.; Yi, J.; Zhou, Y.; Liu, C. Establishment of replication deficient vesicular stomatitis virus for studies of PEDV spike-mediated cell entry and its inhibition. Microorganisms 2023, 11, 2075. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlaoperm, C.; Moyadee, W.; Wongsaengnoi, E.; Klankaew, W.; Rattanasrisomporn, A.; Paemanee, A.; Choowongkomon, K.; Rungsuriyawiboon, O.; Rattanasrisomporn, J. Evaluation of the Dual Antiviral and Immunomodulatory Effects of Phallus indusiatus in a Feline Infectious Peritonitis Model Using PBMCs. Vet. Sci. 2025, 12, 847. https://doi.org/10.3390/vetsci12090847
Hlaoperm C, Moyadee W, Wongsaengnoi E, Klankaew W, Rattanasrisomporn A, Paemanee A, Choowongkomon K, Rungsuriyawiboon O, Rattanasrisomporn J. Evaluation of the Dual Antiviral and Immunomodulatory Effects of Phallus indusiatus in a Feline Infectious Peritonitis Model Using PBMCs. Veterinary Sciences. 2025; 12(9):847. https://doi.org/10.3390/vetsci12090847
Chicago/Turabian StyleHlaoperm, Chularat, Wassamon Moyadee, Emwalee Wongsaengnoi, Wiwat Klankaew, Amonpun Rattanasrisomporn, Atchara Paemanee, Kiattawee Choowongkomon, Oumaporn Rungsuriyawiboon, and Jatuporn Rattanasrisomporn. 2025. "Evaluation of the Dual Antiviral and Immunomodulatory Effects of Phallus indusiatus in a Feline Infectious Peritonitis Model Using PBMCs" Veterinary Sciences 12, no. 9: 847. https://doi.org/10.3390/vetsci12090847
APA StyleHlaoperm, C., Moyadee, W., Wongsaengnoi, E., Klankaew, W., Rattanasrisomporn, A., Paemanee, A., Choowongkomon, K., Rungsuriyawiboon, O., & Rattanasrisomporn, J. (2025). Evaluation of the Dual Antiviral and Immunomodulatory Effects of Phallus indusiatus in a Feline Infectious Peritonitis Model Using PBMCs. Veterinary Sciences, 12(9), 847. https://doi.org/10.3390/vetsci12090847








