Enucleation Due to Ocular Abscess in a Captive Chimpanzee (Pan troglodytes): A Case Report from the Republic of Congo
Simple Summary
Abstract
1. Introduction
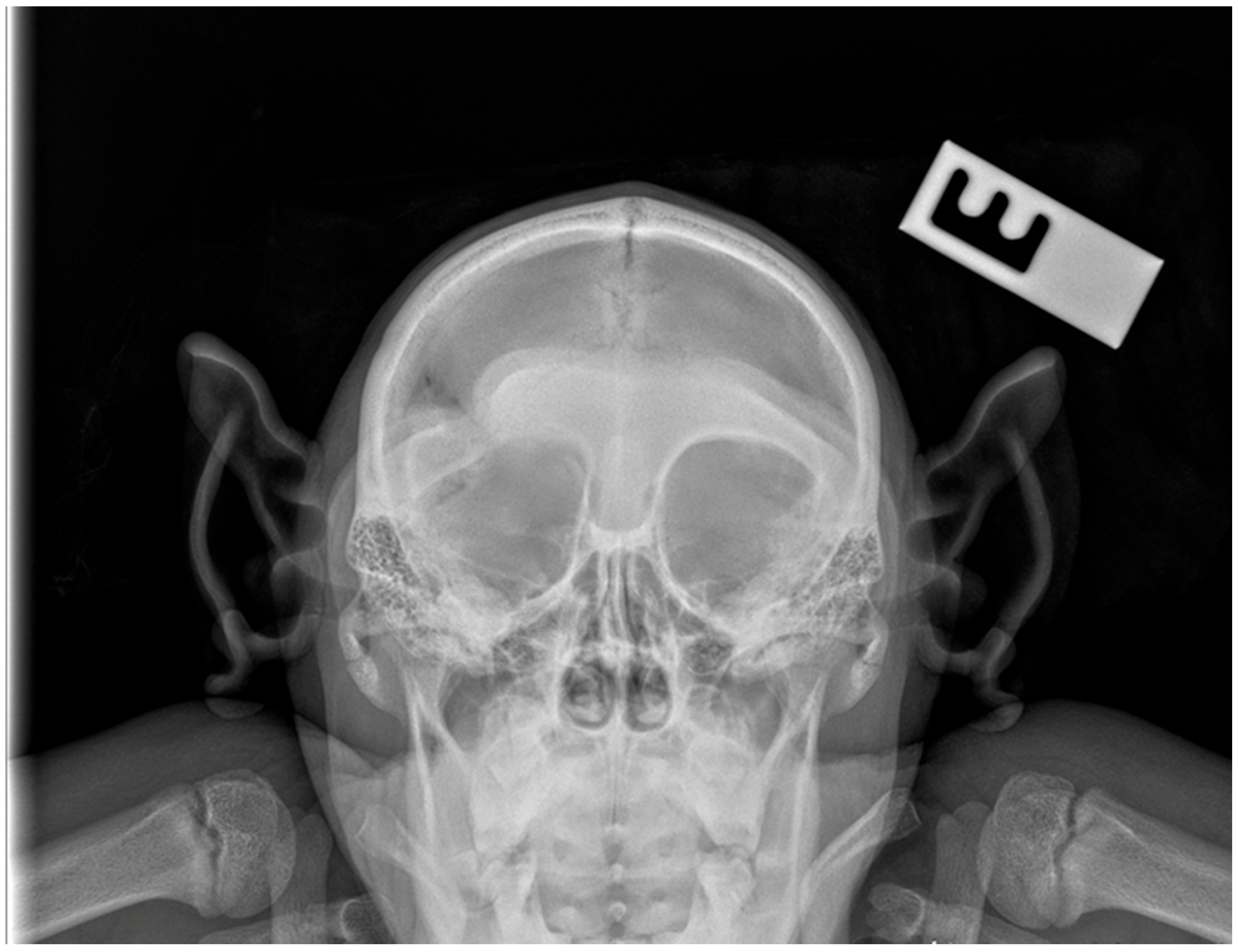
2. Case Presentation
2.1. Anesthesia Technique
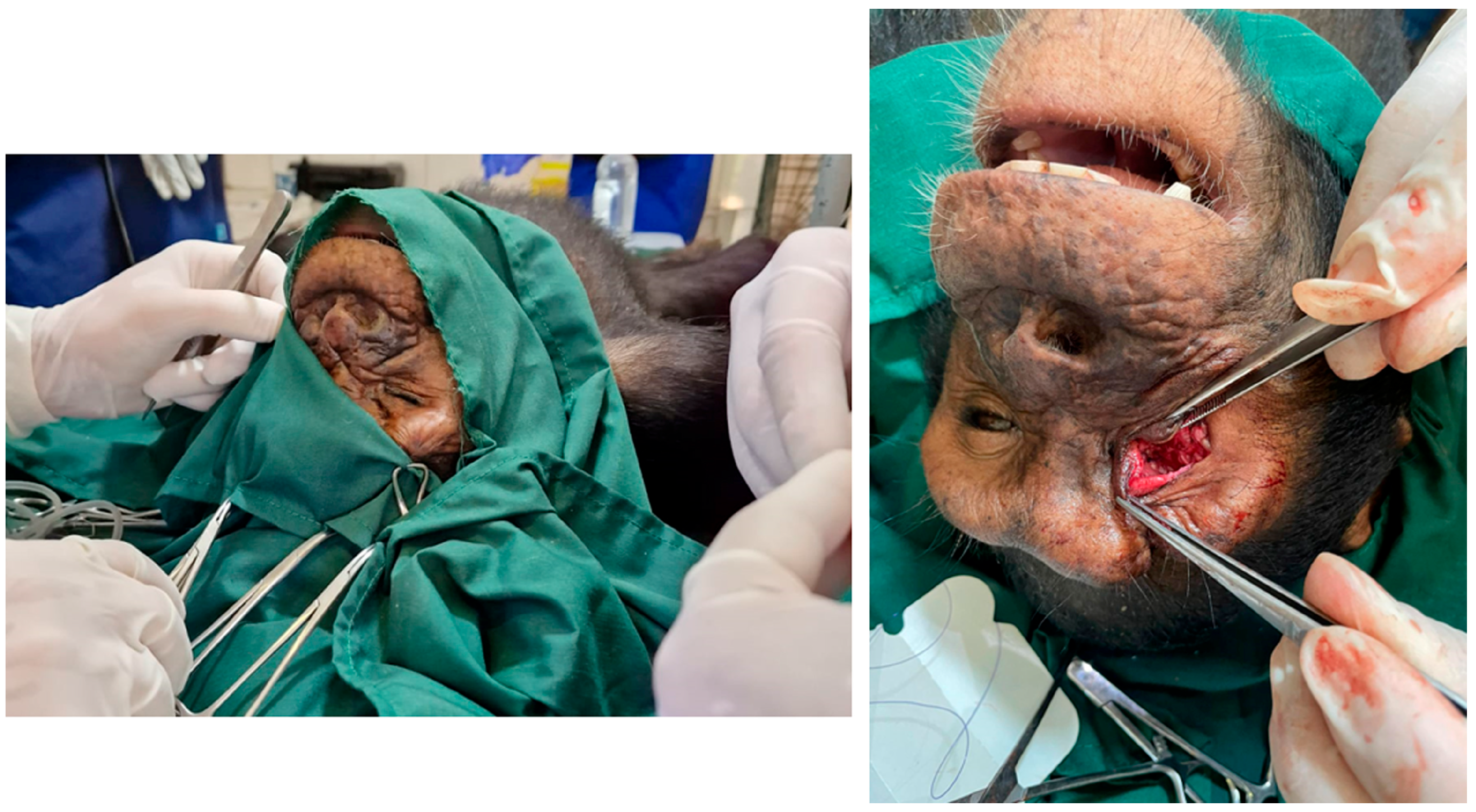
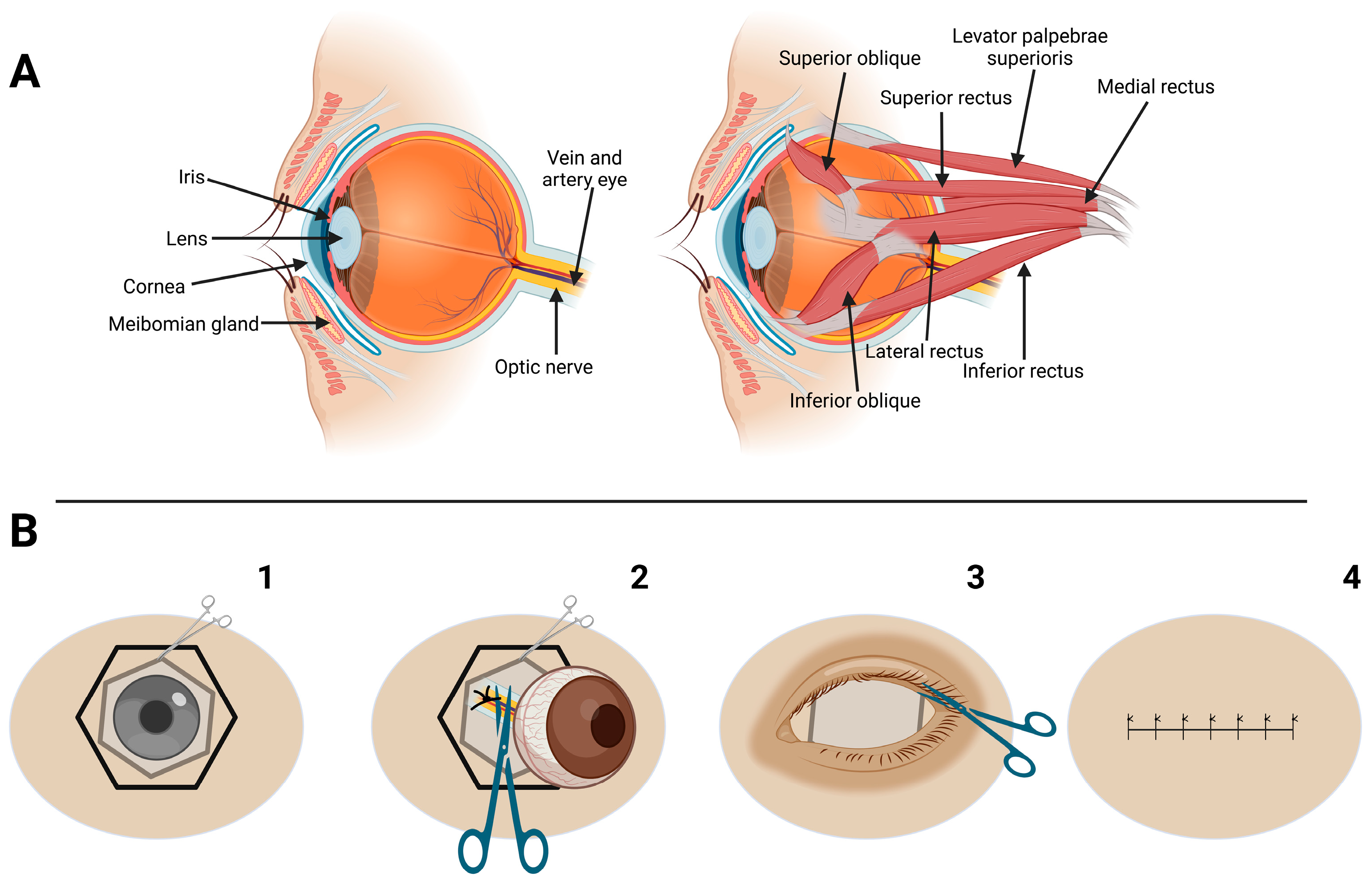
2.2. Surgical Technique
2.3. Postoperative Period
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PO | Oral route of medication administration |
| TID | Three times in a day |
| BID | Two times in a day |
| GOT | Aspartate aminotransferase |
| GPT | Alanine aminotransferase |
References
- Muehlenbein, M.P. Basics in Human Evolution, 1st ed.; Elsevier: Saint Louis, MO, USA, 2015; ISBN 978-0-12-802652-6. [Google Scholar]
- Carlsson, H.-E.; Schapiro, S.J.; Farah, I.; Hau, J. Use of Primates in Research: A Global Overview. Am. J. Primatol. 2004, 63, 225–237. [Google Scholar] [CrossRef]
- Wallace, P.Y.; Asa, C.S.; Agnew, M.; Cheyne, S.M. A Review of Population Control Methods in Captive-Housed Primates. Anim. Welfare 2016, 25, 7–20. [Google Scholar] [CrossRef]
