Self-Adhesive, Human Bandage Contact Lens Versus Conjunctival Transposition Flap for Surgical Repair of Feline Corneal Sequestrum
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
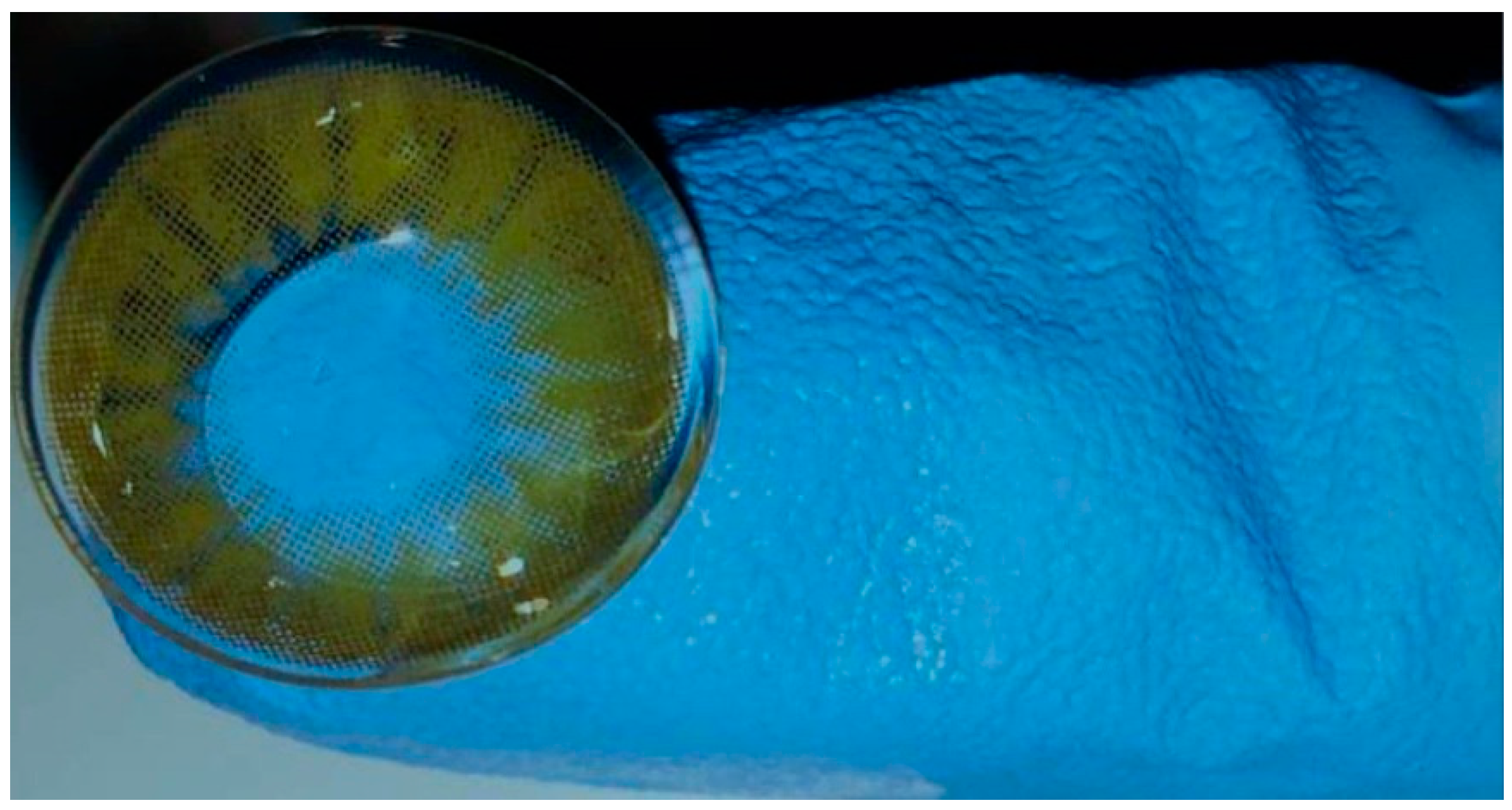
2.2. Contact Lens
2.3. Surgical Procedure
2.4. Statistical Analysis
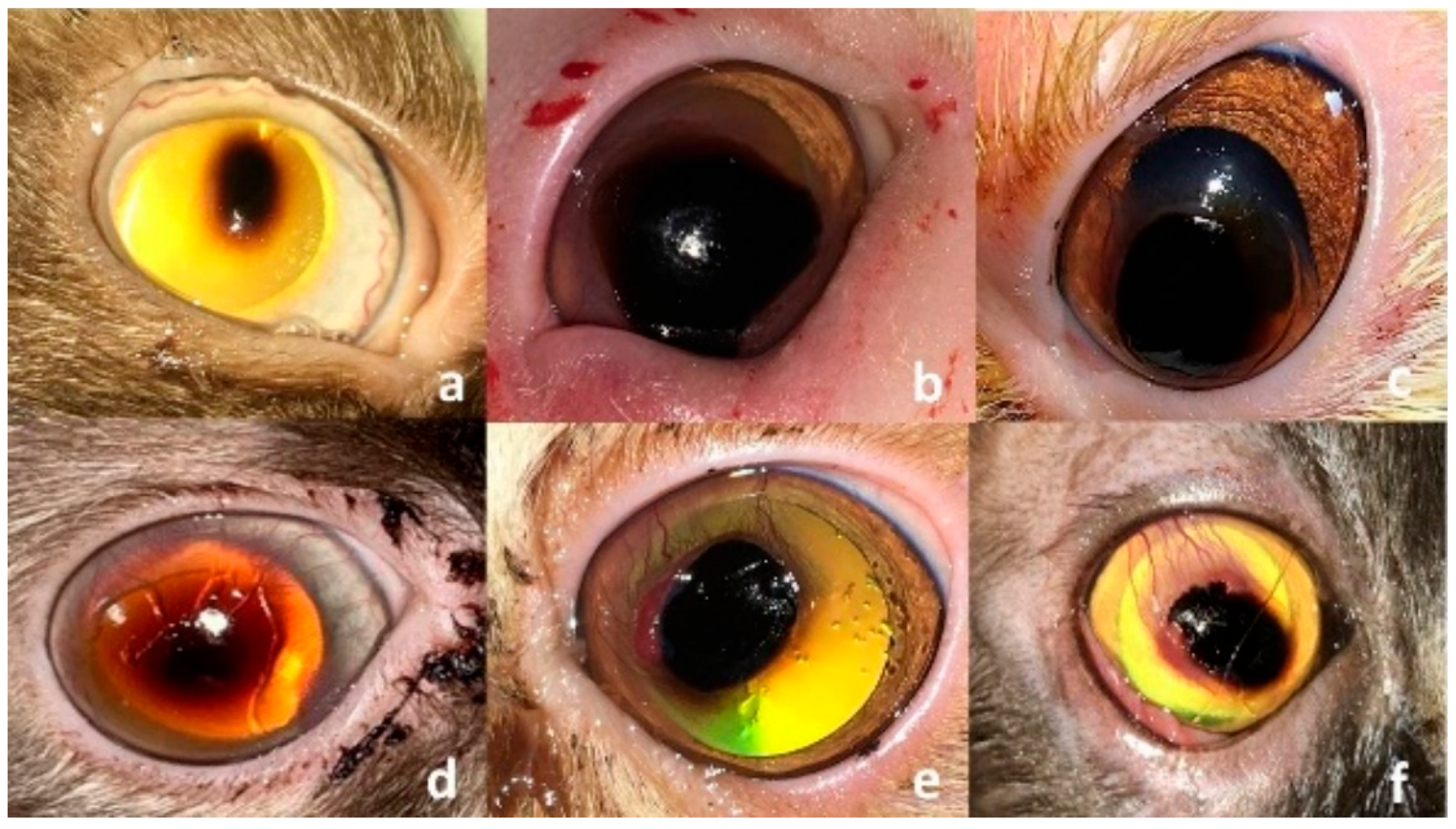
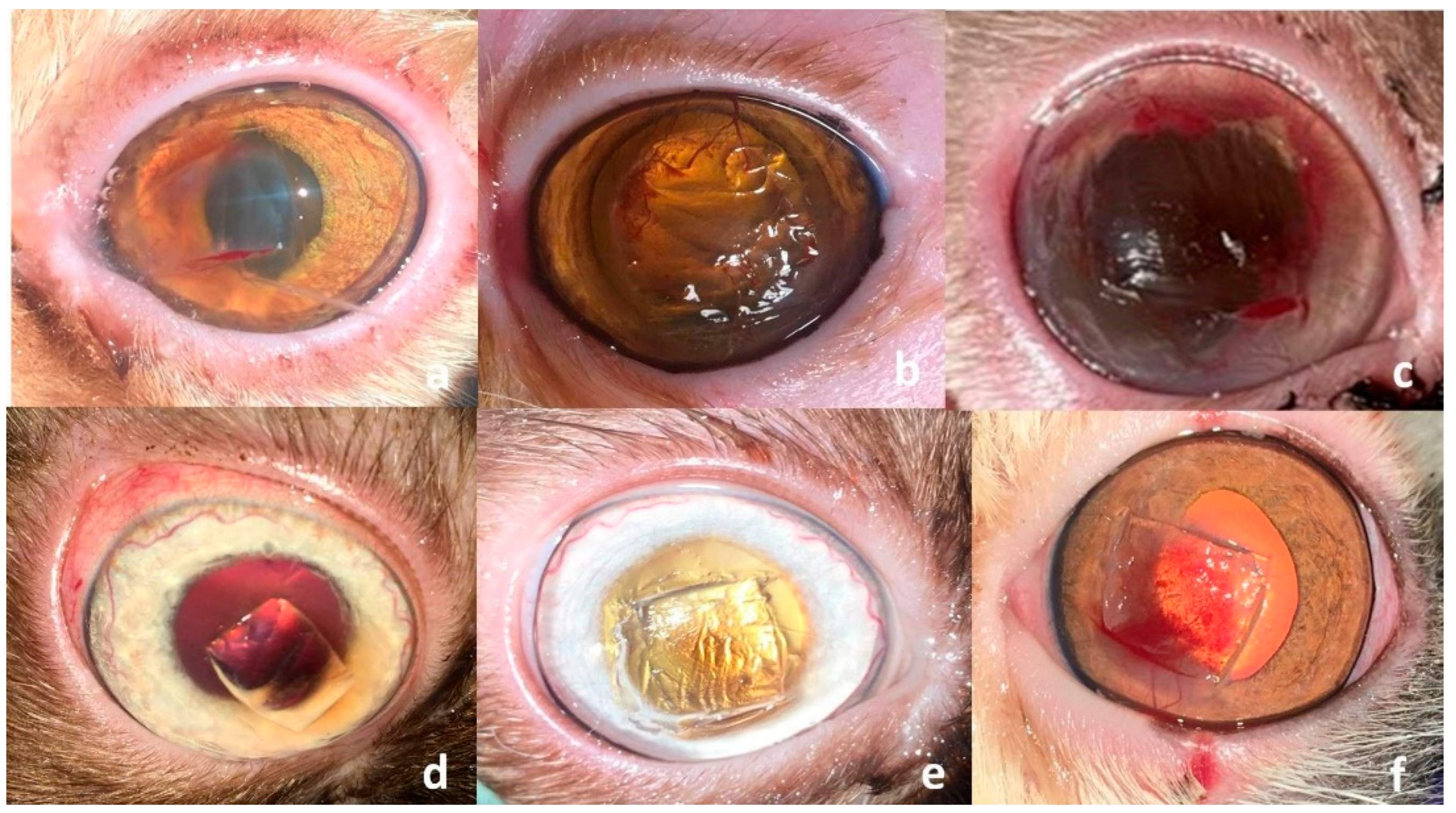
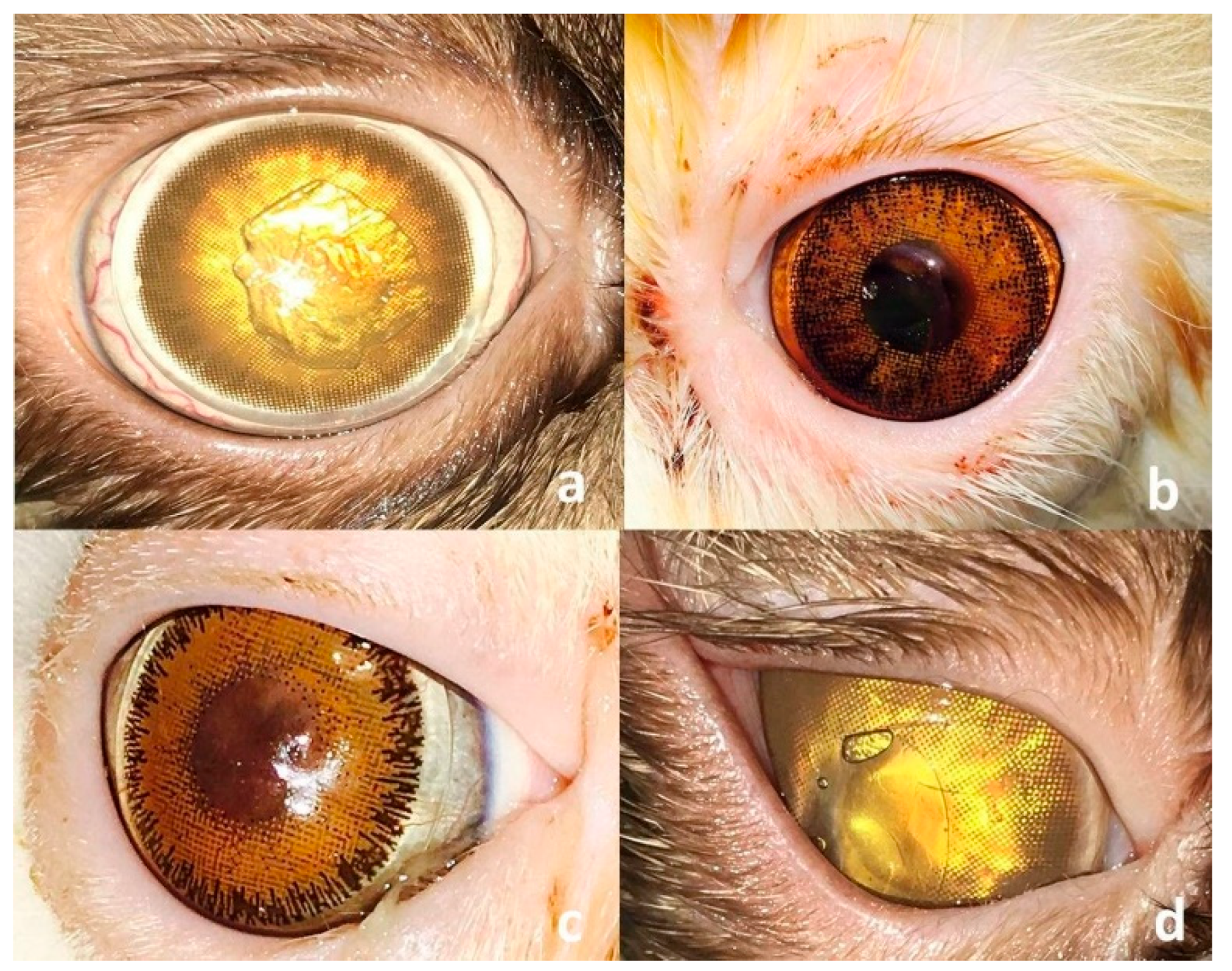
3. Results
3.1. Clinical Findings
3.2. Surgical Outcome
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SHBCL | self-adhesive, human bandage contact lens |
| CTF | conjunctival transposition flap |
| G-CO | control group |
| FHV-1 | feline herpesvirus type-1 |
| DSH | domestic short hair |
| SIS | small intestinal submucosal |
| AMT | amniotic membrane transplantation |
| BP | bovine pericardial |
References
- Nasisse, M.P.; English, R.V.; Tompkins, M.B.; Guy, J.S.; Sussman, W. Immunologic, histologic, and virologic features of herpesvirus-induced stromal keratitis in cats. Am. J. Vet. Res. 1995, 56, 51–55. [Google Scholar] [CrossRef]
- Barachetti, L.; Giudice, C.; Mortellaro, C.M. Amniotic membrane transplantation for the treatment of feline corneal sequestrum: Pilot study. Vet. Ophthalmol. 2010, 13, 326–330. [Google Scholar] [CrossRef]
- Laguna, F.; Leiva, M.; Costa, D.; Lacerda, R.; Gimenez, T.P. Corneal grafting for the treatment of feline corneal sequestrum: A retrospective study of 18 eyes (13 cats). Vet. Ophthalmol. 2015, 18, 291–296. [Google Scholar] [CrossRef]
