Prognostic Factors for Mortality Following Diaphragmatic Herniorrhaphy in Dogs and Cats: Multivariable Logistic Regression and Machine Learning Approaches
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Logistic Regression
3.2. Random Forest
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DH | diaphragmatic hernia |
| NCSS | Number Cruncher Statistical System |
| ROC | receiver operating characteristic |
| AUC | area under the ROC curve |
| OR | odds ratio |
| CI | confidence interval |
| BUN | blood urea nitrogen |
| CART | classification and regression tree |
| ASA | American Society of Anesthesiologists |
| PCV | packed cell volume |
| WBC | white blood cell |
| ALT | alanine aminotransferase |
| Ref | reference |
| OOB | out of bag |
Appendix A
| Parameters | Canine | Feline |
|---|---|---|
| PCV (%) | 35–57 | 30–45 |
| WBC count (×103/µL) | 5.0–14.1 | 5.5–19.5 |
| Platelet count (×103/µL) | 211–621 | 300–800 |
| BUN (mg/dL) | 8–28 | 19–34 |
| Creatinine (mg/dL) | 0.5–1.7 | 0.9–2.2 |
| ALT (U/L) | 10–109 | 25–97 |
| Plasma protein (Biuret) (g/dL) | 5.4–7.5 | 6.0–7.9 |
| Albumin (g/dL) | 2.3–3.1 | 2.8–3.9 |
References
- Minihan, A.C.; Berg, J.; Evans, K.L. Chronic diaphragmatic hernia in 34 dogs and 16 cats. J. Am. Anim. Hosp. Assoc. 2004, 40, 51–63. [Google Scholar] [CrossRef]
- Gibson, T.W.; Brisson, B.A.; Sears, W. Perioperative survival rates after surgery for diaphragmatic hernia in dogs and cats: 92 cases (1990–2002). J. Am. Vet. Med. Assoc. 2005, 227, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Besalti, O.; Pekcan, Z.; Caliskan, M.; Aykut, A.G. A retrospective study on traumatic diaphragmatic hernias in cats. Ankara Univ. Vet. Fak. Derg. 2011, 58, 175–179. [Google Scholar] [CrossRef]
- Legallet, C.; Mankin, K.T.; Selmic, L.E. Prognostic indicators for perioperative survival after diaphragmatic herniorrhaphy in cats and dogs: 96 cases (2001–2013). BMC. Vet. Res. 2017, 13, 16. [Google Scholar] [CrossRef]
- Yaygingül, R.; Bozkan, Z.; Bilgen Şen, Z.; Kibar Kurt, B.; Belge, A. Traumatic diaphragmatic hernia in cats: A retrospective study of 15 cases (2016–2017). Kocatepe Vet. J. 2019, 12, 205–212. [Google Scholar] [CrossRef]
- De Bastiani, D.; Montinaro, V.; Cipolla, E.; Bussadori, R.; Pisani, G.; Cinti, F. Complications and outcome of traumatic diaphragmatic hernia repair without post-operative chest drain: Retrospective study in 90 cats. Open Vet. J. 2023, 13, 677–683. [Google Scholar] [CrossRef]
- Pereira, G.J.; Rahal, S.C.; Melchert, A.; Abibe, R.B.; Brandão, C.V.S.; Quitzan, J.G.; Mesquita, L.R.; Mamprim, M.J. Eleven-year retrospective analysis of acquired diaphragmatic hernia in 49 dogs and 48 cats. Can. Vet. J. 2023, 64, 149–152. [Google Scholar]
- Igna, C.; Schuszler, L.; Sala, A.; Bumb, D.; Proteasa, A.; Dascalu, R. Diaphragmatic hernia in dogs and cats: A report of 43 cases (2001–2013). Lucr. Ştiinţ. Med. Vet. 2014, 47, 48–51. [Google Scholar]
- Robertson, A. In cats and dogs with traumatic diaphragmatic rupture, does surgical timing affect outcome? Vet. Evid. 2021, 6, 1–9. [Google Scholar] [CrossRef]
- Schmiedt, C.W.; Tobias, K.M.; Stevenson, M.A.M. Traumatic diaphragmatic hernia in cats: 34 cases (1991–2001). J. Am. Vet. Med. Assoc. 2003, 222, 1237–1240. [Google Scholar] [CrossRef]
- Boudrieau, R.J.; Muir, W.W. Pathophysiology of traumatic diaphragmatic hernia in dogs. Compend. Contin. Educ. Pract. Vet. 1987, 9, 379–384. [Google Scholar]
- Ozer, K.; Guzel, O.; Devecioglu, Y.; Aksoy, O. Diaphragmatic hernia in cats: 44 cases. Med. Weter. 2007, 63, 1564–1567. [Google Scholar]