- Strong, V.J.; Grindlay, D.; Redrobe, S.; Cobb, M.; White, K. A Systematic Review of the Literature Relating to Captive Great Ape Morbidity and Mortality. J. Zoo Wildl. Med. 2016, 47, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Humle, T.; Maisels, F.; Oates, J.F.; Plumptre, A.; Williamson, E.A. Pan Troglodytes (Errata Version Published in 2018). The IUCN Red List of Threatened Species [IUCN website]. 2016. Available online: http://hdl.handle.net/1893/26837 (accessed on 8 August 2025). [CrossRef]
- Anderson, J.R. Chimpanzees and Death. Phil. Trans. R. Soc. B 2018, 373, 20170257. [Google Scholar] [CrossRef]
- Ferreira Da Silva, M.J.; Regalla, A. The Illegal Trade in Live Western Chimpanzees (Pan troglodytes verus) in Guinea-Bissau and Proposed Conservation Management Actions. Conserv. Lett. 2025, 18, e13087. [Google Scholar] [CrossRef]
- McLennan, M.R.; Mori, H.; Mahittikorn, A.; Prasertbun, R.; Hagiwara, K.; Huffman, M.A. Zoonotic Enterobacterial Pathogens Detected in Wild Chimpanzees. Ecohealth 2018, 15, 143–147. [Google Scholar] [CrossRef]