- Andrew, S.M.; Tou, S.; Brooks, D.E. Corneoconjunctival transposition for the treatment of feline corneal sequestra: A retrospective study of 17 cases (1990–1998). Vet. Ophthalmol. 2001, 4, 107–111. [Google Scholar] [CrossRef]
- Dulaurent, T.; Azoulay, T.; Goulle, F.; Dulaurent, A.; Mentek, M.; Peiffer, R.L.; Isard, P.-F. Use of bovine pericardium (Tutopatch®) graft for surgical repair of deep melting corneal ulcers in dogs and corneal sequestra in cats. Vet. Ophthalmol. 2014, 17, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Cullen, C.L.; Wadowska, D.W.; Singh, A.; Melekhovets, Y. Ultrastructural findings in feline corneal sequestra. Vet. Ophthalmol. 2005, 8, 295–303. [Google Scholar] [CrossRef]
- Volopich, S.; Benetka, V.; Schwendenwein, I.; Möstl, K.; Sommerfeld-Stur, I.; Nell, B. Cytologic findings, and feline herpesvirus DNA and Chlamydophila felis antigen detection rates in normal cats and cats with conjunctival and corneal lesions. Vet. Ophthalmol. 2005, 8, 25–32. [Google Scholar] [CrossRef]
- Grahn, B.H.; Sisler, S.; Storey, E. Qualitative tear film and conjunctival goblet cell assessment of cats with corneal sequestra. Vet. Ophthalmol. 2005, 8, 167–170. [Google Scholar] [CrossRef]
