Effects of Chromium Yeast Supplementation on Serum hsp60 and hsp70, mRNA Expression in Heat-Stressed Lambs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Determination of Chromium in Blood
2.3. Sample Collection for qPCR and Cortisol
2.4. RNA Extraction, cDNA Synthesis and Gene Expression Analysis
2.5. Cortisol Analysis and Glucose Clearance Test
2.6. Data Analysis
3. Results
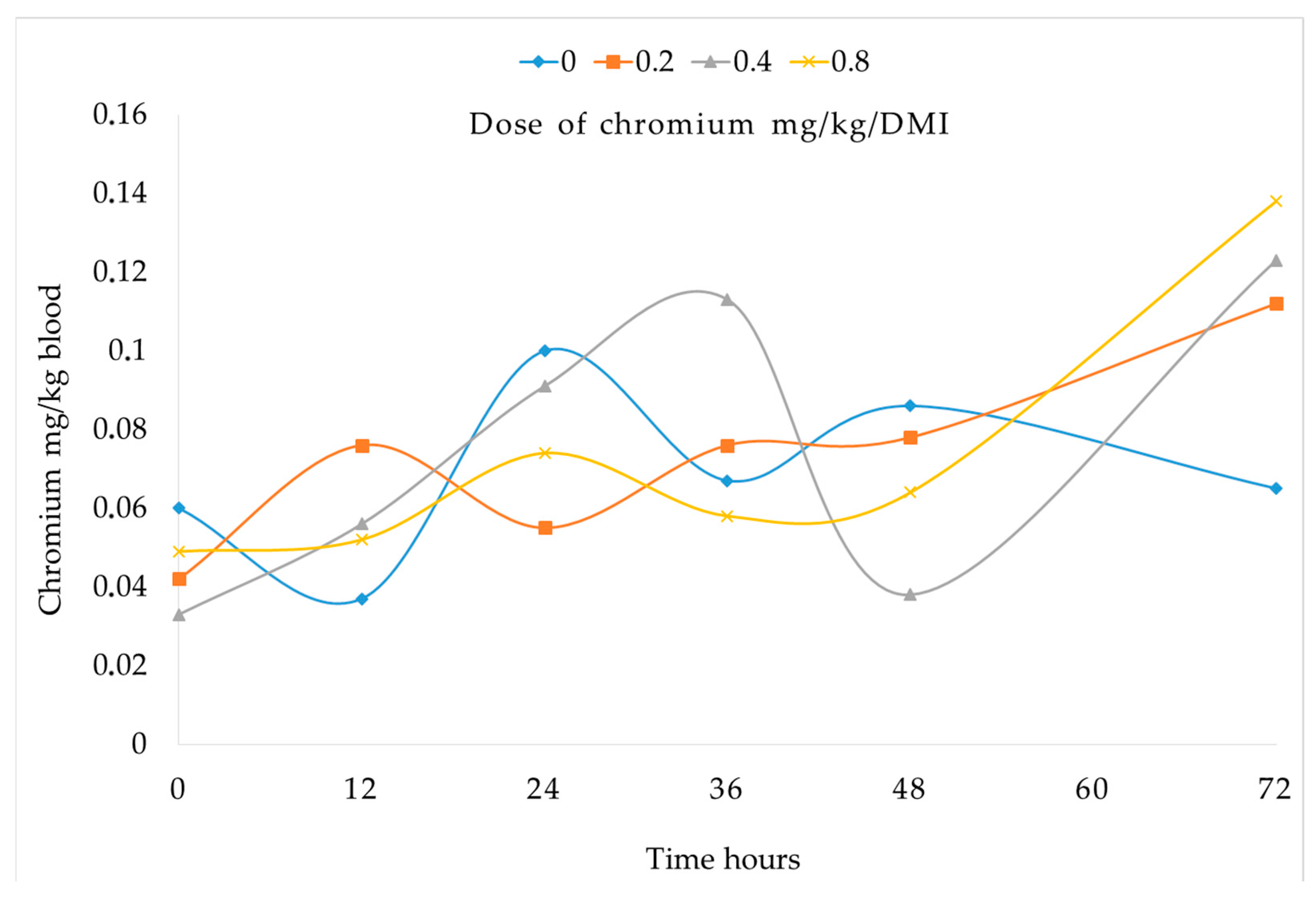
3.1. Chromium Concentration in Blood
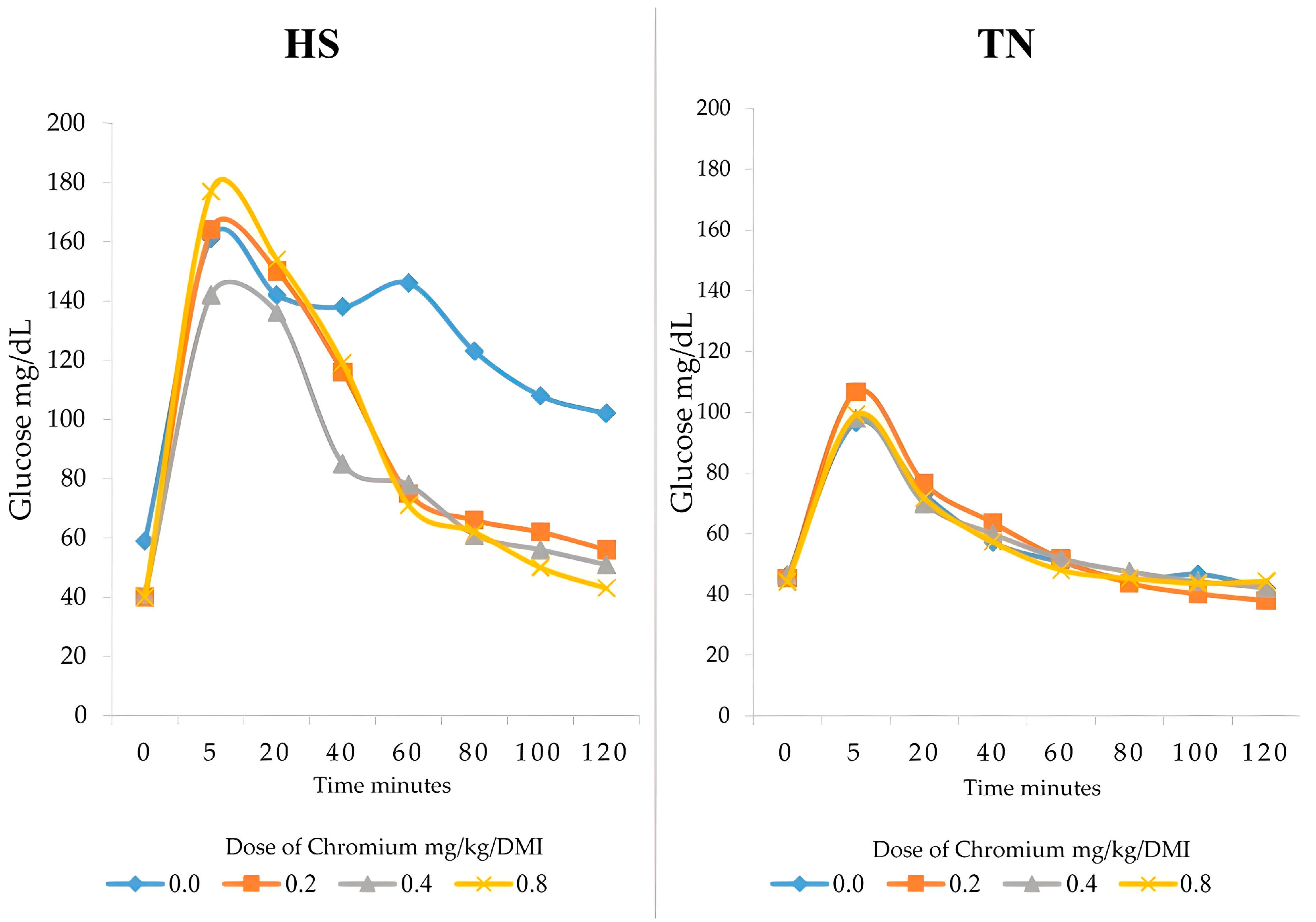
3.2. Glucose Clearance in Blood
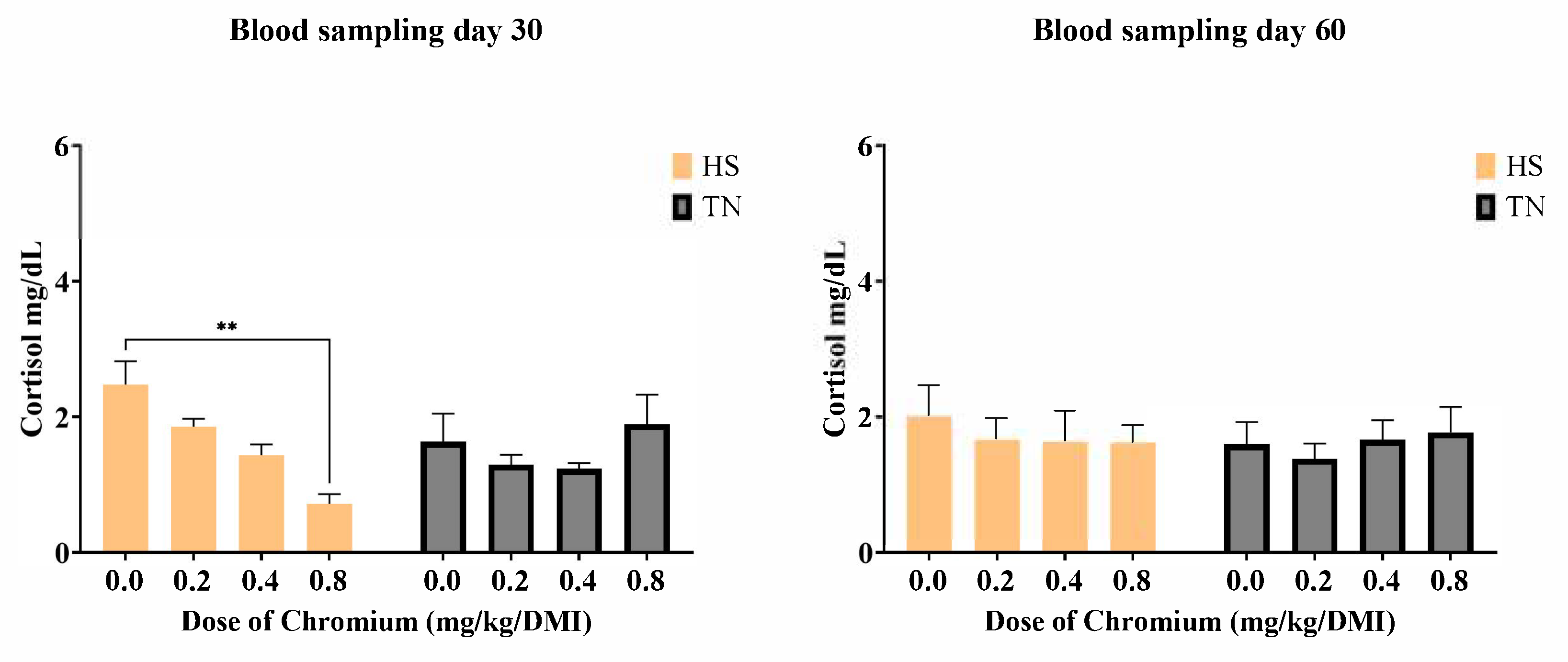
3.3. Cortisol Levels
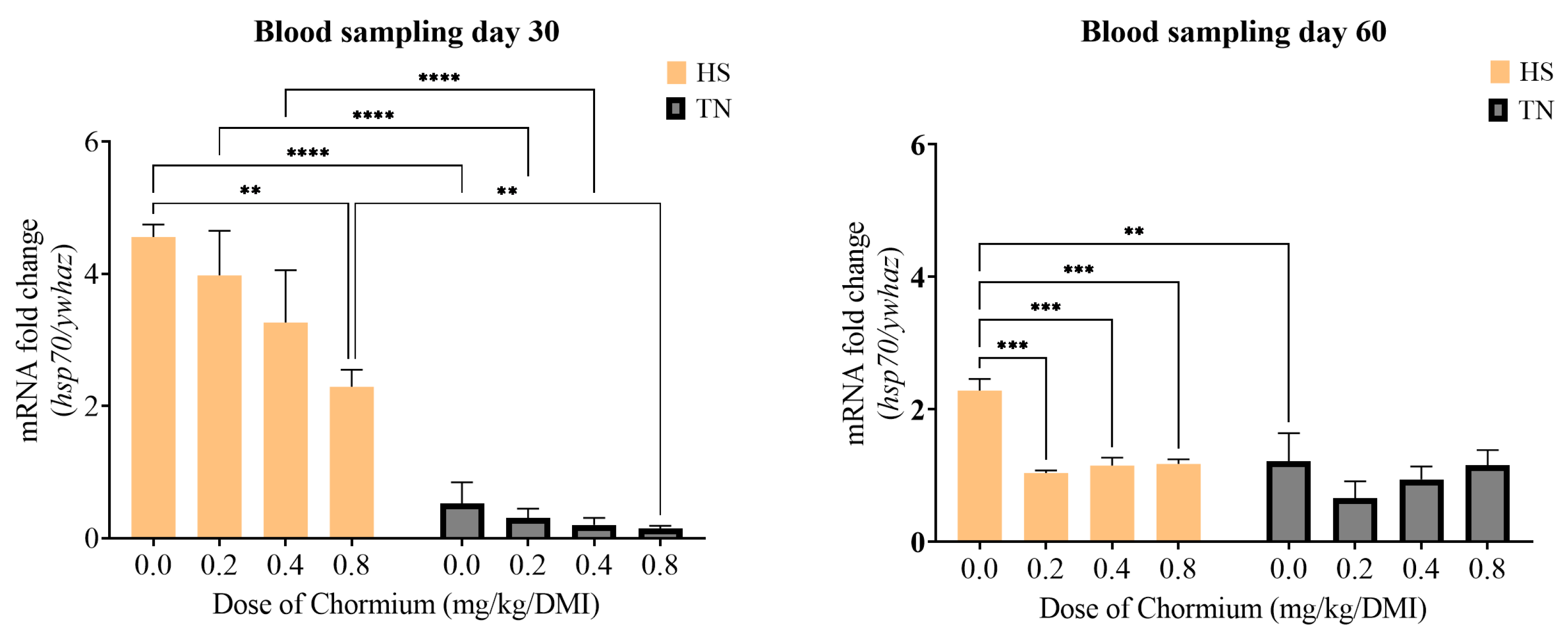
3.4. Gene Expression of hsp60 and hsp70
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arvidsson Segerkvist, K.; Hansson, H.; Sonesson, U.; Gunnarsson, S. A Systematic Mapping of Current Literature on Sustainability at Farm-Level in Beef and Lamb Meat Production. Sustainability 2021, 13, 2488. [Google Scholar] [CrossRef]
- Silva, S.R.; Sacarrão-Birrento, L.; Almeida, M.; Ribeiro, D.M.; Guedes, C.; González Montaña, J.R.; Pereira, A.F.; Zaralis, K.; Geraldo, A.; Tzamaloukas, O.; et al. Extensive Sheep and Goat Production: The Role of Novel Technologies towards Sustainability and Animal Welfare. Animals 2022, 12, 885. [Google Scholar] [CrossRef]
- Primi, R.; Bernabucci, G.; Evangelista, C.; Viola, P.; Girotti, P.; Spina, R.; Compagnucci, S.; Ronchi, B. Ecosystem Services Linked to Extensive Sheep and Goat Farming in Mountain Areas: A Global Literature Analysis Using Text Mining and Topic Analysis. Animals 2025, 15, 350. [Google Scholar] [CrossRef]
- Anim-Jnr, A.S.; Sasu, P.; Bosch, C.; Mabiki, F.P.; Frimpong, Y.O.; Emmambux, M.N.; Greathead, H.M.R. Sustainable Small Ruminant Production in Low- and Middle-Income African Countries: Harnessing the Potential of Agroecology. Sustainability 2023, 15, 15326. [Google Scholar] [CrossRef]
- Mazinani, M.; Rude, B. Population, World Production and Quality of Sheep and Goat Products. Am. J. Anim. Vet. Sci. 2020, 15, 291–299. [Google Scholar] [CrossRef]
- Borthwick, Z.; Quiring, K.; Griffith, S.C.; Leu, S.T. Heat Stress Conditions Affect the Social Network Structure of Free-ranging Sheep. Ecol. Evol. 2024, 14, e10996. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Wei, Y.; Zou, J.; Yang, B.; Wang, Q.; Lu, J.; Lu, J.; Zheng, Z.; Huang, Y.; et al. Effect of Heat Stress on Growth Performance, Carcase Characteristics, Meat Quality and Rumen-Muscle Axis of Hu Sheep. Ital. J. Anim. Sci. 2024, 23, 87–100. [Google Scholar] [CrossRef]
- McManus, C.M.; Faria, D.A.; Lucci, C.M.; Louvandini, H.; Pereira, S.A.; Paiva, S.R. Heat Stress Effects on Sheep: Are Hair Sheep More Heat Resistant? Theriogenology 2020, 155, 157–167. [Google Scholar] [CrossRef]