- Ghobrial, L.; Lankester, F.; Kiyang, J.A.; Akih, A.E.; de Vries, S.; Fotso, R.; Gadsby, E.L.; Jenkins, P.D.; Gonder, M.K. Tracing the Origins of Rescued Chimpanzees Reveals Widespread Chimpanzee Hunting in Cameroon. BMC Ecol. 2010, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Tranquilli, S.; Abedi-Lartey, M.; Abernethy, K.; Amsini, F.; Asamoah, A.; Balangtaa, C.; Blake, S.; Bouanga, E.; Breuer, T.; Brncic, T.M.; et al. Protected Areas in Tropical Africa: Assessing Threats and Conservation Activities. PLoS ONE 2014, 9, e114154. [Google Scholar] [CrossRef] [PubMed]
- Dunay, E.; Owens, L.A.; Dunn, C.D.; Rukundo, J.; Atencia, R.; Cole, M.F.; Cantwell, A.; Emery Thompson, M.; Rosati, A.G.; Goldberg, T.L. Viruses in Sanctuary Chimpanzees across Africa. Am. J. Primatol. 2023, 85, e23452. [Google Scholar] [CrossRef]
- Dunay, E.; Rukundo, J.; Atencia, R.; Cole, M.F.; Cantwell, A.; Emery Thompson, M.; Rosati, A.G.; Goldberg, T.L. Viruses in Saliva from Sanctuary Chimpanzees (Pan troglodytes) in Republic of Congo and Uganda. PLoS ONE 2023, 18, e0288007. [Google Scholar] [CrossRef]