- Featherstone, H.J.; Franklin, V.J.; Sansom, J. Feline corneal sequestrum: Laboratory analysis of ocular samples from 12 cats. Vet. Ophthalmol. 2004, 7, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.K.; Pineda, R.; Albert, D.M.; Shore, J.W. ‘Black cornea’ after long term epinephrine use. Arch. Ophthalmol. 1992, 110, 1273–1275. [Google Scholar] [CrossRef]
- Stiles, J.; Townsend, W.M. Feline ophthalmology. In Veterinary Ophthalmology, 4th ed.; Gelatt, K.N., Ames, I.A., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 1095–1164. [Google Scholar]
- Featherstone, H.J.; Sansom, J. Feline corneal sequestra: A review of 64 cases (80 eyes) from 1993 to 2000. Vet. Ophthalmol. 2004, 7, 213–227. [Google Scholar] [CrossRef]
- Pumphrey, S.A.; Pizzirani, S.; Pirie, C.G. 360-degree conjunctival grafting for management of diffuse keratomalacia in a dog. Vet. Ophthalmol. 2011, 14, 209–213. [Google Scholar] [CrossRef]
- Goulle, F. Use of porcine small intestinal submucosa for corneal reconstruction in dogs and cats: 106 cases. J. Small Anim. Pract. 2012, 53, 34–43. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Choi, H. Surface modification of Polymacon contact lenses for enhanced tear film stability. J. Biomed. Mater. Res. B 2017, 105, 812–819. [Google Scholar]
- Schmidt, G.M.; Blanchard, G.L.; Keller, W.F. The use of hydrophilic contact lenses in corneal disease of the dog and cat: A preliminary report. J. Small Anim. Pract. 1977, 18, 773–777. [Google Scholar] [CrossRef]