- van Wettere, W.H.E.J.; Kind, K.L.; Gatford, K.L.; Swinbourne, A.M.; Leu, S.T.; Hayman, P.T.; Kelly, J.M.; Weaver, A.C.; Kleemann, D.O.; Walker, S.K. Review of the Impact of Heat Stress on Reproductive Performance of Sheep. J. Anim. Sci. Biotechnol. 2021, 12, 26. [Google Scholar] [CrossRef]
- Zhang, M.; Dunshea, F.R.; Warner, R.D.; Digiacomo, K.; Joy, A.; Abhijith, A.; Prathap, P.; Ma, T.; Chauhan, S.S. Short Duration Heatwaves Increase Body Temperature and Alter Blood Gas Balance but May Not Cause Oxidative Stress and Intestinal Structure Variations in Lambs. Small Rumin. Res. 2024, 240, 107367. [Google Scholar] [CrossRef]
- Silver, J.T.; Noble, E.G. Regulation of Survival Gene Hsp70. Cell Stress Chaperones 2012, 17, 1–9. [Google Scholar] [CrossRef]
- Rakib, M.R.H.; Gargiulo, J.I. Potential Use of HSP70 as an Indicator of Heat Stress in Dairy Cows—A Review. J. Dairy Sci. 2024, 107, 11597–11610. [Google Scholar] [CrossRef]
- Lu, Z.; Ma, Y.; Li, Q.; Liu, E.; Jin, M.; Zhang, L.; Wei, C. The Role of N6-Methyladenosine RNA Methylation in the Heat Stress Response of Sheep (Ovis Aries). Cell Stress Chaperones 2019, 24, 333–342. [Google Scholar] [CrossRef]
- Dangi, S.S.; Gupta, M.; Nagar, V.; Yadav, V.P.; Dangi, S.K.; Shankar, O.; Chouhan, V.S.; Kumar, P.; Singh, G.; Sarkar, M. Impact of Short-Term Heat Stress on Physiological Responses and Expression Profile of HSPs in Barbari Goats. Int. J. Biometeorol. 2014, 58, 2085–2093. [Google Scholar] [CrossRef]
- Manjari, R.; Yadav, M.; Ramesh, K.; Uniyal, S.; Rastogi, S.K.; Sejian, V.; Hyder, I. HSP70 as a Marker of Heat and Humidity Stress in Tarai Buffalo. Trop. Anim. Health Prod. 2015, 47, 111–116. [Google Scholar] [CrossRef]
- Akinyemi, M.O.; Osaiyuwu, H.; Eboreime, A.E. Effects of Heat Stress on Physiological Parameters and Serum Concentration of Hsp70 in Indigenous Breeds of Sheep in Nigeria. Slovak J. Anim. Sci 2019, 52, 119–126. [Google Scholar]
- Yang, J.; Zong, Y.; Su, J.; Li, H.; Zhu, H.; Columbus, L.; Zhou, L.; Liu, Q. Conformation Transitions of the Polypeptide-Binding Pocket Support an Active Substrate Release from Hsp70s. Nat. Commun. 2017, 8, 1201. [Google Scholar] [CrossRef]
- Rutledge, B.S.; Choy, W.Y.; Duennwald, M.L. Folding or Holding?—Hsp70 and Hsp90 Chaperoning of Misfolded Proteins in Neurodegenerative Disease. J. Biol. Chem. 2022, 298, 101905. [Google Scholar] [CrossRef]
- Mayer, M.P.; Gierasch, L.M. Recent Advances in the Structural and Mechanistic Aspects of Hsp70 Molecular Chaperones. J. Biol. Chem. 2019, 294, 2085–2097. [Google Scholar] [CrossRef]