- Schmidt, R.E. Ophthalmic Lesions in Non-Human Primates. Vet. Pathol. 1971, 8, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Dubicanac, M.; Radespiel, U.; Zimmermann, E. A Review on Ocular Findings in Mouse Lemurs: Potential Links to Age and Genetic Background. Primate Biol. 2017, 4, 215–228. [Google Scholar] [CrossRef]
- Joosten, G.G.; Gould, M.M.; Anzelmo, M.; Rozzi, F.R. Ontogenia de las órbitas en Pan troglodytes: Implicancias estructurales y funcionales. Rev. Del Mus. De La Plata 2018, 3, 309–323. Available online: https://publicaciones.fcnym.unlp.edu.ar/rmlp/article/view/2332 (accessed on 8 August 2025). [CrossRef]
- Dobbie, K.-L.S. Ocular Findings and Intraocular Lens Power in Captive Chimpanzees (Pan troglodytes); University of Pretoria: Pretoria, South Africa, 2020. [Google Scholar]
- Thomasy, S.M. Ophthalmology of Primatomorpha: Lemurs, Tarsiers, Monkeys, Apes, and Relatives. In Wild and Exotic Animal Ophthalmology: Volume 2: Mammals; Montiani-Ferreira, F., Moore, B.A., Ben-Shlomo, G., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 483–543. ISBN 978-3-030-81273-7. Available online: https://link.springer.com/chapter/10.1007/978-3-030-81273-7_22#citeas (accessed on 8 August 2025).