- Turner, S.M. Eyelids. In Small Animal Ophthalmology, 1st ed.; Turner, S.M., Ed.; Saunders-Elsevier: Shanghai, China, 2008; pp. 15–57, 121–197. [Google Scholar]
- Bossuyt, S.M. The use of therapeutic soft contact bandage lenses in the dog and the cat: A series of 41 cases. Vlaams Diergeneeskd. Tijdschr. 2016, 85, 343–348. [Google Scholar]
- Gosling, A.A.; Labelle, A.L.; Breaux, C.B. Management of spontaneous chronic corneal epithelial defects (SCCEDs) in dogs with diamond burr debridement and placement of a bandage contact lens. Vet. Ophthalmol. 2013, 16, 83–88. [Google Scholar] [CrossRef]
- Pentlarge, V.W. Corneal sequestrum in cats. Compendium for Continuing Education for the Practicing Veterinarian. 1989, 11, 24–32. [Google Scholar]
- Morgan, R.V. Feline corneal sequestrum: A retrospective study of 42 cases (1987–1991). J. Am. Anim. Hosp. Assoc. 1994, 30, 24–28. [Google Scholar]
- Gilger, B.C.; Whitley, R.D. Surgery of the cornea and sclera. In Veterinary Ophthalmology; Gelatt, K.N., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999; pp. 675–700. [Google Scholar]
- Kaup, M.; Redbrake, C.; Plange, N.; Arend, K.O.; Remky, A. Amniotic membrane transplantation in severe ocular surface disorders. Eur. J. Ophthalmol. 2008, 18, 691–694. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Ziegler, K.R.; Model, L.S.; Eghbalieh, S.D.; Brenes, R.A.; Kim, S.T.; Shu, C.; Dardik, A. Current usage and future directions for the bovine pericardial patch. Ann. Vasc. Surg. 2011, 25, 561–568. [Google Scholar] [CrossRef]
