Dietary Supplementation of Methionine, Tryptophan, and Threonine for Pigs Under Sanitary Challenges: Current Knowledge and Future Directions
Simple Summary
Abstract
1. Introduction
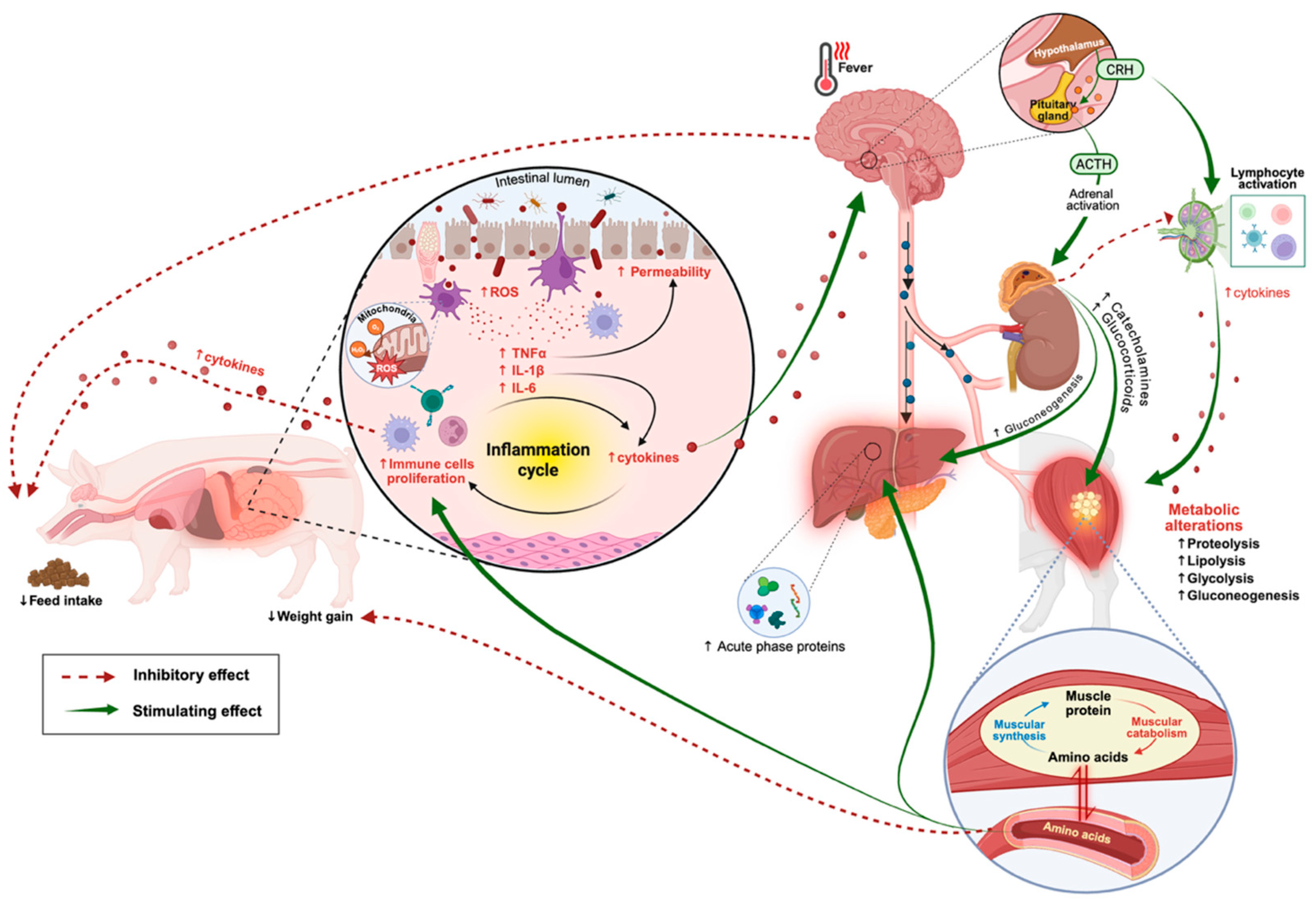
2. Metabolic and Physiological Responses of Pigs Under Sanitary Challenge Conditions
3. Evidence from Experimental Models
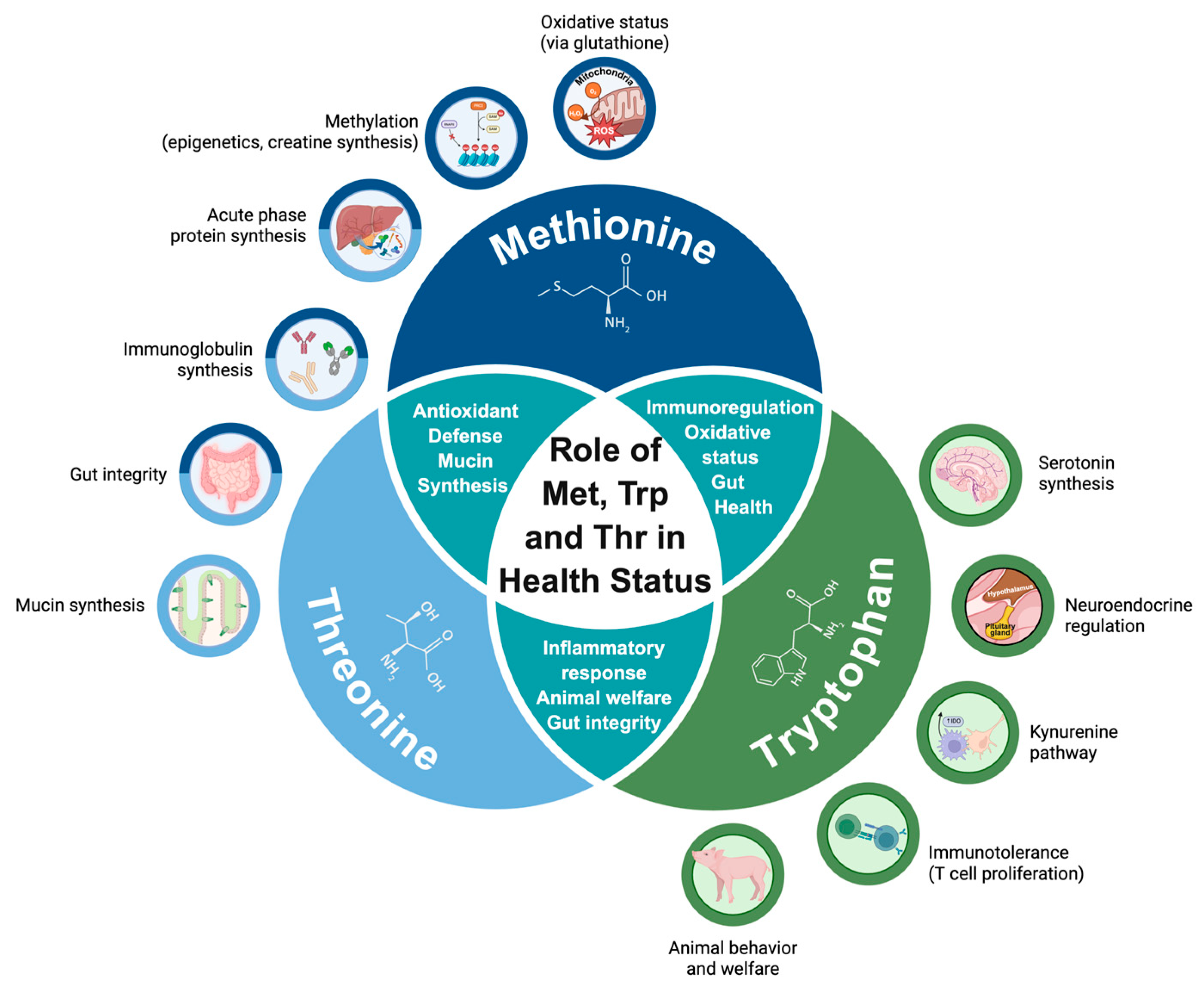
4. Roles of Functional Amino Acids: A Focus on Met, Trp, and Thr
5. Dietary Met, Trp, and Thr Supplementation Mitigates Performance Loss and Behavioral Alterations for Sanitary-Stressed Group-Housed Pigs
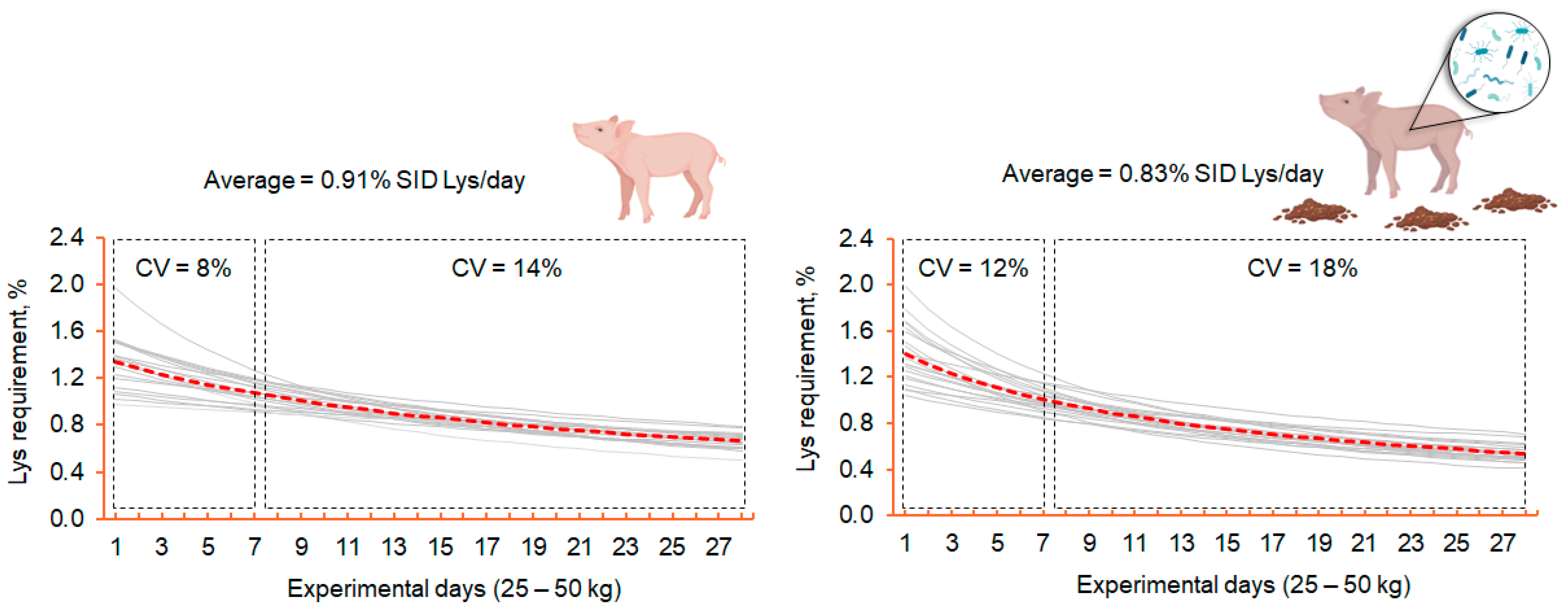
6. Integrating Functional Amino Acids and Precision Feeding to Support Pig Health Under Sanitary Stress
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prunier, A.; Heinonen, M.; Quesnel, H. High Physiological Demands in Intensively Raised Pigs: Impact on Health and Welfare. Animal 2010, 4, 886–898. [Google Scholar] [CrossRef]
- Clapperton, M.; Diack, A.B.; Matika, O.; Glass, E.J.; Gladney, C.D.; Mellencamp, M.A.; Hoste, A.; Bishop, S.C. Traits Associated with Innate and Adaptive Immunity in Pigs: Heritability and Associations with Performance Under Different Health Status Conditions. Genet. Sel. Evol. 2009, 41, 54. [Google Scholar] [CrossRef]
- Campos, P.H.R.F.; Floc’h, N.; Noblet, J.; Renaudeau, D. Physiological Responses of Growing Pigs to High Ambient Temperature and/or Inflammatory Challenges. Rev. Bras. Zootec. 2017, 46, 537–544. [Google Scholar] [CrossRef]
- Van der Peet-Schwering, C.M.C.; Koopmans, S.J.; Jansman, A.J.M. Amino Acid Requirements in Relation to Health Status in Growing and Finishing Pigs; Wageningen Livestock Research Report; Wageningen Livestock Research: Wageningen, The Netherlands, 2019; p. 1168. [Google Scholar] [CrossRef]
- Cornelison, A.S.; Karriker, L.A.; Williams, N.H.; Haberl, B.J.; Stalder, K.J.; Schulz, L.L.; Patience, J.F. Impact of Health Challenges on Pig Growth Performance, Carcass Characteristics and Net Returns Under Commercial Conditions. Transl. Anim. Sci. 2018, 2, 50–61. [Google Scholar] [CrossRef]
- Larour, G. Les Dépenses de Santé 2008 dans 89 Élevages Naisseur-Engraisseurs Bretons; Chambre d’Agriculture de Bretagne: Rennes, France, 2010; 48p. [Google Scholar]
- Lee, C.; Giles, L.R.; Bryden, W.L.; Downing, J.L.; Owens, P.C.; Kirby, A.C.; Wynn, P.C. Performance and Endocrine Responses of Group Housed Weaner Pigs Exposed to the Air Quality of a Commercial Environment. Livest. Prod. Sci. 2005, 93, 255–262. [Google Scholar] [CrossRef]
- Renaudeau, D. Effect of housing conditions (clean vs. dirty) on growth performance and feeding behaviour in growing pigs in a tropical climate. Trop. Anim. Health Prod. 2009, 41, 559–563. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Wessels, A.; Corrent, E.; Wu, G.; Bosi, P. The Relevance of Functional Amino Acids to Support the Health of Growing Pigs. Anim. Feed Sci. Technol. 2018, 245, 104–116. [Google Scholar] [CrossRef]
- Kipper, M.; Andretta, I.; Monteiro, S.G.; Lovatto, P.A.; Lehnen, C.R. Meta-Analysis of the Effects of Endoparasites on Pig Performance. Vet. Parasitol. 2011, 181, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Chatelet, A.; Gondret, F.; Merlot, E.; Gilbert, H.; Friggens, N.C.; Le Floc’h, N. Impact of Hygiene of Housing Conditions on Performance and Health of Two Pig Genetic Lines Divergent for Residual Feed Intake. Animal 2017, 12, 350–358. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Melchior, D.; Obled, C. Modifications of Protein and Amino Acid Metabolism During Inflammation and Immune System Activation. Livest. Prod. Sci. 2004, 87, 37–45. [Google Scholar] [CrossRef]