- Ben-Khoud, Y.; Chen, C.S.; Ali, M.M.U. Alternative ATPase Domain Interactions in Eukaryotic Hsp70 Chaperones. Front. Mol. Biosci. 2023, 10, 1155784. [Google Scholar] [CrossRef]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular Chaperones in Protein Folding and Proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Saibil, H. Chaperone Machines for Protein Folding, Unfolding and Disaggregation. Nat. Rev. Mol. Cell Biol. 2013, 14, 630–642. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Kang, D.; Park, J.; Khan, M.; Shim, K. Chronic Heat Stress Regulates the Relation between Heat Shock Protein and Immunity in Broiler Small Intestine. Sci. Rep. 2020, 10, 18872. [Google Scholar] [CrossRef]
- Lee, T.Y.; Huang, L.J.; Dong, H.P.; Tohru, Y.; Liu, B.H.; Yang, R.C. Impairment of Mitochondrial Unfolded Protein Response Contribute to Resistance Declination of H2O2-Induced Injury in Senescent MRC-5 Cell Model. Kaohsiung J. Med. Sci. 2020, 36, 89–97. [Google Scholar] [CrossRef]
- Fauvet, B.; Rebeaud, M.E.; Tiwari, S.; De Los Rios, P.; Goloubinoff, P. Repair or Degrade: The Thermodynamic Dilemma of Cellular Protein Quality-Control. Front. Mol. Biosci. 2021, 8, 768888. [Google Scholar] [CrossRef]
- Kim, W.S.; Peng, D.Q.; Jo, Y.H.; Nejad, J.G.; Lee, H.G. Responses of Beef Calves to Long-Term Heat Stress Exposure by Evaluating Growth Performance, Physiological, Blood and Behavioral Parameters. J. Therm. Biol. 2021, 100, 103033. [Google Scholar] [CrossRef]
- Christison, G.I.; Johnson, H.D. Cortisol Turnover in Heat-Stressed Cows. J. Anim. Sci. 1972, 35, 1005–1010. [Google Scholar] [CrossRef]
- Kregel, K.C. Invited Review: Heat Shock Proteins: Modifying Factors in Physiological Stress Responses and Acquired Thermotolerance. J. Appl. Physiol. 2002, 92, 2177–2186. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Luo, Y.; Zhu, L.; Li, G. Effect of Acute Heat Stress on Adrenocorticotropic Hormone, Cortisol, Interleukin-2, Interleukin-12 and Apoptosis Gene Expression in Rats. Biomed. Rep. 2015, 3, 425–429. [Google Scholar] [CrossRef]
- Hernández-García, P.A.; Orzuna-Orzuna, J.F.; Chay-Canul, A.J.; Silva, G.V.; Galván, C.D.; Ortíz, P.B.R. Meta-Analysis of Organic Chromium Dietary Supplementation on Growth Performance, Carcass Traits, and Serum Metabolites of Lambs. Small Rumin. Res. 2024, 233, 107254. [Google Scholar] [CrossRef]
- Shen, J.; Hao, Z.; Wang, J.; Hu, J.; Liu, X.; Li, S.; Ke, N.; Song, Y.; Lu, Y.; Hu, L.; et al. Comparative Transcriptome Profile Analysis of Longissimus Dorsi Muscle Tissues from Two Goat Breeds with Different Meat Production Performance Using RNA-Seq. Front. Genet. 2021, 11, 619399. [Google Scholar] [CrossRef]
- Levina, A.; Pham, T.H.N.; Lay, P.A. Binding of Chromium (III) to Transferrin Could Be Involved in Detoxification of Dietary Chromium (III) Rather Than Transport of an Essential Trace Element. Angew. Chem. Int. Ed. 2016, 55, 8104–8107. [Google Scholar] [CrossRef]