- Blogg, J.R.; Stanley, R.G.; Dutton, A.G. Use of conjunctival pedicle grafts in the management of feline keratitis nigrum. J. Small Anim. Pract. 1989, 30, 678–684. [Google Scholar] [CrossRef]
- Braus, B.K.; Riedler, D.; Tichy, A.; Spergser, J.; Schwendenwein, I. The effects of two different types of bandage contact lenses on the healthy canine eye. Vet. Ophthalmol. 2018, 21, 477–486. [Google Scholar] [CrossRef]
- Diehl, K.A.; Bowden, A.C.; Knudsen, D. Bandage contact lens retention in dogs. A pilot study. Vet. Ophthalmol. 2019, 22, 584–590. [Google Scholar] [CrossRef]
- Denk, N.; Fritsche, J.; Reese, S. The effect of UV-blocking contact lenses as a therapy for canine chronic superficial keratitis. Vet. Ophthalmol. 2011, 14, 186–194. [Google Scholar] [CrossRef]
- Anastassiadis, Z.; Bayley, K.D.; Read, R.A. Corneal diamond burr debridement for superficial non-healing corneal ulcers in cats. Vet. Ophthalmol. 2022, 25, 476–482. [Google Scholar] [CrossRef]
- Dees, D.D.; Fritz, K.J.; Wagner, L.; Paglia, D.; Knollinger, A.M.; Madsen, R. Effect of bandage contact lens wear and postoperative medical therapies on corneal healing rate after diamond burr debridement in dogs. Vet. Ophthalmol. 2017, 20, 382–389. [Google Scholar] [CrossRef]