- Van der Meer, Y.; Lammers, A.; Jansman, A.J.M.; Rijnen, M.M.J.A.; Hendriks, W.H.; Gerrits, W.J.J. Performance of pigs kept under different sanitary conditions affected by protein intake and amino acid supplementation. J. Anim. Sci. 2016, 94, 4704–4719. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Wellington, M.O.; González-Vega, J.C.; Htoo, J.K.; van Kessel, A.G.; Columbus, D.A. Functional amino acid supplementation, regardless of dietary protein content, improves growth performance and immune status of weaned pigs challenged with Salmonella Typhimurium. J. Anim. Sci. 2021, 99, skaa365. [Google Scholar] [CrossRef]
- Wellington, M.O.; Agyekum, A.K.; Hamonic, K.; Htoo, J.K.; Van Kessel, A.G.; Columbus, D.A. Effect of supplemental threonine above requirement on growth performance of Salmonella Typhimurium challenged pigs fed high-fiber diets. J. Anim. Sci. 2019, 97, 3636–3647. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Le Bellego, L.; Matte, J.J.; Melchior, D.; Sève, B. The Effect of Sanitary Status Degradation and Dietary Tryptophan Content on Growth Rate and Tryptophan Metabolism in Weaning Pigs. J. Anim. Sci. 2009, 87, 1686–1694. [Google Scholar] [CrossRef]
- Trevisi, P.; Corrent, E.; Mazzoni, M.; Messori, S.; Priori, D.; Gherpelli, Y.; Simongiovanni, A.; Bosi, P. Effect of added dietary threonine on growth performance, health, immunity and gastrointestinal function of weaning pigs with differing genetic susceptibility to Escherichia coli infection and challenged with E. coli K88. J. Anim. Physiol. Anim. Nutr. 2015, 99, 511–520. [Google Scholar] [CrossRef]
- Jayaraman, B.J.; Htoo, J.K.; Nyachoti, C.M. Effects of Dietary Threonine: Lysine Ratios and Sanitary Conditions on Performance, Plasma Urea Nitrogen, Plasma Free Threonine and Lysine of Weaned Pigs. Anim. Nutr. 2015, 1, 283–288. [Google Scholar] [CrossRef]
- Kahindi, R.K.; Regassa, A.; Htoo, J.K.; Nyachoti, C.M. Growth Performance and Expression of Genes Encoding Enzymes Involved in Methionine and Cysteine Metabolism in Piglets Fed Increasing Sulphur Amino Acid to Lysine Ratio During Enterotoxigenic Escherichia coli Challenge. Can. J. Anim. Sci. 2018, 98, 333–340. [Google Scholar] [CrossRef]
- Capozzalo, M.M.; Kim, J.C.; Htoo, J.K.; de Lange, C.F.M.; Mullan, B.P.; Resink, J.W.; Hansen, C.F.; Stumbles, P.A.; Hampson, D.J.; Ferguson, N.; et al. Estimating the Standardized Ileal Digestible Tryptophan Requirement of Pigs Kept Under Commercial Conditions in the Immediate Post-Weaning Period. Anim. Feed Sci. Technol. 2020, 259, 114342. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012; 380p. [Google Scholar]
- França, I.; Valini, G.A.D.C.; Arnaut, P.R.; Ortiz, M.T.; Silva, C.A.; de Oliveira, M.J.K.; Paulino, G.S.C.; Marçal, D.A.; Melo, A.D.B.; Htoo, J.K.; et al. Dietary Supplementation with Functional Amino Acids Improves the Capacity of Growing Pigs to Cope with a Health Challenge. Anim. Feed Sci. Technol. 2024, 318, 116148. [Google Scholar] [CrossRef]
- Valini, G.A.C.; Arnaut, P.R.; Franca, I.; Ortiz, M.T.; Oliveira, M.J.K.; Melo, A.D.B.; Marcal, D.A.; Campos, P.H.R.F.; Htoo, J.K.; Brand, H.B.; et al. Increased dietary Trp, Thr, and Met supplementation improves growth performance and protein deposition of salmonella-challenged growing pigs under poor housing conditions. J. Anim. Sci. 2023, 101, skad141. [Google Scholar] [CrossRef]
- Valini, G.A.C.; Méthot, S.; Pomar, C.; Hauschild, L.; Remus, A. Size matters: Lower body weight pigs have a different response to immune challenge and amino acids supplementation above the estimated requirement compared to heavy pigs. J. Anim. Sci. 2024, 102, skae255. [Google Scholar] [CrossRef]
- Badaoui, B.; Tuggle, C.K.; Hu, Z.; Reecy, J.M.; Ait-Ali, T.; Alselmo, A.; Botti, S. Pig Immune Response to General Stimulus and to Porcine Reproductive and Respiratory Syndrome Virus Infection: A Meta-Analysis Approach. BMC Genom. 2013, 14, 220. [Google Scholar] [CrossRef]
- Riera, R.M.; Pérez-Martínez, D.; Castillo Ferrer, C. Innate immunity in vertebrates: An overview. Immunology 2016, 148, 125–139. [Google Scholar] [CrossRef]