- Hung, A.T.; Leury, B.J.; Sabin, M.A.; Fahri, F.; DiGiacomo, K.; Lien, T.F.; Dunshea, F.R. Dietary Nano Chromium Picolinate Can Ameliorate Some of the Impacts of Heat Stress in Cross-Bred Sheep. Anim. Nutr. 2021, 7, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Ma, F.T.; Jin, Y.H.; Gao, D.; Li, H.Y.; Sun, P. Chromium Yeast Alleviates Heat Stress by Improving Antioxidant and Immune Function in Holstein Mid-Lactation Dairy Cows. Anim. Feed Sci. Technol. 2020, 269, 114635. [Google Scholar] [CrossRef]
- Haldar, S.; Samanta, S.; Banarjee, R.; Sharma, B.; Ghosh, T.K. Glucose Tolerance and Serum Concentrations of Hormones and Metabolites in Goats (Capra hircus) Fed Diets Supplemented with Inorganic and Organic Chromium Salts. Animal 2007, 1, 347–356. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Small Ruminants; National Academies Press: Washington, DC, USA, 2007; ISBN 978-0-309-10213-1. [Google Scholar]
- Spears, J.W.; Lloyd, K.E.; Krafka, K. Chromium Concentrations in Ruminant Feed Ingredients. J. Dairy Sci. 2017, 100, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Dallago, B.S.L.; Braz, S.V.; Marçola, T.G.; McManus, C.; Caldeira, D.F.; Campeche, A.; Gomes, E.F.; Paim, T.P.; Borges, B.O.; Louvandini, H. Blood Parameters and Toxicity of Chromium Picolinate Oral Supplementation in Lambs. Biol. Trace Elem. Res. 2015, 168, 91–102. [Google Scholar] [CrossRef]
- Rodríguez-Gaxiola, M.A.; Domínguez-Vara, I.A.; Barajas-Cruz, R.; Contreras-Andrade, I.; Morales-Almaráz, E.; Bórquez-Gastelum, J.L.; Sánchez-Torres, J.E.; Trujillo-Gutiérrez, D.; Salem, A.Z.M.; Ramírez-Bribiesca, E.; et al. Effect of Enriched-Chromium Yeast on Growth Performance, Carcass Characteristics and Fatty Acid Profile in Finishing Rambouillet Lambs. Small Rumin. Res. 2020, 188, 106118. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, H.; Luo, G.; Niu, R.; Wang, J. Effect of Dietary Yeast Chromium and L-Carnitine on Lipid Metabolism of Sheep. Biol. Trace Elem. Res. 2013, 155, 221–227. [Google Scholar] [CrossRef]
- Nicolás-López, P.; Macías-Cruz, U.; Mellado, M.; Correa-Calderón, A.; Meza-Herrera, C.A.; Avendaño-Reyes, L. Growth Performance and Changes in Physiological, Metabolic and Hematological Parameters Due to Outdoor Heat Stress in Hair Breed Male Lambs Finished in Feedlot. Int. J. Biometeorol. 2021, 65, 1451–1459. [Google Scholar] [CrossRef]
- Zhang, M.; Warner, R.D.; Dunshea, F.R.; DiGiacomo, K.; Joy, A.; Abhijith, A.; Prathap, P.; Ma, T.; Chauhan, S.S. Impact of Heatwaves on the Physiology and Retail Meat Quality of Lambs. Foods 2022, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, A.A.; Osman, S.F.; Teama, F.E.I.; Gad, A.E. The Detrimental Impact of High Environmental Temperature on Physiological Response, Growth, Milk Production, and Reproductive Efficiency of Ruminants. Trop. Anim. Health Prod. 2023, 55, 388. [Google Scholar] [CrossRef]
- Schüller, L.K.; Burfeind, O.; Heuwieser, W. Short Communication: Comparison of Ambient Temperature, Relative Humidity, and Temperature-Humidity Index between on-Farm Measurements and Official Meteorological Data. J. Dairy Sci. 2013, 96, 7731–7738. [Google Scholar] [CrossRef] [PubMed]