- Desai, R.J.; Wang, S.V.; Vaduganathan, M.; Evers, T.; Schneeweiss, S. Comparison of machine learning methods with traditional models for use of administrative claims with electronic medical records to predict heart failure outcomes. JAMA. Netw. Open 2020, 3, e1918962. [Google Scholar] [CrossRef]
- Cox, A.P.; Raluy-Callado, M.; Wang, M.; Bakheit, A.M.; Moore, A.P.; Dinet, J. Predictive analysis for identifying potentially undiagnosed post-stroke spasticity patients in United Kingdom. J. Biomed. Inform. 2016, 60, 328–333. [Google Scholar] [CrossRef]
- Desbordes, P.; Ruan, S.; Modzelewski, R.; Pineau, P.; Vauclin, S.; Gouel, P.; Michel, P.; Fiore, F.D.; Vera, P.; Gardin, I. Predictive value of initial FDG-PET features for treatment response and survival in esophageal cancer patients treated with chemo-radiation therapy using a random forest classifier. PLoS ONE 2017, 12, e0173208. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, Y.; Zhu, Y.; Zhou, T.; Li, J.; Ding, K.; Li, J. A multicenter random forest model for effective prognosis prediction in collaborative clinical research network. Artif. Intell. Med. 2020, 103, 101814. [Google Scholar] [CrossRef] [PubMed]
- Toyama, Y.; Hotta, M.; Motoi, F.; Takanami, K.; Minamimoto, R.; Takase, K. Prognostic value of FDG-PET radiomics with machine learning in pancreatic cancer. Sci. Rep. 2020, 10, 17024. [Google Scholar] [CrossRef]
- Talebi, A.; Celis-Morales, C.A.; Borumandnia, N.; Abbasi, S.; Pourhoseingholi, M.A.; Akbari, A.; Yousefi, J. Predicting metastasis in gastric cancer patients: Machine learning-based approaches. Sci. Rep. 2023, 13, 4163. [Google Scholar] [CrossRef]
- Watanabe, M.; Ashida, R.; Miyakoshi, C.; Arizono, S.; Suga, T.; Kanao, S.; Kitamura, K.; Ogawa, T.; Ishikura, R. Prognostic analysis of curatively resected pancreatic cancer using harmonized positron emission tomography radiomic features. Eur. J. Hybrid Imaging 2023, 7, 8. [Google Scholar] [CrossRef]
- Touw, W.G.; Bayjanov, J.R.; Overmars, L.; Backus, L.; Boekhorst, J.; Wels, M.; van Hijum, S.A.F.T. Data mining in the Life Sciences with Random Forest: A walk in the park or lost in the jungle? Brief. Bioinform. 2013, 14, 315–326. [Google Scholar] [CrossRef]
- Bi, Q.; Goodman, K.E.; Kaminsky, J.; Lessler, J. What is machine learning? A primer for the epidemiologist. Am. J. Epidemiol. 2019, 188, 2222–2239. [Google Scholar] [CrossRef]
- Shoop-Worrall, S.J.; O’Neill, D.G.; Viscasillas, J.; Brodbelt, D.C. Mortality related to general anaesthesia and sedation in dogs under UK primary veterinary care. Vet. Anaesth. Analg. 2022, 49, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Brodbelt, D.C.; Pfeiffer, D.U.; Young, L.E.; Wood, J.L. Results of the confidential enquiry into perioperative small animal fatalities regarding risk factors for anesthetic-related death in dogs. J. Am. Vet. Med. Assoc. 2008, 233, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Deveci, M.Z.Y.; Yurtal, Z.; İşler, C.T.; Emiroğlu, S.B.; Alakuş, I.; Altuğ, M.E. Herniorrhaphy and surgical outcomes of diaphragmatic hernias in cats. Slov. Vet. Res. 2022, 59, 47–57. [Google Scholar] [CrossRef]
- Garson, H.; Dodman, N.; Baker, G. Diaphragmatic hernia: Analysis of fifty-six cases in dogs and cats. J. Sm. Anim. Pract. 1980, 21, 469–481. [Google Scholar] [CrossRef]
- Gounden, V.; Bhatt, H.; Jialal, I. Renal Function Tests; StatPearls Publishing: St. Petersburg, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507821/ (accessed on 24 April 2025).