- Celi, P.; Cowieson, A.J.; Fru-Nji, F.; Steinert, R.E.; Kluenter, A.M.; Verlhac, V. Gastrointestinal Functionality in Animal Nutrition and Health: New Opportunities for Sustainable Animal Production. Anim. Feed Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Deblanc, C.; Cariolet, R.; Gautier Bouchardon, A.V.; Merlot, E.; Simon, G. Effect of Feed Restriction on Performance and Postprandial Nutrient Metabolism in Pigs Co-Infected with Mycoplasma hyopneumoniae and Swine Influenza Virus. PLoS ONE 2014, 9, e104605. [Google Scholar] [CrossRef]
- Lu, X.J.; Chen, Q.; Rong, Y.J.; Chen, F.; Chen, J. CXCR3.1 and CXCR3.2 Differentially Contribute to Macrophage Polarization in Teleost Fish. J. Immunol. 2017, 198, 4692–4706. [Google Scholar] [CrossRef]
- Cruvinel, W.M.; Junior, D.M.; Araújo, J.A.P.; Catelan, T.T.T.; Souza, A.W.S.; Silva, N.P.; Andrade, L.E.C. Sistema Imunitário—Parte I. Fundamentos da Imunidade Inata com Ênfase nos Mecanismos Moleculares e Celulares da Resposta Inflamatória. Rev. Bras. Reumatol. 2010, 50, 434–461. [Google Scholar] [CrossRef]
- Castro, I.; Quisenberry, L.; Calvo, R.M.; Obregon, M.J.; Lado-Abeal, J. Septic Shock Non-Thyroidal Illness Syndrome Causes Hypothyroidism and Conditions for Reduced Sensitivity to Thyroid Hormone. J. Mol. Endocrinol. 2013, 50, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Kullberg, B.J.; van der Meer, J.W.M. Circulating Cytokines as Mediators of Fever. Clin. Infect. Dis. 2000, 31, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Black, J.L.; Pluske, J.R. Review of Innate Immunity in Pigs; Final Report: APL Project 2010/1029; Australian Pork Limited: Canberra, Australia, 2011; p. 97. [Google Scholar]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino Acids and Immune Function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Volf, J.; Stepanova, H.; Matiasovic, J.; Kyrova, K.; Sisak, F.; Havlickova, H.; Leva, L.; Faldyna, M.; Rychlik, I. Salmonella enterica serovar Typhimurium and Enteritidis infection of pigs and cytokine signalling in palatine tonsils. Vet. Microbiol. 2012, 156, 127–135. [Google Scholar] [CrossRef]
- Nicholson, L.B. The Immune System. Essays Biochem. 2016, 60, 275–301. [Google Scholar] [CrossRef]
- Uehara, A.; Sekiya, C.; Takasugi, Y.; Namiki, M.; Arimura, A. Anorexia induced by interleukin 1: Involvement of corticotropin-releasing factor. Am. J. Physiol. 1989, 257, R613–R617. [Google Scholar] [CrossRef]
- Balaji, R.; Wright, K.J.; Hill, C.M.; Dritz, S.S.; Knoppel, E.L.; Minton, J.E. Acute Phase Responses of Pigs Challenged Orally with Salmonella Typhimurium. J. Anim. Sci. 2000, 78, 1885–1891. [Google Scholar] [CrossRef]
- Mormede, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the Hypothalamic–Pituitary–Adrenal Function as a Tool to Evaluate Animal Welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef]
- Campos, P.H.R.F.; Merlot, E.; Renaudeau, D.; Noblet, J.; Le Floc’h, N. Postprandial Insulin and Nutrient Concentrations in Lipopolysaccharide-Challenged Growing Pigs Reared in Thermoneutral and High Ambient Temperatures. J. Anim. Sci. 2019, 97, 3354–3368. [Google Scholar] [CrossRef] [PubMed]
- Colditz, I.G. Effects of the Immune System on Metabolism: Implications for Production and Disease Resistance in Livestock. Livest. Sci. 2002, 75, 257–268. [Google Scholar] [CrossRef]
- Plata-Salamán, C.R. Anorexia During Acute and Chronic Disease: Relevance of Neurotransmitter Peptide Cytokine Interactions. Nutrition 1997, 13, 159–160. [Google Scholar] [CrossRef]
- Sonti, G.; Ilyin, S.E.; Plata-Salamán, C.R. Neuropeptide Y blocks and reverses interleukin-1-induced anorexia in rats. Peptides 1996, 17, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Exton, M.S.; Lightfoot, J.B.; Stanton, M.W.; Bull, D.F.; King, M.G.; Husband, A.J. Behaviorally Conditioned Anorexia: Role of Gastric Emptying and Prostaglandins. Physiol. Behav. 1995, 58, 471–476. [Google Scholar] [CrossRef]