- Committee on Physiological Effects of Environmental Factors on Animals; Agricultural Board; National Research Council. A Guide to Environmental Research on Animals; National Academy of Sciences: Washington, DC, USA, 1971; ISBN 9780309018692. [Google Scholar]
- Marai, I.F.M.; El-Darawany, A.A.; Fadiel, A.; Abdel-Hafez, M.A.M. Physiological Traits as Affected by Heat Stress in Sheep—A Review. Small Rumin. Res. 2007, 71, 1–12. [Google Scholar] [CrossRef]
- Bhateshwar, V.; Rai, D.C.; Datt, M. Heat Stress Responses in Small Ruminants under Arid and Semi-Arid Regions of Western India: A Review. Agric. Rev. 2022, 44, 164–172. [Google Scholar] [CrossRef]
- Bermejo-Barrera, P.; Moreda-Piñeiro, A.; Moreda-Piñeiro, J.; Kauppila, T.; Bermejo-Barrera, A.; Huang, Y.; Chuang, I.; Pan, C.; Schlemmer, G.; Petek, M. Slurry sampling for electrothermal AAS determination of cadmium in seafood products. At. Spectrosc. 2000, 21, 5–9. [Google Scholar]
- Dandare, A.; Rafiq, M.; Liaquat, A.; Jawad Khan, M. Two Hours Method for RNA and DNA Co-Extraction from Blood of Coronary Artery Disease Patients: Fast, Simple and Economical Technique. Pak. J. Med. Sci. 2022, 38, 1754–1759. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate–Phenol–Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Desjardins, P.; Conklin, D. NanoDrop Microvolume Quantitation of Nucleic Acids. J. Vis. Exp. 2010, 45, 2565. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real- Time Quantitative PCR and the 2−ΔΔCT Method. Method 2001, 408, 402–408. [Google Scholar] [CrossRef]
- Chen, G.; Liu, P.; Pattar, G.R.; Tackett, L.; Bhonagiri, P.; Strawbridge, A.B.; Elmendorf, J.S. Chromium Activates Glucose Transporter 4 Trafficking and Enhances Insulin-Stimulated Glucose Transport in 3T3-L1 Adipocytes via a Cholesterol-Dependent Mechanism. Mol. Endocrinol. 2006, 20, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Latheef, S.K.; Dadar, M.; Samad, H.A.; Munjal, A.; Khandia, R.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; et al. Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values. Front. Mol. Biosci. 2019, 6, 91. [Google Scholar] [CrossRef]
- Adam, T.C.; Hasson, R.E.; Ventura, E.E.; Toledo-Corral, C.; Le, K.A.; Mahurkar, S.; Lane, C.J.; Weigensberg, M.J.; Goran, M.I. Cortisol Is Negatively Associated with Insulin Sensitivity in Overweight Latino Youth. J. Clin. Endocrinol. Metab. 2010, 95, 4729–4735. [Google Scholar] [CrossRef]
- Ngala, R.A.; Awe, M.A.; Nsiah, P. The Effects of Plasma Chromium on Lipid Profile, Glucose Metabolism and Cardiovascular Risk in Type 2 Diabetes Mellitus. A Case—Control Study. PLoS ONE 2018, 13, e0197977. [Google Scholar] [CrossRef]