- Prause, L.C.; Grauer, G.F. Association of gastrointestinal hemorrhage with increased blood urea nitrogen and BUN/creatinine ratio in dogs: A literature review and retrospective study. Vet. Clin. Pathol. 1998, 27, 107–111. [Google Scholar] [CrossRef]
- Kazory, A. Emergence of blood urea nitrogen as a biomarker of neurohormonal activation in heart failure. Am. J. Cardiol. 2010, 106, 694–700. [Google Scholar] [CrossRef]
- Testani, J.M.; Coca, S.G.; Shannon, R.P.; Kimmel, S.E.; Cappola, T.P. Influence of renal dysfunction phenotype on mortality in the setting of cardiac dysfunction: Analysis of three randomized controlled trials. Eur. J. Heart Fail. 2011, 13, 1224–1230. [Google Scholar] [CrossRef]
- Hartz, A.J.; Kuhn, E.M.; Kayser, K.L.; Johnson, W.D. BUN as a risk factor for mortality after coronary artery bypass grafting. Ann. Thora.c Surg. 1995, 60, 398–404. [Google Scholar] [CrossRef]
- Felker, G.M.; Leimberger, J.D.; Califf, R.M.; Cuffe, M.S.; Massie, B.M.; Adams, K.F., Jr.; Gheorghiade, M.; O’Connor, C.M. Risk stratification after hospitalization for decompensated heart failure. J. Card. Fail. 2004, 10, 460–466. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Adams, K.F., Jr.; Abraham, W.T.; Yancy, C.W.; Boscardin, W.J. ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA. 2005, 293, 572–580. [Google Scholar] [CrossRef]
- Shenkman, H.J.; Zareba, W.; Bisognano, J.D. Comparison of prognostic significance of amino-terminal pro-brain natriuretic Peptide versus blood urea nitrogen for predicting events in patients hospitalized for heart failure. Am. J. Cardiol. 2007, 99, 1143–1145. [Google Scholar] [CrossRef] [PubMed]
- Cauthen, C.A.; Lipinski, M.J.; Abbate, A.; Appleton, D.; Nusca, A.; Varma, A.; Goudreau, E.; Cowley, M.J.; Vetrovec, G.W. Relation of blood urea nitrogen to long-term mortality in patients with heart failure. Am. J. Cardiol. 2008, 101, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.U.; Johannes, R.S.; Sun, X.; Conwell, D.L.; Banks, P.A. Early changes in blood urea nitrogen predict mortality in acute pancreatitis. Gastroenterology 2009, 137, 129–135. [Google Scholar] [CrossRef]
- Ye, B.; Deng, H.; Zhao, H.; Liang, J.; Ke, L.; Li, W. Association between an increase in blood urea nitrogen at 24 h and worse outcomes in COVID-19 pneumonia. Ren. Fail. 2021, 43, 347–350. [Google Scholar] [CrossRef]