- Plata-Salamán, C.R. Cytokine Induced Anorexia: Behavioral, Cellular, and Molecular Mechanisms. Ann. N. Y. Acad. Sci. 1998, 856, 160–170. [Google Scholar] [CrossRef]
- Finck, B.N.; Kelley, K.W.; Dantzer, R.; Johnson, R.W. In Vivo and In Vitro Evidence for the Involvement of Tumor Necrosis Factor α in the Induction of Leptin by Lipopolysaccharide. Endocrinology 1998, 139, 2278–2283. [Google Scholar] [CrossRef]
- Kyriazakis, I.; Sandberg, F.B.; Brindle, W. The Prediction of the Consequences of Pathogen Challenges on the Performance of Growing Pigs. In Mathematical Modelling in Animal Nutrition; France, J., Kebreab, E., Eds.; CAB International: Wallingford, UK, 2008; pp. 398–418. [Google Scholar] [CrossRef]
- Cooney, R.; Owens, E.; Jurasinski, C.; Gray, K.; Vannice, J.; Vary, T. Interleukin 1 Receptor Antagonist Prevents Sepsis Induced Inhibition of Protein Synthesis. Am. J. Physiol. 1994, 267, E636–E641. [Google Scholar] [CrossRef]
- Zamir, O.; O’Brien, W.; Thompson, R.; Bloedow, D.C.; Fisher, J.E.; Hasselgren, P.O. Reduced muscle protein breakdown in septic rats following treatment with interleukin-1 receptor antagonist. Int. J. Biochem. 1994, 26, 943–950. [Google Scholar] [CrossRef]
- Wellington, M.O.; Htoo, J.K.; Van Kessel, A.G.; Columbus, D.A. Impact of dietary fiber and immune system stimulation on threonine requirement for protein deposition in growing pigs. J. Anim. Sci. 2018, 96, 5222–5232. [Google Scholar] [CrossRef]
- Huntley, N.F.; Nyachoti, C.M.; Patience, J.F. Immune System Stimulation Increases Nursery Pig Maintenance Energy Requirements. J. Anim. Sci. 2017, 95, 68–69. [Google Scholar] [CrossRef]
- Reeds, P.J.; Jahoor, F. The Amino Acid Requirements of Disease. Clin. Nutr. 2001, 20, 15–22. [Google Scholar] [CrossRef]
- Williams, N.H.; Stahly, T.S.; Zimmerman, D.R. Effect of chronic immune system activation on the rate, efficiency, and composition of growth and lysine needs of pigs fed from 6 to 27 kg. J. Anim. Sci. 1997, 75, 2463–2471. [Google Scholar] [CrossRef]
- Williams, N.H.; Stahly, T.S.; Zimmerman, D.R. Effect of chronic immune system activation on body nitrogen retention, partial efficiency of lysine utilization, and lysine needs of pigs. J. Anim. Sci. 1997, 75, 2472–2480. [Google Scholar] [CrossRef]
- Black, J.L. Models to Predict Feed Intake. In Voluntary Feed Intake in Pigs; Torrallardona, D., Roura, E., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2009; pp. 323–351. [Google Scholar] [CrossRef]
- Sandberg, F.B.; Emmans, G.C.; Kyriazakis, I. The effects of pathogen challenges on the performance of native and immune animals: The problem of prediction. Animal 2007, 1, 67–86. [Google Scholar] [CrossRef]
- Kyriazakis, I.; Doeschl-Wilson, A. Anorexia During Infection in Mammals: Variation and Its Sources. In Voluntary Feed Intake in Pigs; Torrallardona, D., Roura, E., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2009; pp. 307–321. [Google Scholar] [CrossRef]
- Adewole, D.I.; Kim, I.H.; Nyachoti, C.M. Gut Health of Pigs: Challenge Models and Response Criteria with a Critical Analysis of the Effectiveness of Selected Feed Additives—A Review. Asian-Australas. J. Anim. Sci. 2016, 29, 909–924. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Jondreville, C.; Matte, J.J.; Sève, B. Importance of Sanitary Environment for Growth Performance and Plasma Nutrient Homeostasis During the Post Weaning Period in Piglets. Arch. Anim. Nutr. 2006, 60, 23–34. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; He, X.; Yuan, J.; Yang, Y.; Wang, Z. Growth performance and immune responses in chickens after challenge with lipopolysaccharide and modulation by dietary different oils. Animal 2008, 2, 216–223. [Google Scholar] [CrossRef]
- Chapman, M.E.; Wang, W.; Erf, G.F.; Widemand-Junior, R.F. Pulmonary Hypertensive Responses of Broilers to Bacterial Lipopolysaccharide (LPS); Evaluation of LPS Source and Dose and Impact of Pre-Existing Pulmonary Hypertension and Cellulose Micro-Particles Selection. Poult. Sci. 2005, 84, 432–441. [Google Scholar] [CrossRef]