- Hendrix, D.V.H. Diseases of the cornea and sclera in the cat. In Veterinary Ophthalmology, 6th ed.; Gelatt, K.N., Gilger, B.C., Kern, T.J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 1202–1226. [Google Scholar]


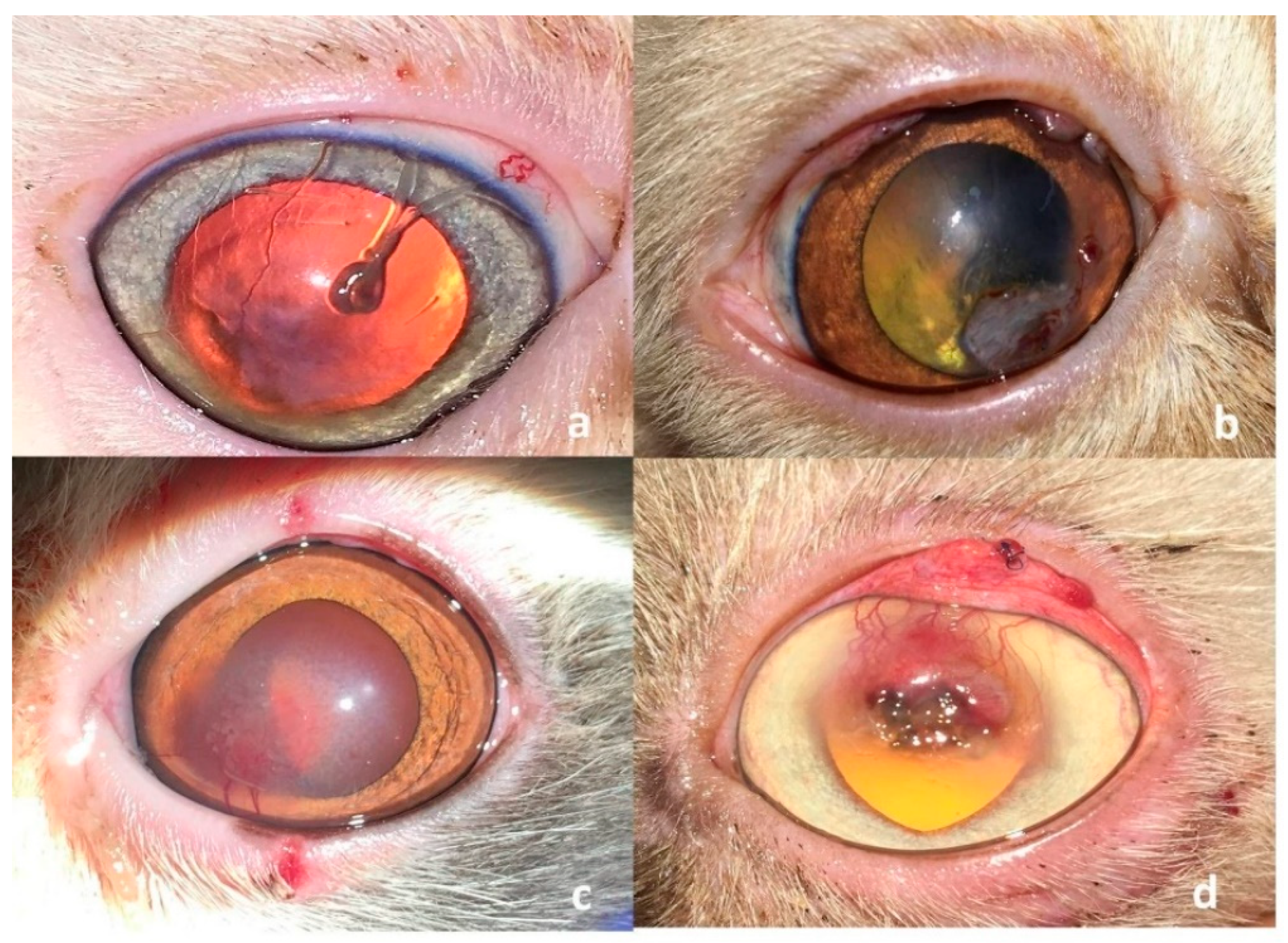
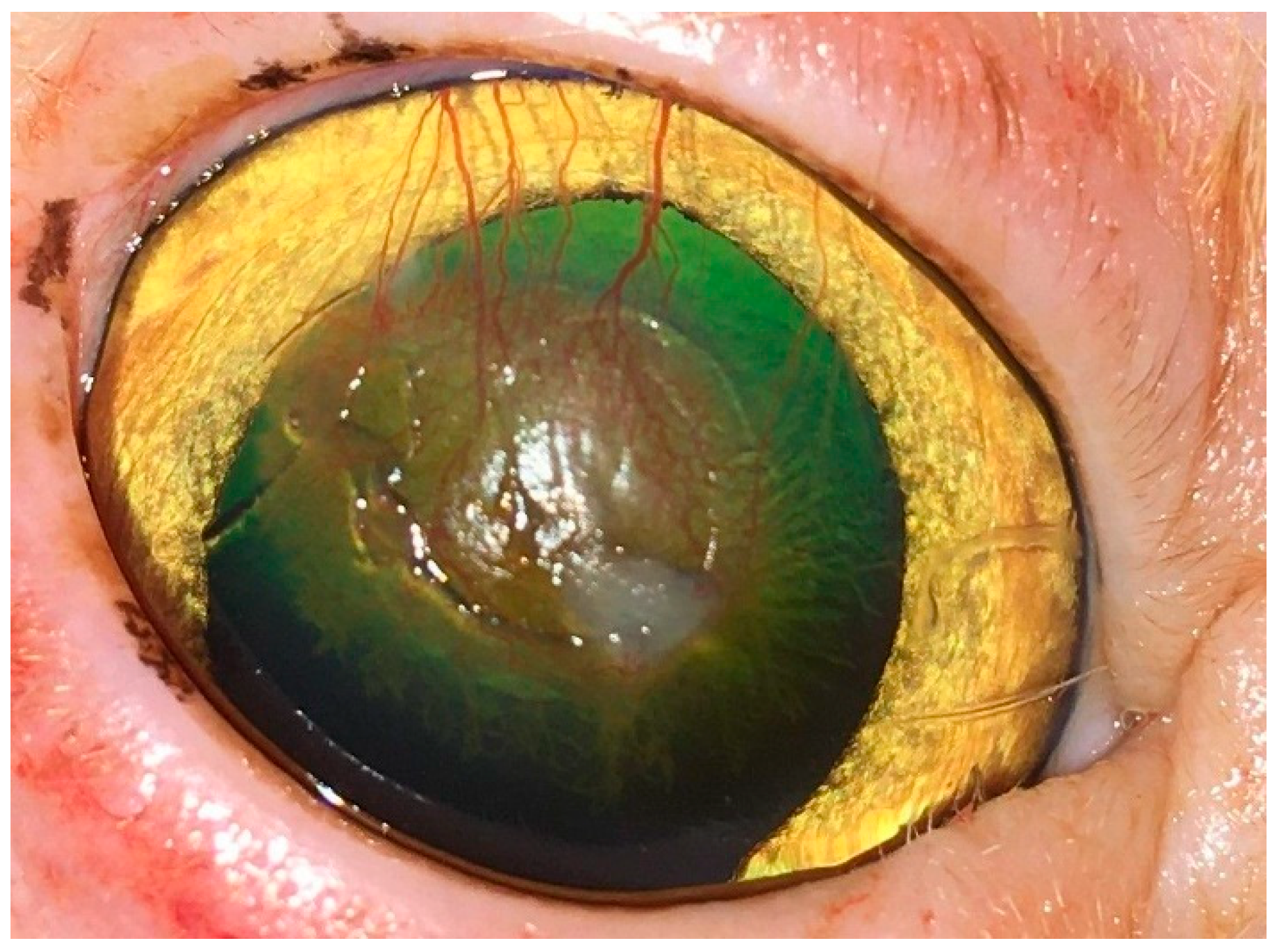
| Group of Cats | No. | Breed | Patient Data Sex | Age (m) | Eye | Duration (m) | Site | Characterization of the Sequestrum Diameter (mm) | Depth |
|---|---|---|---|---|---|---|---|---|---|
| SHBCL (10 cats) | 1 | Persian | M | 24 | OD | 2 | CC | 7 | A 1/3 |
| 2 | Persian | M | 28 | OD | 6 | CC | 4 | M 1/3 | |
| 3 | Himalayan | M | 36 | OS | 7 | CC | 4 | M 1/3 | |
| 4 | Himalayan | F | 34 | OD | 8 | PC | 5 | M 1/3 | |
| 5 | Himalayan | M | 60 | OD | 12 | CC | 6 | M 1/3 | |
| 6 | Persian | F | 42 | OS | 12 | CC | 8 | A 1/3 | |
| 7 | Himalayan | M | 54 | OS | 5 | CC | 6 | M 1/3 | |
| 8 | Persian | F | 16 | OS | 7 | PC | 5 | M 1/3 | |
| 9 | Persian | M | 24 | OS | 8 | PC | 6 | A 1/3 | |
| 10 | DSH | F | 42 | OD | 8 | CC | 4 | A 1/3 | |
| CTF (10 cats) | 1 | Himalayan | M | 36 | OS | 9 | CC | 6 | M 1/3 |
| 2 | DSH | F | 54 | OD | 12 | CC | 5 | M 1/3 | |
| 3 | Himalayan | F | 72 | OS | 14 | CC | 6 | A 1/3 | |
| 4 | Persian | M | 36 | OD | 14 | CC | 4 | M 1/3 | |
| 5 | Persian | F | 12 | OS | 12 | CC | 5 | M 1/3 | |
| 6 | Himalayan | M | 28 | OS | 14 | CC | 7 | M 1/3 | |