- Beier, K.; Eppanapally, S.; Bazick, H.S.; Chang, D.; Mahadevappa, K.; Gibbons, F.K.; Christopher, K.B. Elevation of blood urea nitrogen is predictive of long-term mortality in critically ill patients independent of "normal" creatinine. Crit. Care. Med. 2011, 39, 305–313. [Google Scholar] [CrossRef]
- Arihan, O.; Wernly, B.; Lichtenauer, M.; Franz, M.; Kabisch, B.; Muessig, J.; Masyuk, M.; Lauten, A.; Schulze, P.C.; Hoppe, U.C.; et al. Blood Urea Nitrogen (BUN) is independently associated with mortality in critically ill patients admitted to ICU. PLoS ONE 2018, 13, e0191697. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, H.; Su, X.; Fang, Z.; Dong, Z.; Yu, C.; Luo, K. Role of elevated liver transaminase levels in the diagnosis of liver injury after blunt abdominal trauma. Exp. Ther. Med. 2012, 4, 255–260. [Google Scholar] [CrossRef]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef]
- Toloşi, L.; Lengauer, T. Classification with correlated features: Unreliability of feature ranking and solutions. Bioinformatics 2011, 27, 1986–1994. [Google Scholar] [CrossRef]
- Gregorutti, B.; Michel, B.; Saint-Pierre, P. Correlation and variable importance in random forests. Stat. Comput. 2017, 27, 659–678. [Google Scholar] [CrossRef]
- Voges, L.F.; Jarren, L.C.; Seifert, S. Exploitation of surrogate variables in random forests for unbiased analysis of mutual impact and importance of features. Bioinformatics 2023, 39, btad471. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.L.; Mentch, L.; Wheeler, B.J.; Tapia, A.L.; Richards, M.; Zhou, S.; Yi, L.; Redline, S.; Buysse, D.J. Use and misuse of random forest variable importance metrics in medicine: Demonstrations through incident stroke prediction. BMC. Med. Res. Methodol. 2023, 23, 144. [Google Scholar] [CrossRef]
- Williamson, B.D.; Gilbert, P.B.; Simon, N.R.; Carone, M. A general framework for inference on algorithm-agnostic variable importance. J. Am. Stat. Assoc. 2023, 118, 1645–1658. [Google Scholar] [CrossRef]
- Krimer, P.M. Generating and Interpreting Test Results: Test Validity, Quality Control, Reference Values, and Basic Epidemiology. In Duncan & Prasse’s Veterinary Laboratory Medicine: Clinical Pathology, 5th ed.; Latimer, K.S., Ed.; Wiley-Blackwell: Chichester, UK, 2011; pp. 374–375. [Google Scholar]
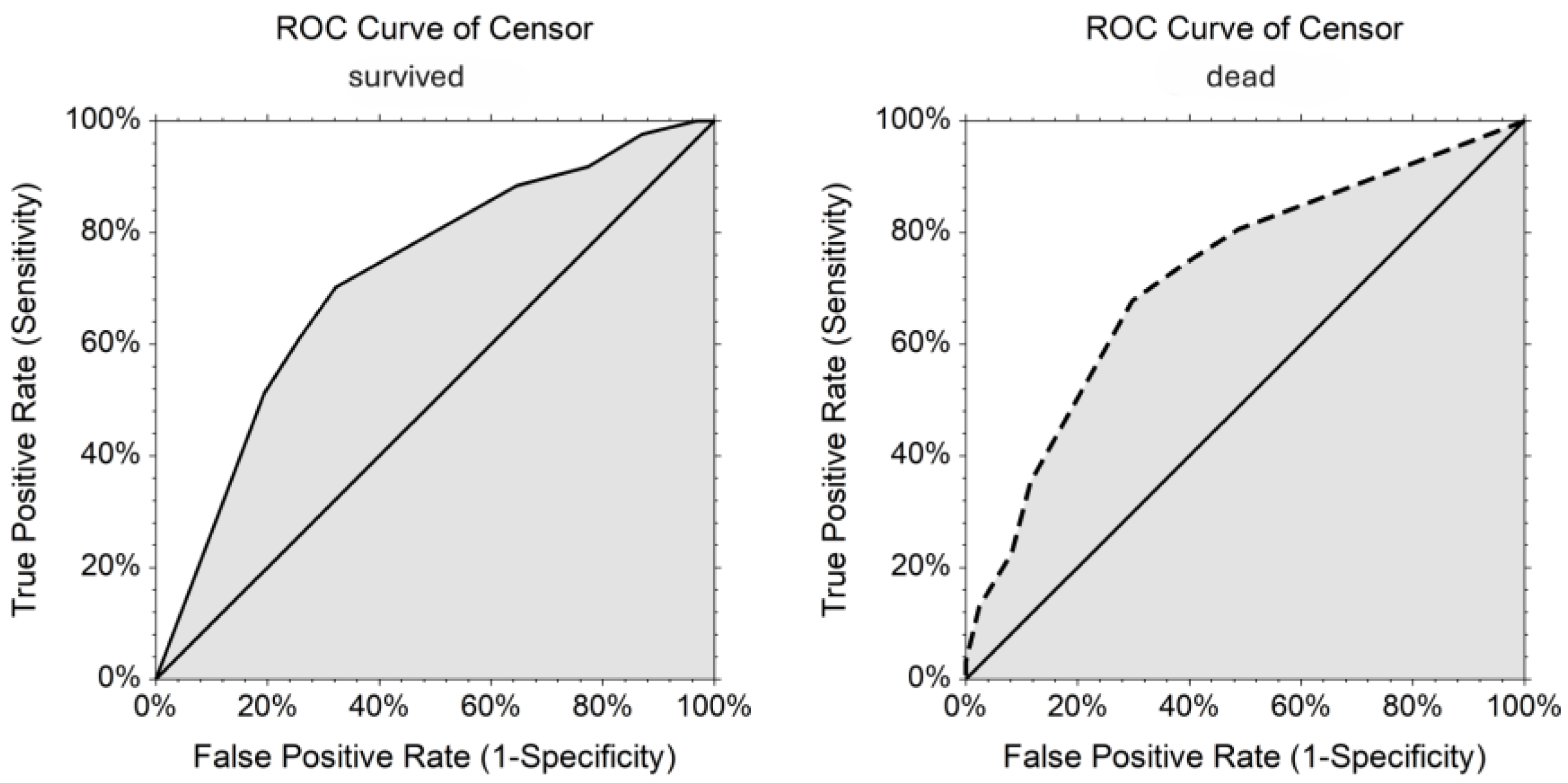
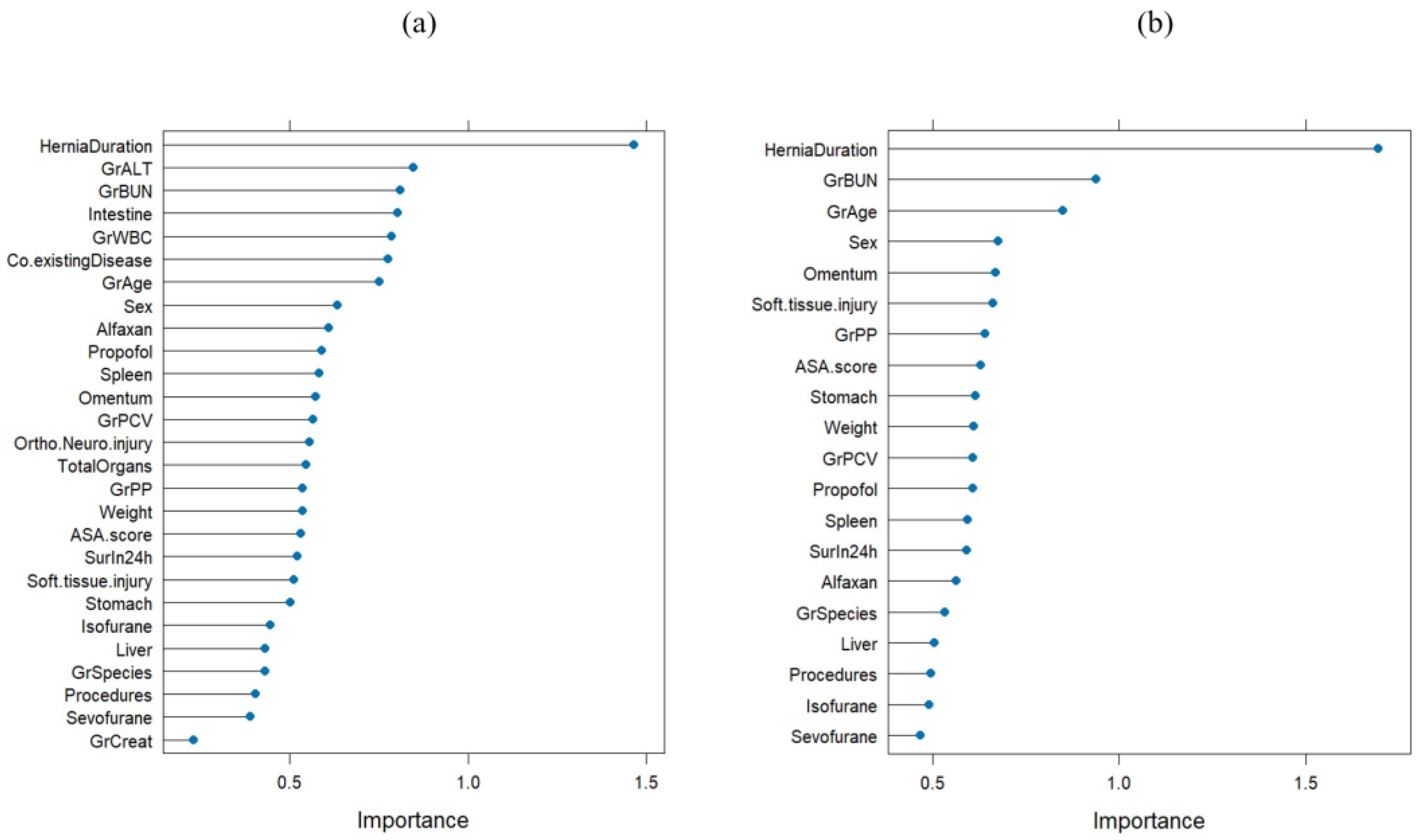