- Parrott, R.F.; Vellucci, S.V. Comparison of the Antipyretic Actions of Indomethacin and L-745,337, a Selective Cyclooxygenase-2 Inhibitor, in Endotoxin-Treated Prepubertal Pigs. Gen. Pharmacol. 1998, 30, 65–69. [Google Scholar] [CrossRef]
- Webel, D.M.; Finck, B.N.; Baker, D.H.; Johnson, R.W. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 1997, 75, 1514–1520. [Google Scholar] [CrossRef]
- Johnson, R.W. Inhibition of Growth by Pro Inflammatory Cytokines: An Integrated View. J. Anim. Sci. 1997, 75, 1244–1255. [Google Scholar] [CrossRef]
- Liu, Y.L.; Li, D.F.; Gong, L.M.; Yi, G.F.; Gaines, A.M.; Carrol, J.A. Effects of Fish Oil Supplementation on the Performance and the Immunological, Adrenal, and Somatotrophic Responses of Weaned Pigs After an Escherichia coli Lipopolysaccharide Challenge. J. Anim. Sci. 2003, 81, 2758–2765. [Google Scholar] [CrossRef]
- Kegley, E.B.; Spears, J.W.; Auman, S.K. Dietary Phosphorus and an Inflammatory Challenge Affect Performance and Immune Function of Weanling Pigs. J. Anim. Sci. 2001, 79, 413–419. [Google Scholar] [CrossRef]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post-weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef]
- Luise, D.; Lauridsen, C.; Bosi, P.; Trevisi, P. Methodology and Application of Escherichia coli F4 and F18 Encoding Infection Models in Post-Weaning Pigs. J. Anim. Sci. Biotechnol. 2019, 10, 53. [Google Scholar] [CrossRef]
- Trevisi, P.; Melchior, D.; Mazzoni, M.; Casini, L.; De Filippi, S.; Minieri, L.; Lalata-Costerbosa, G.; Bosi, P. A tryptophan-enriched diet improves feed intake and growth performance of susceptible weanling pigs orally challenged with Escherichia coli K88. J. Anim. Sci. 2009, 87, 148–156. [Google Scholar] [CrossRef]
- Kiarie, E.; Slominski, B.A.; Krause, D.O.; Nyachoti, C.M. Acute Phase Response of Piglets Fed Diets Containing Nonstarch Polysaccharide Hydrolysis Products and Egg Yolk Antibodies Following an Oral Challenge with Escherichia coli (K88). Can. J. Anim. Sci. 2009, 89, 353–360. [Google Scholar] [CrossRef]
- Lee, J.S.; Awji, E.G.; Lee, S.J.; Tassew, D.D.; Park, Y.B.; Park, K.S.; Kim, M.K.; Kim, B.; Park, S.C. Effect of Lactobacillus plantarum CJLP243 on the Growth Performance and Cytokine Response of Weaning Pigs Challenged with Enterotoxigenic Escherichia coli. J. Anim. Sci. 2012, 90, 3709–3717. [Google Scholar] [CrossRef]
- Li, H.; Zhao, P.; Lei, Y.; Li, T.; Kim, I. Response to an Escherichia coli K88 Oral Challenge and Productivity of Weanling Pigs Receiving a Dietary Nucleotide Supplement. J. Anim. Sci. Technol. 2015, 6, 49. [Google Scholar] [CrossRef]
- Luppi, A. Swine Enteric Colibacillosis: Diagnosis, Therapy and Antimicrobial Resistance. Porc. Health Manag. 2017, 3, 16. [Google Scholar] [CrossRef]
- Stokes, C.R.; Bailey, M.; Haverson, K.; Harris, C.; Jones, P.; Inman, C.; Pié, S.; Oswald, I.; Williamns, B.; Akkermans, A.; et al. Postnatal development of intestinal immune system in piglets: Implications for the process of weaning. Anim. Res. 2004, 53, 325–334. [Google Scholar] [CrossRef]
- Haley, C.A.; Dargatz, D.A.; Bush, E.J.; Erdman, M.M.; Fedorka-Cray, P.J. Salmonella Prevalence and Antimicrobial Susceptibility from the National Animal Health Monitoring System Swine 2000 and 2006 Studies. J. Food Prot. 2012, 75, 428–436. [Google Scholar] [CrossRef]
- Davies, P.; Morrow, W.M.; Jones, F.; Deen, J.; Fedorka-Cray, P.; Harris, I. Prevalence of Salmonella in Finishing Swine Raised in Different Production Systems in North Carolina, USA. Epidemiol. Infect. 1997, 119, 237–244. [Google Scholar] [CrossRef]
- Funk, J.; Davies, P.; Nichols, M. Longitudinal Study of Salmonella enterica in Growing Pigs Reared in Multiple Site Swine Production Systems. Vet. Microbiol. 2001, 83, 45–60. [Google Scholar] [CrossRef]