- Bodarski, R.; Kinal, S.; Król, B.; Bodarski, R.; Słupczyńska, M.; Mońka, M.; Tronina, W. Bioavailability of Organic and Inorganic Sources of Chromium in Broiler Chicken Feeds. J. Elem. 2016, 22, 283–294. [Google Scholar] [CrossRef]
- Laschinsky, N.; Kottwitz, K.; Freund, B.; Dresow, B.; Fischer, R.; Nielsen, P. Bioavailability of Chromium(III)-Supplements in Rats and Humans. BioMetals 2012, 25, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Frazzoli, C.; Cubadda, F. Organic Forms of Trace Elements as Feed Additives: Assessment of Risks and Benefits for Farm Animals and Consumers. Pure Appl. Chem. 2010, 82, 393–407. [Google Scholar] [CrossRef]
- Ducros, V. Chromium Metabolism—A Literature Review. Biol. Trace Elem. Res. 1992, 32, 65–77. [Google Scholar] [CrossRef]
- Gaebel, G.; Martens, H.; Suendermann, M.; Galfi, P. The Effect of Diet, Intraruminal PH and Osmolarity on Sodium, Chloride and Magnesium Absorption from the Temporarily Isolated and Washed Reticulo-rumen of Sheep. Q. J. Exp. Physiol. 1987, 72, 501–511. [Google Scholar] [CrossRef]
- Byrne, L.; Murphy, R.A. Relative Bioavailability of Trace Minerals in Production Animal Nutrition: A Review. Animals 2022, 12, 1981. [Google Scholar] [CrossRef]
- Moreno-Camarena, L.; Arturo Domínguez-Vara, I.; Morales-Almaráz, E.; Bórquez-Gastelum, J.L.; Trujillo-Gutiérrez, D.; Acosta-Dibarrat, J.P.; Sánchez-Torres, J.E.; Pinos-Rodríguez, J.M.; Modragón-Ancelmo, J.; Barajas-Cruz, R.; et al. Effects of Dietary Chromium-Yeast Level on Growth Performance, Blood Metabolites, Meat Traits and Muscle Fatty Acids Profile, and Microminerals Content in Liver and Bone of Lambs. Ital. J. Anim. Sci. 2020, 19, 1542–1551. [Google Scholar] [CrossRef]
- Hall, L.; Martinus, R.D. Hyperglycaemia and Oxidative Stress Upregulate HSP60 & HSP70 Expression in HeLa Cells. Springerplus 2013, 2, 431. [Google Scholar] [CrossRef]
- Mehaba, N.; Coloma-Garcia, W.; Such, X.; Caja, G.; Salama, A.A.K. Heat Stress Affects Some Physiological and Productive Variables and Alters Metabolism in Dairy Ewes. J. Dairy Sci. 2021, 104, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, J.J.; Furness, J.B.; Wijesiriwardana, U.A.; Ringuet, M.; Liu, F.; Digiacomo, K.; Leury, B.J.; J.clarke, I.; Dunshea, F.R. The Effect of Heat Stress on Respiratory Alkalosis and Insulin Sensitivity in Cinnamon Supplemented Pigs. Animals 2020, 10, 690. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Ghorbani, G.R.; Khorvash, M.; Rahmani, H.R.; Nikkhah, A. Chromium Improves Production and Alters Metabolism of Early Lactation Cows in Summer. J. Anim. Physiol. Anim. Nutr. 2011, 95, 81–89. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of Animals to Heat Stress. Animal 2018, 12, s431–s444. [Google Scholar] [CrossRef]
- Trevisi, E.; Bertoni, G. Some Physiological and Biochemical Methods for Acute and Chronic Stress Evaluationin Dairy Cows. Ital. J. Anim. Sci. 2009, 8, 265–286. [Google Scholar] [CrossRef]