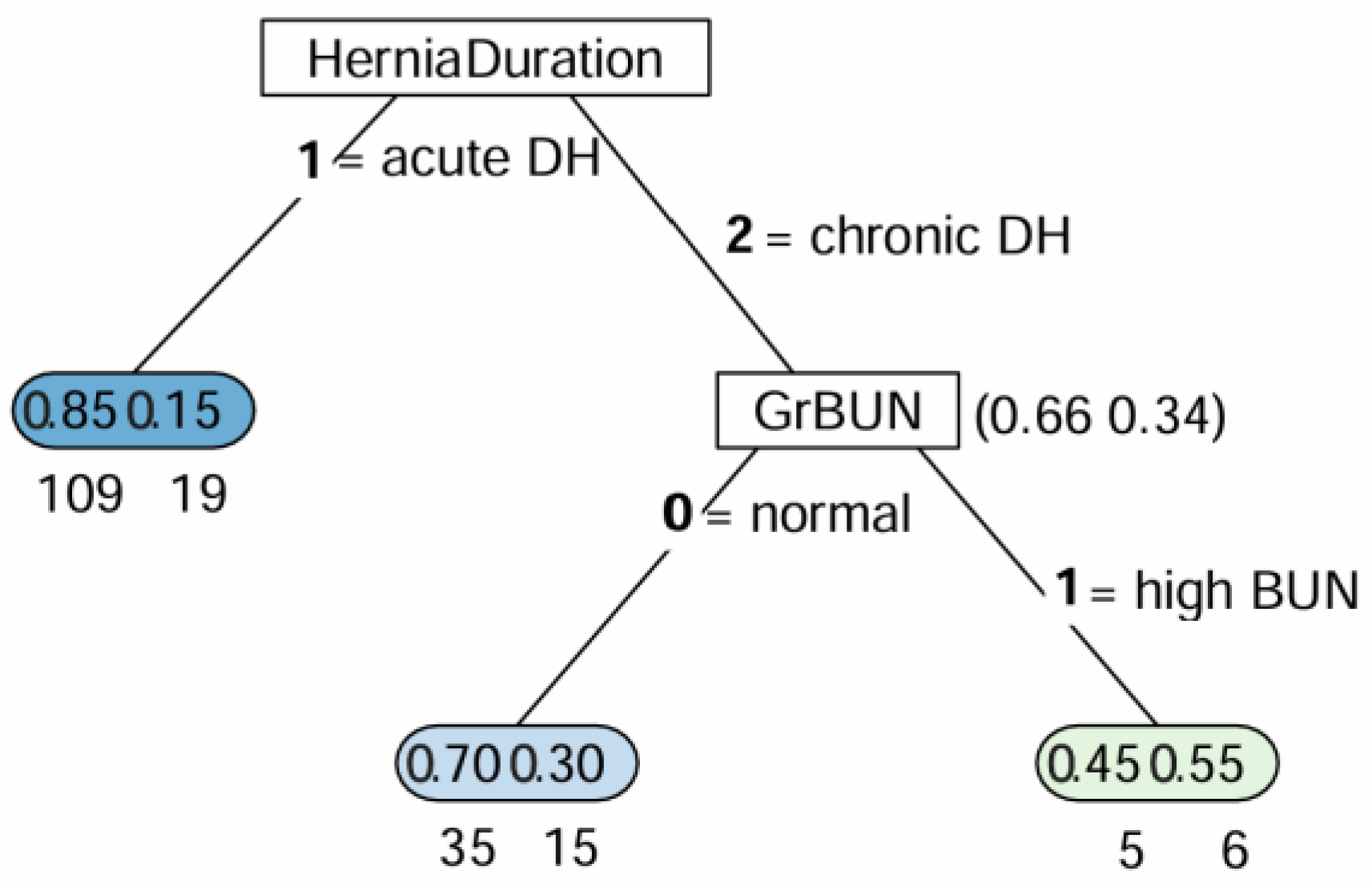

| Variables | Dogs (n = 63) | Cats (n = 126) | Both Dogs and Cats (N = 189) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survival n (%) | Death n (%) | p Value | Survival | Death | p Value | Survival | Death | p Value | ||
| Sex | Female | 23 (76.7%) | 7 (23.3%) | 0.325 | 52 (75.4%) | 17 (24.6%) | 0.634 | 75 (75.8%) | 24 (24.2%) | 0.277 |
| Male | 29 (87.9%) | 4 (12.1%) | 45 (79.0%) | 12 (21.0%) | 74 (82.2%) | 16 (17.8%) | ||||
| Age | <5 years | 36 (90.0%) | 4 (10.0%) | 0.081 a | 90 (77.6%) | 26 (22.4%) | 0.695 a | 126 (80.8%) | 30 (19.2%) | 0.157 |
| ≥5 years | 16 (69.6%) | 7 (30.4%) | 7 (70.0%) | 3 (30.0%) | 23 (69.7%) | 10 (30.3%) | ||||
| Weight (kg) | Dog <10/cat <3 | 33 (86.8%) | 5 (13.2%) | 0.267 | 62 (76.5%) | 19 (23.5%) | 0.875 | 95 (79.8%) | 24 (20.2%) | 0.662 |
| Dog ≥10/cat ≥3 | 19 (76.0%) | 6 (24.0%) | 35 (77.8%) | 10 (22.2%) | 54 (77.1%) | 16 (22.8%) | ||||
| Duration of hernia | Acute | 39 (88.6%) | 5 (11.4%) | 0.052 | 70 (83.3%) | 14 (16.7%) | 0.017* | 109 (85.2%) | 19 (14.8%) | 0.002 * |
| Chronic | 13 (68.4%) | 6 (31.6%) | 27 (64.3%) | 15 (35.7%) | 40 (65.6%) | 21 (34.4%) | ||||
| Blood profiles | ||||||||||
| PCV | Normal | 32 (84.2%) | 6 (15.8%) | 0.667 | 62 (72.9%) | 23 (27.1%) | 0.121 | 94 (76.4%) | 29 (23.6%) | 0.268 |
| Anemia | 20 (80.0%) | 5 (20.0%) | 35 (85.4%) | 6 (14.6%) | 55 (83.3%) | 11 (16.7%) | ||||
| WBC | Normal | 20 (87.0%) | 3 (13.0%) | 0.732 a | 47 (68.1%) | 22 (31.9%) | 0.009 * | 67 (72.8%) | 25 (27.2%) | 0.049 * |
| Leukocytosis | 32 (80.0%) | 8 (20.0%) | 50 (87.7%) | 7 (12.3%) | 82 (84.5%) | 15 (15.5%) | ||||
| Plasma protein | Normal | 29 (87.9%) | 4 (12.1%) | 0.197 a | 62 (76.5%) | 19 (23.5%) | 0.718 | 91 (79.8%) | 23 (20.2%) | 0.686 |
| Abnormal | 20 (74.1%) | 7 (25.9%) | 31 (79.5%) | 8 (20.5%) | 51 (77.3%) | 15 (22.7%) | ||||
| BUN | Normal | 39 (83.0%) | 8 (17.0%) | 0.387 a | 64 (83.1%) | 13 (16.9%) | 0.039 * | 103 (83.1%) | 21 (16.9%) | 0.026 * |
| Elevated | 7 (70.0) | 3 (30.0%) | 11 (61.1%) | 7 (38.9%) | 18 (64.3%) | 10 (35.7%) | ||||
| Creatinine | Normal | 43 (82.7%) | 9 (17.3%) | 0.631 a | 80 (78.4%) | 22 (21.6%) | 1.000 a | 123 (79.9%) | 31 (20.1%) | 0.712 a |
| Elevated | 6 (75.0%) | 2 (25.0%) | 3 (75.0%) | 1 (25.0%) | 9 (75.0%) | 3 (25.0%) | ||||