- Turner, J.L.; Dritz, S.S.; Higgins, J.J.; Herkelman, K.L.; Minton, J.E. Effects of a Quillaja saponaria extract on growth performance and immune function of weanling pigs challenged with Salmonella Typhimurium. J. Anim. Sci. 2002, 80, 1939–1946. [Google Scholar] [CrossRef]
- Collado-Romero, M.; Arce, C.; Ramirez-Boo, M.; Carvajal, A.; Garrido, J.J. Quantitative Analysis of the Immune Response upon Salmonella Typhimurium Infection Along the Porcine Intestinal Gut. J. Vet. Res. 2010, 41, 23. [Google Scholar] [CrossRef]
- Santos, L.R.; Zhang, S.; Tsolis, R.M.; Kingsley, R.A.; Adams, L.G.; Baumler, A.J. Animal models of Salmonella infections: Enteritis versus typhoid fever. Microbes Infect. 2001, 3, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Rostagno, M.H.; Gardiner, G.E.; Sutton, A.L.; Richert, B.T.; Radcliffe, S.T. Controlling Salmonella infection in weanling pigs through water delivery of direct-fed microbials or organic acids. Part I: Effects on growth performance, microbial populations, and immune status. J. Anim. Sci. 2012, 90, 261–271. [Google Scholar] [CrossRef]
- Moura, E.A.G.; Silva, D.G.D.; Turco, C.H.; Sanches, T.V.C.; Storino, G.Y.; Almeida, H.M.S.; Mechler Dreibi, M.L.; Rabelo, I.P.; Sonalio, K.; Oliveira, L.G. Salmonella Bacterin Vaccination Decreases Shedding and Colonization of Salmonella Typhimurium in Pigs. Microorganisms 2021, 9, 1163. [Google Scholar] [CrossRef]
- Davis, E.; Wallace, K.; Petry, A.; Broaday, R.; Burdick Sanchez, N.; Carroll, J.; Ballou, M. A Dose Response Investigation of a Micronized Porous Ceramic Particle to Improve the Health and Performance of Post Weaned Pigs Infected with Salmonella enterica Serotype Typhimurium. Front. Vet. Sci. 2022, 3, 872776. [Google Scholar] [CrossRef]
- Spiehs, M.; Shurson, G.; Johnston, L. Effects of two direct-fed microbials on the ability of pigs to resist an infection with Salmonella enterica serovar Typhimurium. J. Swine Health Prod. 2008, 16, 27–36. [Google Scholar] [CrossRef]
- Casey, P.G.; Gardiner, G.E.; Casey, G.; Bradshaw, B.; Lawlor, P.G.; Lynch, P.B.; Leonard, F.C.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F.; et al. A Five-Strain Probiotic Combination Reduces Pathogen Shedding and Alleviates Disease Signs in Pigs Challenged with Salmonella enterica Serovar Typhimurium. Appl. Environ. Microbiol. 2007, 73, 1858–1863. [Google Scholar] [CrossRef] [PubMed]
- Fraga, A.Z.; Campos, P.H.R.F.; Hauschild, L.; Chalvon-Demersay, T.; Beaumont, M.; Le Floc’h, N. A Blend of Functional Amino Acids and Grape Polyphenols Improves the Pig Capacity to Cope with an Inflammatory Challenge Caused by Poor Hygiene of Housing Conditions. BMC Vet. Res. 2023, 19, 25. [Google Scholar] [CrossRef]
- Jayaraman, B.J.; Htoo, J.K.; Nyachoti, C.M. Effects of Different Dietary Tryptophan: Lysine Ratios and Sanitary Conditions on Growth Performance, Plasma Urea Nitrogen, Serum Haptoglobin and Ileal Histomorphology of Weaned Pigs. Anim. Sci. J. 2016, 88, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Kahindi, R.K.; Htoo, J.K.; Nyachoti, C.M. Short Communication: Effect of Dietary Lysine Content and Sanitation Conditions on Performance of Weaned Pigs Fed Antibiotic Free Diets. Can. J. Anim. Sci. 2014, 94, 115–118. [Google Scholar] [CrossRef]
- Kim, J.C.; Pluske, J. Improving Protein Utilization Efficiency Through Better Understanding of Immune and Stress Responses in Pigs. In Proceedings of the Book of Abstracts: World Nutrition Forum, Vancouver, BC, Canada, 12–15 October 2016; pp. 1–12. [Google Scholar]
- Chalvon-Demersay, T.; Luise, D.; Le Floc’h, N.; Tesseraud, S.; Lambert, W.; Bosi, P.; Trevisi, P.; Beaumont, M.; Corrent, E. Functional Amino Acids in Pigs and Chickens: Implication for Gut Health. Front. Vet. Sci. 2021, 8, 663727. [Google Scholar] [CrossRef]
- Martínez-Aispuro, J.; Figueroa-Velasco, J.; Sánchez-Torres, M.; Cordero-Mora, J.; Ayala-Monter, M. Supplementation of functional amino acids in pig diets and its impact on the intestine. Abanico Vet. 2023, 13, e2022-14. [Google Scholar] [CrossRef]