- Wu, J.; Liu, W.; Zhu, S.; Liu, H.; Chen, K.; Zhu, Y.; Li, Z.; Yang, C.; Pan, L.; Li, R.; et al. Design, Methodology, and Preliminary Results of the Non-Human Primates Eye Study. BMC Ophthalmol. 2023, 23, 53. [Google Scholar] [CrossRef]
- Pletcher, J.S.; Zimmer, J.L.; Liu, C.-C.; Beierschmitt, A.; Lewin, A.C. Ocular Examination Findings and Selected Ophthalmic Diagnostic Tests in African Green Monkeys (Chlorocebus Aethiops Sabaeus). Vet. Ophthalmol. 2024, 27, 158–169. [Google Scholar] [CrossRef]
- Debenham, J.J.; Atencia, R. Homologous whole blood transfusion during treatment of severe anemia in a chimpanzee (Pan troglodytes). J. Zoo Wildl. Med. 2014, 45, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Feltrer-Rambaud, Y.; Moresco, A.; Ange-van Heugten, K.; Pizarro, A.; Tomeo-Martín, B.; Carrasco Pesquera, L.; Moresco, N.; Atencia, R. Serum Vitamin D in Sanctuary Chimpanzees (Pan troglodytes) in Range Countries: A Pilot Study. Vet. Med. Sci. 2023, 9, 2937–2945. [Google Scholar] [CrossRef]
- Gumber, S.; Stovall, M.I.; Breding, E.; Crane, M.M. Spontaneous Leiomyomas of the Gastroesophageal Junction in a Chimpanzee (Pan troglodytes). Comp. Med. 2014, 64, 230–233. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4067588/ (accessed on 8 August 2025). [PubMed]
- Popilskis, S.J.; Kohn, D.F. Chapter 11—Anesthesia and Analgesia in Nonhuman Primates. In Anesthesia and Analgesia in Laboratory Animals; Kohn, D.F., Wixson, S.K., White, W.J., John Benson, G., Eds.; American College of Laboratory Animal Medicine; Academic Press: San Diego, CA, USA, 1997; pp. 233–255. ISBN 978-0-12-417570-9. [Google Scholar]
- April, M.; Tabor, E.; Gerety, R.J. Combination of Ketamine and Xylazine for Effective Anaesthesia of Juvenile Chimpanzees (Pan troglodytes). Lab Anim. 1982, 16, 116–118. [Google Scholar] [CrossRef]
- Lewis, J.C.M. Medetomidine-Ketamine Anaesthesia in the Chimpanzee (Pan troglodytes). J. Vet. Anaesth. 1993, 20, 18–20. [Google Scholar] [CrossRef]
- Melis, S.; Schauvliege, S.; van Bolhuis, H.; Hoyer, M.; Gasthuys, F. Chemical Immobilization of Chimpanzees (Pan troglodytes) Using a Combination of Detomidine and Ketamine. Vet. Anaesth. Analg. 2012, 39, 520–528. [Google Scholar] [CrossRef]
- Murphy, K.; Baxter, M.; Flecknell, P. Anesthesia and Analgesia in Nonhuman Primates. In Nonhuman Primates in Biomedical Research; Academic Press: Cambridge, MA, USA, 2012; pp. 403–435. [Google Scholar] [CrossRef]
- West, G.; Heard, D.; Caulkett, N. (Eds.) Zoo Animal and Wildlife Immobilization and Anesthesia; Wiley-Blackwell: Ames, IA, USA, 2014; ISBN 978-0-8138-1183-3. [Google Scholar]
- Sutton, J.B. On Some Points in the Anatomy of the Chimpanzee (Anthropopithecus Troglodytes). J. Anat. Physiol. 1883, 18, 66–85. [Google Scholar]
- Schmittbuhl, M.; Le Minor, J.-M.; Alienbach, B.; Schaaf, A. Shape of the Orbital Opening: Individual Characterization and Analysis of Variability in Modern Humans, Gorilla gorilla, and Pan troglodytes. Ann. Anat. Anat. Anz. 1999, 181, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Denion, E.; Hitier, M.; Guyader, V.; Dugué, A.-E.; Mouriaux, F. Unique Human Orbital Morphology Compared with That of Apes. Sci. Rep. 2015, 5, 11528. [Google Scholar] [CrossRef]
- Atencia Fernandez, R.D.L.T. Parámetros Clínicos de Chimpancé (“Pan troglodytes”) en Programas de Rehabilitación y Reintroducción en su Medio Natural. Universidad Complutense de Madrid. 2016. Available online: https://docta.ucm.es/entities/publication/9f13d04f-1061-4b31-b817-1d8c7737fa26 (accessed on 8 August 2025).