| 7 | DSH | M | 46 | OD | 10 | CC | 5 | M 1/3 | |
| 8 | Persian | F | 24 | OD | 12 | PC | 4 | A 1/3 | |
| 9 | DSH | M | 24 | OD | 8 | PC | 7 | A 1/3 | |
| 10 | Persian | F | 54 | OD | 2 | PC | 4 | A 1/3 | |
| CO (5 cats) | 1 | DSH | M | 72 | OS | 4 | CC | 4 | M 1/3 |
| 2 | Persian | M | 46 | OS | 12 | CC | 6 | M 1/3 | |
| 3 | Himalayan | F | 18 | OD | 6 | PC | 5 | A 1/3 | |
| 4 | Persian | F | 24 | OD | 12 | PC | 7 | A 1/3 | |
| 5 | Persian | F | 36 | OD | 14 | CC | 8 | A 1/3 |
| Ophthalmic Findings | First Recheck Exam (7.6 ± 0.8 Days) | Second Recheck Exam (15.7 ± 1.2 Days) | Third Recheck Exam (31.2 ± 1.3 Days) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| G-SHBCL (n = 10) | G-CTF (n = 10) | G-CO (n = 5) | G-SHBCL (n = 10) | G-CTF (n = 10) | G-CO (n = 5) | G-SHBCL (n = 10) | G-CTF (n = 10) | G-CO (n = 5) | |
| Ocular discharge | 6 (60%) | 9/10 (90%) | 5 (100%) | None | 8 (80%) | 5 (100%) | None | 7 (70%) | 5 (100%) |
| Blepharospasm | 3 (30%) | 4/10 (40%) | 4 (80%) | None | 2 (20%) | 4 (80%) | None | None | 4 (80%) |
| Corneal vascularization | 2 (20%) | 9/10 (45%) | 5 (100%) | None | 7 (70%) | 4 (80%) | 2 (20%) | 2 (20%) | 4 (80%) |
| Corneal edema | 3 (30%) | 8/10 (40%) | 3 (60%) | 2 (20%) | 3 (30%) | 4 (80%) | None | None | 2 (40%) |
| Squinting | 1 (10%) | 4/10 (40%) | None | 5 (50%) | None | None | None | None | None |