- Morales, A.; Buenabad, L.; Castillo, G.; Arce, N.; Araiza, B.A.; Htoo, J.K.; Cervantes, M. Low Protein Amino Acid–Supplemented Diets for Growing Pigs: Effect on Expression of Amino Acid Transporters, Serum Concentration, Performance, and Carcass Composition. J. Anim. Sci. 2015, 93, 2154–2164. [Google Scholar] [CrossRef]
- Yen, J.T.; Kerr, B.J.; Easter, R.A.; Parkhurst, A.M. Difference in rates of net portal absorption between crystalline and protein-bound lysine and threonine in growing pigs fed once daily. J. Anim. Sci. 2004, 82, 1079–1090. [Google Scholar] [CrossRef]
- Sun, F.; Cao, Y.; Cai, C.; Li, S.; Yu, D.; Yao, Y. Regulation of nutritional metabolism in transition dairy cows: Energy homeostasis and health in response to post-ruminal choline and methionine. PLoS ONE 2016, 11, e0160659. [Google Scholar] [CrossRef]
- Zhou, Z.; Vailati-Riboni, M.; Trevisi, E.; Drackley, J.K.; Luchini, D.N.; Loor, J.J. Better postpartal performance in dairy cows supplemented with rumen-protected methionine compared with choline during the peripartal period. J. Dairy Sci. 2016, 99, 8716–8732. [Google Scholar] [CrossRef]
- Sierzant, K.; Perruchot, M.H.; Merlot, E.; Floc’h, L.; Gondret, F. Tissue-Specific Responses of Antioxidant Pathways to Poor Hygiene Conditions in Growing Pigs Divergently Selected for Feed Efficiency. BMC Vet. Res. 2019, 15, 341. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandeh, A.; de Lange, C.F.M. Immune System Stimulation Increases Reduced Glutathione Synthesis Rate in Growing Pigs. In Energy and Protein Metabolism and Nutrition; Crovetto, G.M., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2010; pp. 501–502. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Matte, J.J.; Melchior, D.; Van Milgen, J.; Sève, B. A Moderate Inflammation Caused by the Deterioration of Housing Conditions Modifies Trp Metabolism but Not Trp Requirement for Growth of Post-Weaned Piglets. Animal 2010, 4, 1891–1898. [Google Scholar] [CrossRef]
- Popov, A.; Schultze, J.L. IDO Expressing Regulatory Dendritic Cells in Cancer and Chronic Infection. J. Mol. Med. 2008, 86, 145–160. [Google Scholar] [CrossRef]
- Sharma, M.D.; Baban, B.; Chandler, P.; Hou, D.Y.; Singh, N.; Yagita, H.; Azuma, M.; Blazar, B.R.; Mellor, A.L.; Munn, D.H. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2, dioxygenase. J. Clin. Investig. 2007, 117, 2570–2582. [Google Scholar] [CrossRef]
- Mor, A.; Tankiewicz Kwedlo, A.; Krupa, A.; Pawlak, D. Role of Kynurenine Pathway in Oxidative Stress During Neurodegenerative Disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The Biological Stress of Early Weaned Piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Wu, G.; Sun, Y.; Wang, B.; He, B.; Dai, Z.; Wu, Z. Glutamine enhances tight junction protein expression and modulates corticotropin-releasing factor signaling in the jejunum of weanling piglets. J. Nutr. 2015, 145, 25–31. [Google Scholar] [CrossRef]
- Ruth, M.R.; Field, C.J. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J. Anim. Sci. Biotechnol. 2013, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.; Kim, J.C.; Blank, J.L. Manipulating the Immune System for Pigs to Optimise Performance. Anim. Prod. Sci. 2017, 58, 666–680. [Google Scholar] [CrossRef]
- Pluske, J.R.; Turpin, D.L.; Kim, J.C. Gastrointestinal Tract (Gut) Health in the Young Pig. Anim. Nutr. 2018, 12, 187–196. [Google Scholar] [CrossRef]
- Rakhshandeh, A.; Htoo, J.K.; Karrow, N.; Miller, S.P.; de Lange, C.F.M. Impact of Immune System Stimulation on the Ileal Nutrient Digestibility and Utilisation of Methionine plus Cysteine Intake for Whole Body Protein Deposition in Growing Pigs. Br. J. Nutr. 2013, 111, 101–110. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Eicher, S.D.; Ajuwon, K.M.; Applegate, T.J. Effect of threonine on secretory immune system using a chicken intestinal ex vivo model with lipopolysaccharide challenge. Poult. Sci. 2017, 96, 3043–3051. [Google Scholar] [CrossRef]