| ALT | Normal | 11 (78.6%) | 3 (21.4%) | 0.698 a | 18 (66.7%) | 9 (33.3%) | 0.096 | 29 (70.7%) | 12 (29.3%) | 0.107 |
| Elevated | 35 (83.3%) | 7 (16.7%) | 64 (82.0%) | 14 (18.0%) | 99 (82.5%) | 21 (17.5%) | ||||
| Anesthetics | Propofol | 46 (83.6%) | 9 (16.4%) | 0.620 a | 88 (77.9%) | 25 (22.1%) | 0.494 a | 134 (79.8%) | 34 (20.2%) | 0.378 |
| Alfaxalone | 6 (75.0%) | 2 (25.0%) | 0.620 a | 8 (66.7%) | 4 (33.3%) | 0.469 a | 14 (70.0%) | 6 (30.0%) | 0.306 | |
| Isoflurane | 23 (85.2%) | 4 (14.8%) | 0.745 a | 48 (76.2%) | 15 (23.8%) | 0.832 | 71 (78.9%) | 19 (21.1%) | 0.987 | |
| Sevoflurane | 29 (80.6%) | 7 (19.4%) | 0.745 a | 49 (77.8%) | 14 (22.2%) | 0.832 | 78 (78.8%) | 21 (21.2%) | 0.987 | |
| Surgical procedure | 1 procedure | 42 (85.7%) | 7 (14.3%) | 0.243 a | 83 (74.8%) | 28 (25.2%) | 0.188 a | 125 (78.1%) | 35 (21.9%) | 0.574 |
| >1 procedure | 10 (71.4%) | 4 (28.6%) | 14 (93.3%) | 1 (6.7%) | 24 (82.8% | 5 (17.2%) | ||||
| Herniated organ | ||||||||||
| Stomach | no | 29 (87.9%) | 4 (12.1%) | 0.325 a | 51 (73.9%) | 18 (26.1%) | 0.440 | 80 (78.4%) | 22 (21.3%) | 0.959 |
| yes | 23 (76.7%) | 7 (23.3%) | 40 (80.0%) | 10 (20.0%) | 63 (78.8%) | 17 (21.3%) | ||||
| Liver | no | 16 (76.2%) | 5 (23.8%) | 0.348 | 23 (85.2%) | 4 (14.8%) | 0.307 a | 39 (81.3%) | 9 (18.8%) | 0.629 |
| yes | 36 (85.7%) | 6 (14.3%) | 70 (74.5%) | 24 (25.5%) | 106 (77.9%) | 30 (22.1%) | ||||
| Spleen | no | 34 (85.0%) | 6 (15.0%) | 0.498 | 63 (74.1%) | 22 (25.9%) | 0.272 | 97 (77.6%) | 28 (22.4%) | 0.561 |
| yes | 18 (78.3%) | 5 (21.7%) | 30 (83.3%) | 6 (16.7) | 48 (81.4%) | 11 (18.6%) | ||||
| Intestine | no | 27 (90.0%) | 3 (10.0%) | 0.189 a | 38 (88.4%) | 5 (11.6%) | 0.026 * | 65 (89.0%) | 8 (11.0%) | 0.006 * |
| yes | 25 (75.8%) | 8 (24.2%) | 55 (70.5%) | 23 (29.5%) | 80 (72.1%) | 31 (27.9%) | ||||
| Omentum | no | 41 (85.4%) | 7 (14.6%) | 0.435 a | 58 (75.3%) | 19 (24.7%) | 0.596 | 99 (79.2%) | 26 (20.8%) | 0.848 |
| yes | 11 (73.3%) | 4 (26.7%) | 35 (79.6%) | 9 (20.4%) | 46 (78.0%) | 13 (22.0%) | ||||
| Concurrent injuries | ||||||||||
| Soft tissue | no | 39 (86.7%) | 6 (13.3%) | 0.173 | 84 (77.8%) | 24 (22.2%) | 0.604 | 123 (80.4%) | 30 (19.6%) | 0.280 |
| yes | 13 (72.2%) | 5 (27.8%) | 13 (72.2%) | 5 (27.8%) | 26 (72.2%) | 10 (27.8%) | ||||
| Ortho/neuro | no | 33 (80.5%) | 8 (19.5%) | 0.733 a | 68 (77.3%) | 20 (22.7%) | 0.892 | 101 (78.3%) | 28 (21.7%) | 0.631 |