- Feng, C.; Wuren, Q.; Zhang, X.; Sun, X.; Na, Q. Effects of Dietary Chromium Picolinate Supplementation on Broiler Growth Performance: A Meta-Analysis. PLoS ONE 2021, 16, e0249527. [Google Scholar] [CrossRef]
- Sadri, H.; Larki, N.N.; Kolahian, S. Hypoglycemic and Hypolipidemic Effects of Leucine, Zinc, and Chromium, Alone and in Combination, in Rats with Type 2 Diabetes. Biol. Trace Elem. Res. 2017, 180, 246–254. [Google Scholar] [CrossRef]
- Kircheva, N.; Toshev, N.; Dudev, T. Holo-Chromodulin: Competition between the Native Cr3+ and Other Biogenic Cations (Fe3+, Fe2+, Mg2+, and Zn2+) for the Binding Sites. Metallomics 2022, 14, mfac082. [Google Scholar] [CrossRef]
- Vincent, J.B. Is the Pharmacological Mode of Action of Chromium(III) as a Second Messenger? Biol. Trace Elem. Res. 2015, 166, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cui, W.M.; Zhang, S.W.; Kong, F.H.; Pedersen, M.A.; Wen, Y.; Lv, J.P. Effect of Glucose Tolerance Factor (GTF) from High Chromium Yeast on Glucose Metabolism in Insulin-Resistant 3T3-L1 Adipocytes. RSC Adv. 2015, 5, 3482–3490. [Google Scholar] [CrossRef]
- Dalólio, F.S.; Albino, L.F.T.; de Oliveira, H.C.; Fireman, A.K.B.A.T.; Burin, A., Jr.; Busanello, M.; Rohloff Junior, N.; Silva Tesser, G.L.; Nunes, R.V. Dietary Chromium-Methionine Supplementation and Broiler (22–43 Days) Responses during Heat Stress. 2—Physiological Variables, and Heat Shock Protein 70 and Insulin-like Growth Factor-1 Gene Expression. Anim. Prod. Sci. 2024, 64, An23354. [Google Scholar] [CrossRef]
- Liang, H.; Ge, X.; Xia, D.; Ren, M.; Mi, H.; Pan, L. The Role of Dietary Chromium Supplementation in Relieving Heat Stress of Juvenile Blunt Snout Bream Megalobrama Amblycephala. Fish Shellfish Immunol. 2022, 120, 23–30. [Google Scholar] [CrossRef]
- Mousaie, A.; Valizadeh, R.; Chamsaz, M. Selenium-Methionine and Chromium-Methionine Supplementation of Sheep around Parturition: Impacts on Dam and Offspring Performance. Arch. Anim. Nutr. 2017, 71, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Sulzbacher, M.M.H.; Ludwig, M.S.; Heck, T.G. Oxidative Stress and Decreased Tissue HSP70 Are Involved in the Genesis of Sepsis: HSP70 as a Therapeutic Target. Rev. Bras. Ter. Intensiva 2020, 32, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, S.; Muthuraman, P. Cortisol Effect on Heat Shock Proteins in the C2C12 and 3T3-L1 Cells. Vitr. Cell. Dev. Biol.—Anim. 2014, 50, 581–586. [Google Scholar] [CrossRef]
- Amini, M.R.; Sheikhhossein, F.; Djafari, F.; Jafari, A.; Djafarian, K.; Shab-Bidar, S. Effects of Chromium Supplementation on Oxidative Stress Biomarkers. Int. J. Vitam. Nutr. Res. 2023, 93, 241–251. [Google Scholar] [CrossRef]