- Atencia, R.; Stöhr, E.J.; Drane, A.L.; Stembridge, M.; Howatson, G.; Del Rio, P.R.L.; Feltrer, Y.; Tafon, B.; Redrobe, S.; Peck, B.; et al. Heart rate and indirect blood pressure responses to four different field anesthetic protocols in wild-born captive chimpanzees (Pan troglodytes). J. Zoo Wildl. Med. 2017, 48, 636–644. [Google Scholar] [CrossRef]
- Atencia, R.; Revuelta, L.; Somauroo, J.D.; Shave, R.E. Electrocardiogram Reference Intervals for Clinically Normal Wild-Born Chimpanzees (Pan troglodytes). Am. J. Vet. Res. 2015, 76, 688–693. [Google Scholar] [CrossRef]
- Tiefenbacher, S.; Fahey, M.A.; Rowlett, J.K.; Meyer, J.S.; Pouliot, A.L.; Jones, B.M.; Novak, M.A. The Efficacy of Diazepam Treatment for the Management of Acute Wounding Episodes in Captive Rhesus Macaques. Comp. Med. 2005, 55, 387–392. [Google Scholar]
- Dortzbach, R.K.; Woog, J.J. Choice of Procedure. Enucleation, Evisceration, or Prosthetic Fitting over Globes. Ophthalmology 1985, 92, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Moshfeghi, D.M.; Moshfeghi, A.A.; Finger, P.T. Enucleation. Surv. Ophthalmol. 2000, 44, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Obuchowska, I.; Mariak, Z.; Elmdhm, S. Surgical technique and complications of enucleation. Klin. Oczna 2005, 107, 163–166. [Google Scholar]
- Fu, L.; Patel, B.C. Enucleation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Clark, I.R.; Sandel, A.A.; Reddy, R.B.; Langergraber, K.E. A Preliminary Analysis of Wound Care and Other-Regarding Behavior in Wild Chimpanzees at Ngogo, Kibale National Park, Uganda. Primates 2021, 62, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Association of Primate Veterinarians Guidelines for Wound Management of Nonhuman Primates. J. Am. Assoc. Lab. Anim. Sci. 2022, 61, 315–319.