| Residues of the sequestrum | 4 (40%) | NA | 2 (40%) | 2 (20%) | NA | 2 (40%) | None | 2 (20%) | 2 (40%) |
| Granulation tissue | None | NA | 4 (80%) | None | NA | 5 (100%) | None | 5 (50%) | 5 (100%) |
| Corneal scarring | None | NA | 3 (60%) | 3 (30%) | NA | 5 (100%) | 2 (20%) | 2 (20%) | 5 (100%) |
| Positive fluorescein test | None | NA | 2 (40%) | None | NA | None | None | None | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, K.M.; Mostafa, A.A. Self-Adhesive, Human Bandage Contact Lens Versus Conjunctival Transposition Flap for Surgical Repair of Feline Corneal Sequestrum. Vet. Sci. 2025, 12, 839. https://doi.org/10.3390/vetsci12090839
Ali KM, Mostafa AA. Self-Adhesive, Human Bandage Contact Lens Versus Conjunctival Transposition Flap for Surgical Repair of Feline Corneal Sequestrum. Veterinary Sciences. 2025; 12(9):839. https://doi.org/10.3390/vetsci12090839
Chicago/Turabian StyleAli, Khaled M., and Ayman A. Mostafa. 2025. "Self-Adhesive, Human Bandage Contact Lens Versus Conjunctival Transposition Flap for Surgical Repair of Feline Corneal Sequestrum" Veterinary Sciences 12, no. 9: 839. https://doi.org/10.3390/vetsci12090839
APA StyleAli, K. M., & Mostafa, A. A. (2025). Self-Adhesive, Human Bandage Contact Lens Versus Conjunctival Transposition Flap for Surgical Repair of Feline Corneal Sequestrum. Veterinary Sciences, 12(9), 839. https://doi.org/10.3390/vetsci12090839