| yes | 19 (86.4%) | 3 (13.6%) | 29 (78.4%) | 8 (21.6%) | 48 (81.4%) | 11 (18.6%) | ||||
| Coexisting disease | no | 41 (87.2%) | 6 (12.8%) | 0.093 | 81 (77.9%) | 23 (22.1%) | 0.602 | 122 (80.8%) | 29 (19.2%) | 0.189 |
| yes | 11 (68.8%) | 5 (31.2%) | 16 (72.7%) | 6 (27.3%) | 27 (71.1%) | 11 (28.9%) | ||||
| Surgery within 24 h | no | 45 (83.3%) | 9 (16.7%) | 0.650 a | 86 (76.1%) | 27 (23.9%) | 0.731 a | 131 (78.4%) | 36 (21.6%) | 1.000 a |
| yes | 7 (77.8%) | 2 (22.2%) | 11 (84.6%) | 2 (15.4%) | 18 (81.8%) | 4 (18.2%) | ||||
| ASA score | 2–3 | 30 (88.2%) | 4 (11.8%) | 0.319 a | 54 (77.1%) | 16 (22.9%) | 0.962 | 84 (80.8%) | 20 (19.2%) | 0.472 |
| 4–5 | 22 (75.9%) | 7 (24.1%) | 43 (76.8%) | 13 (23.2%) | 65 (76.5%) | 20 (23.5%) | ||||
| Total number of organs | 0–2 | 34 (85.0%) | 6 (15.0%) | 0.497 | 44 (75.9%) | 14 (24.1%) | 0.879 | 78 (79.6%) | 20 (20.4%) | 0.717 |
| ≥3 | 18 (78.3%) | 5 (21.7%) | 47 (77.1%) | 14 (22.9%) | 65 (77.4%) | 19 (22.6%) | ||||
| Variables | Odds Ratio (95% CI) | p Value | |
|---|---|---|---|
| Species | Dog | Ref | 0.379 |
| Cat | 1.41 (0.65, 3.06) | ||
| Sex | Female | Ref | 0.279 |
| Male | 0.68 (0.33, 1.37) | ||
| Age | <5 years | Ref | 0.161 * |
| ≥5 years | 1.83 (0.79, 4.24) | ||
| Weight (kg) | Dog <10/cat <3 | Ref | 0.662 |
| Dog ≥10/cat ≥3 | 1.17 (0.57, 2.40) | ||
| Duration of hernia | Acute | Ref | 0.003 * |
| Chronic | 3.01 (1.47, 6.18) | ||
| Blood profiles | |||
| PCV | Normal | Ref | 0.270 |
| Anemia | 0.65 (0.30, 1.40) | ||
| WBC | Normal | Ref | 0.051 * |
| Leukocytosis | 0.49 (0.24, 1.00) | ||
| Plasma protein | Normal | Ref | 0.686 |
| Abnormal | 1.16 (0.56, 2.43) | ||
| BUN | Normal | Ref | 0.030 * |
| Elevated | 2.72 (1.10, 6.73) | ||
| Creatinine | Normal | Ref | 0.688 |
| Elevated | 1.32 (0.33, 5.18) | ||
| ALT | Normal | Ref | 0.112 * |
| Elevated | 0.51 (0.22, 1.17) | ||
| Anesthetics | Propofol | 0.63 (0.23, 1.76) | 0.381 |
| Alfaxalone | 1.70 (0.61, 4.76) | 0.311 | |
| Isoflurane | 0.99 (0.49, 2.00) | 0.986 | |
| Sevoflurane | 1.00 (0.50, 2.02) | 0.986 | |
| Surgical procedure | 1 procedure | Ref | 0.575 |
| >1 procedure | 0.74 (0.26, 2.09) | ||
| Herniated organ | Stomach | 0.98 (0.48, 2.00) | 0.959 |
| Liver | 1.22 (0.53, 2.81) | 0.630 | |
| Spleen | 0.79 (0.36, 1.73) | 0.561 | |
| Intestine | 3.15 (1.35, 7.32) | 0.008 * | |
| Omentum | 1.08 (0.51, 2.28) | 0.848 | |