- Mutlow, A.G.; Mauch, W.D.; Carpenter, J.W.; Smith, T. Surgical Procedure and Postoperative Management of a Perineal Urethrostomy in a Chimpanzee (Pan troglodytes). J. Zoo Wildl. Med. 2006, 37, 381–386. [Google Scholar] [CrossRef]
- Sasseville, V.G.; Mansfield, K.G.; Mankowski, J.L.; Tremblay, C.; Terio, K.A.; Mätz-Rensing, K.; Gruber-Dujardin, E.; Delaney, M.A.; Schmidt, L.D.; Liu, D.; et al. Meeting Report: Spontaneous Lesions and Diseases in Wild, Captive-Bred, and Zoo-Housed Nonhuman Primates and in Nonhuman Primate Species Used in Drug Safety Studies. Vet. Pathol. 2012, 49, 1057–1069. [Google Scholar] [CrossRef]
- Walter, W.L. Update on Enucleation and Evisceration Surgery. Ophthalmic. Plast. Reconstr. Surg. 1985, 1, 243–252. [Google Scholar] [CrossRef]
- Gelatt, K.; Gelatt, J.; Plummer, G. Veterinary Ophthalmic Surgery; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-7020-3429-9. [Google Scholar]
- Allgoewer, I.; Soukup, P. Modified Lateral Enucleation Technique-Surgery without Ligation or Clamping of the Optic Nerve: Technique Description, Complication Rate and Risk Factors, and Intraoperative Blood Loss Estimation in Companion Animals. Vet. Ophthalmol. 2025, 28, 425–437. [Google Scholar] [CrossRef]
- Duke-Elder, S. System of Ophthalmology, Vol. XII: Neuro-Ophthalmology; Henry Kimpton: London, UK, 1973. [Google Scholar]
- Blanc, V.F.; Feinstein, R. The Oculocardiac Reflex: A Review. Can. J. Anaesth. 1991, 38, 836–849. [Google Scholar]
- Bourgeois, S.R.; Vazquez, M.; Brasky, K. Combination Therapy Reduces Self-Injurious Behavior in a Chimpanzee (Pan Troglodytes Troglodytes): A Case Report. J. Appl. Anim. Welf. Sci. 2007, 10, 123–140. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Values of the Chimpanzee | Normal Values (Young Chimpanzee ~2 Years) |
|---|---|---|
| Heart rate (bpm) | 71 | 53–89 |
| Respiratory rate (breaths/min) | 27 | 25–40 |
| Rectal temperature (°C) | 37.4 | 37.0–38.5 |
| Hematocrit (%) | 42 | 36–46 |
| Hemoglobin (g/dL) | 12.8 | 11–14 |
| Red blood cells (106/µL) | 5.2 | 4.5–6.0 |
| White blood cells (103/µL) | 16 | 7–12 |
| Platelets (103/µL) | 320 | 250–450 |
| Urea (mg/dL) | 11 | 5–15 |
| Creatinine (mg/dL) | 0.9 | 0.5–1.2 |
| Glucose (mg/dL) | 98 | 70–120 |
| ALT (U/L) | 34 | 20–50 |
| AST (U/L) | 46 | 25–60 |
| Alkaline phosphatase (U/L) | 280 | 150–400 |
| Total proteins (g/dL) | 7.2 | 6.0–8.0 |
| Globulin (g/dL) | 4.1 | 2.5–3.5 |
| Albumin (g/dL) | 4.0 | 3.0–4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuertes-Recuero, M.; López-Hernández, J.L.; Ramírez-Lago, A.; Gutiérrez-Cepeda, L.; De Pablo-Moreno, J.A.; Morón-Elorza, P.; Revuelta, L.; Atencia, R. Enucleation Due to Ocular Abscess in a Captive Chimpanzee (Pan troglodytes): A Case Report from the Republic of Congo. Vet. Sci. 2025, 12, 805. https://doi.org/10.3390/vetsci12090805
Fuertes-Recuero M, López-Hernández JL, Ramírez-Lago A, Gutiérrez-Cepeda L, De Pablo-Moreno JA, Morón-Elorza P, Revuelta L, Atencia R. Enucleation Due to Ocular Abscess in a Captive Chimpanzee (Pan troglodytes): A Case Report from the Republic of Congo. Veterinary Sciences. 2025; 12(9):805. https://doi.org/10.3390/vetsci12090805
Chicago/Turabian StyleFuertes-Recuero, Manuel, José L. López-Hernández, Alejandra Ramírez-Lago, Luna Gutiérrez-Cepeda, Juan A. De Pablo-Moreno, Pablo Morón-Elorza, Luis Revuelta, and Rebeca Atencia. 2025. "Enucleation Due to Ocular Abscess in a Captive Chimpanzee (Pan troglodytes): A Case Report from the Republic of Congo" Veterinary Sciences 12, no. 9: 805. https://doi.org/10.3390/vetsci12090805
APA StyleFuertes-Recuero, M., López-Hernández, J. L., Ramírez-Lago, A., Gutiérrez-Cepeda, L., De Pablo-Moreno, J. A., Morón-Elorza, P., Revuelta, L., & Atencia, R. (2025). Enucleation Due to Ocular Abscess in a Captive Chimpanzee (Pan troglodytes): A Case Report from the Republic of Congo. Veterinary Sciences, 12(9), 805. https://doi.org/10.3390/vetsci12090805