- Hoffman, N.J.; Penque, B.A.; Habegger, K.M.; Sealls, W.; Tackett, L.; Elmendorf, J.S. Chromium Enhances Insulin Responsiveness via AMPK. J. Nutr. Biochem. 2014, 25, 565–572. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Estêvão, M.D.; Qorbani, M.; Heydari, H.; Hosseini, A.S.; Fazelian, S.; Belančić, A.; Persad, E.; Rezamand, G.; Heshmati, J. The Effect of Chromium Intake on Oxidative Stress Parameters: A Systematic Review and Meta-Analysis. J. Trace Elem. Med. Biol. 2022, 69, 126879. [Google Scholar] [CrossRef]
- Collier, R.J.; Collier, J.L.; Rhoads, R.P.; Baumgard, L.H. Invited Review: Genes Involved in the Bovine Heat Stress Response. J. Dairy Sci. 2008, 91, 445–454. [Google Scholar] [CrossRef] [PubMed]

| Ingredients (as Fed Basis) | % |
|---|---|
| Soybean meal | 9.20 |
| Glycerol | 4 |
| Rice flour | 21 |
| Corn | 15.20 |
| Urea | 0.25 |
| Sulfur | 0.05 |
| Salt | 0.30 |
| Silage | 50 |
| Chemical composition of the basal diet used in the experiment DM (%) | |
| Nutrient | |
| Dry matter (DM) | 49.61 |
| Organic matter (OM) | 89.17 |
| Ash | 10.82 |
| Crude protein (CP) | 12.12 |
| Ethereal extract (EE) | 7.65 |
| Neutral detergent fiber (NDF) | 40.90 |
| Acid detergent fiber (ADF) | 11.03 |
| Non-structural carbohydrates (NSC) | 28.50 |
| Gene | Gene Name | Accession Number | Sequence | Size of the Amplicon (bp) |
|---|---|---|---|---|
| ywhaz | 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide | NM_001267887.1 | F-AGCAGGCTGAGCGATATGAT | 180 |
| R-TCTCAGCACCTTCCGTCTTT | ||||
| hsp60 | Heat shock protein 60 | XM_027965061.1 | F-ACTGGCTCCTCATCTCACTC | 147 |
| R-TGTTCAATAATCACTGTCCTTCC | ||||
| hsp70 | Heat shock protein 70 | NM_001267874.1 | F-CGGAGAAGGACGAGTTTGAG | 165 |
| R-AATCCACCTCCTCAATGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval-Lozano, E.; Barragán, I.S.R.; Sandoval-Lozano, A.; Castañeda-Serrano, R.D. Effects of Chromium Yeast Supplementation on Serum hsp60 and hsp70, mRNA Expression in Heat-Stressed Lambs. Vet. Sci. 2025, 12, 801. https://doi.org/10.3390/vetsci12090801
Sandoval-Lozano E, Barragán ISR, Sandoval-Lozano A, Castañeda-Serrano RD. Effects of Chromium Yeast Supplementation on Serum hsp60 and hsp70, mRNA Expression in Heat-Stressed Lambs. Veterinary Sciences. 2025; 12(9):801. https://doi.org/10.3390/vetsci12090801
Chicago/Turabian StyleSandoval-Lozano, Edwin, Iang S. Rondón Barragán, Andrés Sandoval-Lozano, and Román David Castañeda-Serrano. 2025. "Effects of Chromium Yeast Supplementation on Serum hsp60 and hsp70, mRNA Expression in Heat-Stressed Lambs" Veterinary Sciences 12, no. 9: 801. https://doi.org/10.3390/vetsci12090801
APA StyleSandoval-Lozano, E., Barragán, I. S. R., Sandoval-Lozano, A., & Castañeda-Serrano, R. D. (2025). Effects of Chromium Yeast Supplementation on Serum hsp60 and hsp70, mRNA Expression in Heat-Stressed Lambs. Veterinary Sciences, 12(9), 801. https://doi.org/10.3390/vetsci12090801