| Concurrent injuries | Soft tissue | 1.58 (0.69, 3.62) | 0.283 |
| Ortho/neuro | 0.83 (0.38, 1.80) | 0.631 | |
| Coexisting disease | No | Ref | 0.192 * |
| Yes | 1.71 (0.76, 3.85) | ||
| Surgery within 24 h | No | Ref | 0.716 |
| Yes | 0.81 (0.26, 2.54) | ||
| ASA score | 2–3 | Ref | 0.472 |
| 4–5 | 1.29 (0.64, 2.60) | ||
| Total number of organs | 0–2 | Ref | 0.717 |
| ≥3 | 1.14 (0.56, 2.32) | ||
| Variables | Adjusted Odds Ratio (95%CI) | p Value | |
|---|---|---|---|
| Age | <5 years | Ref | 0.1707 |
| ≥5 years | 1.91 (0.76, 4.85) | ||
| Duration of hernia | Acute | Ref | 0.0016 * |
| Chronic | 4.01 (1.69, 9.53) | ||
| BUN level | Normal | Ref | 0.0181 * |
| Elevated | 3.24 (1.22, 8.57) | ||
| Variables | Adjusted Hazard Ratio (95%CI) | p Value | |
|---|---|---|---|
| Age | <5 years | Ref | 0.367 |
| ≥5 years | 1.49 (0.63, 3.55) | ||
| Duration of hernia | Acute | Ref | 0.003 * |
| Chronic | 3.31 (1.51, 7.30) | ||
| BUN level | Normal | Ref | 0.015 * |
| Elevated | 2.88 (1.23, 6.77) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwananocha, I.; Niyom, S.; Budsayaplakorn, P.; Kasemsuwan, S.; Theerapan, W.; Warrit, K. Prognostic Factors for Mortality Following Diaphragmatic Herniorrhaphy in Dogs and Cats: Multivariable Logistic Regression and Machine Learning Approaches. Vet. Sci. 2025, 12, 819. https://doi.org/10.3390/vetsci12090819
Kwananocha I, Niyom S, Budsayaplakorn P, Kasemsuwan S, Theerapan W, Warrit K. Prognostic Factors for Mortality Following Diaphragmatic Herniorrhaphy in Dogs and Cats: Multivariable Logistic Regression and Machine Learning Approaches. Veterinary Sciences. 2025; 12(9):819. https://doi.org/10.3390/vetsci12090819
Chicago/Turabian StyleKwananocha, Irin, Sirirat Niyom, Pharkpoom Budsayaplakorn, Suwicha Kasemsuwan, Wutthiwong Theerapan, and Kanawee Warrit. 2025. "Prognostic Factors for Mortality Following Diaphragmatic Herniorrhaphy in Dogs and Cats: Multivariable Logistic Regression and Machine Learning Approaches" Veterinary Sciences 12, no. 9: 819. https://doi.org/10.3390/vetsci12090819
APA StyleKwananocha, I., Niyom, S., Budsayaplakorn, P., Kasemsuwan, S., Theerapan, W., & Warrit, K. (2025). Prognostic Factors for Mortality Following Diaphragmatic Herniorrhaphy in Dogs and Cats: Multivariable Logistic Regression and Machine Learning Approaches. Veterinary Sciences, 12(9), 819. https://doi.org/10.3390/vetsci12090819