- Mao, X.; Lai, X.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Tian, G.; Zhang, K. Effects of dietary threonine supplementation on immune challenge induced by swine Pseudorabies live vaccine in weaned pigs. Anim. Prod. Sci. 2014, 54, 1024–1031. [Google Scholar] [CrossRef]
- Ren, M.; Liu, X.T.; Wang, X.; Zhang, G.J.; Qiao, S.Y.; Zeng, X.F. Increased levels of standardized ileal digestible threonine attenuate intestinal damage and immune responses in Escherichia coli K88+ challenged weaned piglets. Anim. Feed Sci. Technol. 2014, 195, 67–75. [Google Scholar] [CrossRef]
- Capozzalo, M.M.; Kim, J.C.; Htoo, J.K.; de Lange, C.F.; Mullan, B.P.; Hansen, C.F.; Pluske, J.R. Effect of increasing the dietary tryptophan to lysine ratio on plasma levels of tryptophan, kynurenine and urea and on production traits in weaner pigs experimentally infected with an enterotoxigenic strain of Escherichia coli. Arch. Anim. Nutr. 2015, 69, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.W.; Fontes, D.D.O.; Silva, F.C.D.O.; Scottá, B.A.; Silva, M.A.E.; Souza, L.P.O.; Vidal, T.Z.B. Níveis de metionina + cistina para leitões dos 6 aos 16 kg submetidos a diferentes graus de ativação do sistema imune. Rev. Bras. Saúde Prod. Anim. 2015, 16, 827–838. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, Y.; Shen, J.; Lin, Y.; Fang, Z.; Che, L.; Wu, D. Threonine and tryptophan supplementation enhance porcine reproductive and respiratory syndrome (PRRS) vaccine-induced immune responses of growing pigs. Anim. Sci. J. 2015, 86, 294–304. [Google Scholar] [CrossRef]
- Jayaraman, B.; Regassa, A.; Htoo, J.K.; Nyachoti, C.M. Effects of dietary standardized ileal digestible tryptophan:lysine ratio on performance, plasma urea nitrogen, ileal histomorphology and immune responses in weaned pigs challenged with Escherichia coli K88. Livest. Sci. 2017, 203, 114–119. [Google Scholar] [CrossRef]
- Kahindi, R.K.; Regassa, A.; Htoo, J.K.; Nyachoti, C.M. Optimal sulfur amino acid to lysine ratio for post-weaning piglets reared under clean or unclean sanitary conditions. Anim. Nutr. 2017, 3, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, Y.; Jansman, A.J.M.; Gerrits, W.J.J. Low sanitary conditions increase energy expenditure for maintenance and decrease incremental protein efficiency in growing pigs. Animal 2020, 14, 1811–1820. [Google Scholar] [CrossRef]
- Sterndale, S.O.; Miller, D.W.; Mansfield, J.P.; Kim, J.C.; Pluske, J.R. Increasing dietary tryptophan in conjunction with decreasing other large neutral amino acids increases weight gain and feed intake in weaner pigs regardless of experimental infection with enterotoxigenic Escherichia coli. J. Anim. Sci. 2020, 98, skaa190. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Wellington, M.O.; González-Vega, J.C.; Htoo, J.K.; van Kessel, A.G.; Columbus, D.A. A longer adaptation period to a functional amino acid–supplemented diet improves growth performance and immune status of Salmonella Typhimurium-challenged pigs. J. Anim. Sci. 2021, 99, skab146. [Google Scholar] [CrossRef]
- Gonçalves, J.P.R.; Melo, A.D.B.; Yang, Q.; de Oliveira, M.J.K.; Marçal, D.A.; Ortiz, M.T.; Righetti Arnaut, P.; França, I.; Alves da Cunha Valini, G.; Silva, C.A.; et al. Increased dietary Trp, Thr, and Met supplementation improves performance, health, and protein metabolism of weaned piglets under mixed management and poor housing conditions. Animals 2024, 14, 1143. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Panisson, J.C.; Kpogo, L.A.; González-Vega, J.C.; Htoo, J.K.; van Kessel, A.G.; Columbus, D.A. Functional amino acid supplementation postweaning mitigates the response of normal birth weight more than for low birth weight pigs to a subsequent Salmonella challenge. Animal 2022, 16, 100566. [Google Scholar] [CrossRef]
- Jensen, M.B.; Kyriazakis, I.; Lawrence, A.B. The Activity and Straw Directed Behaviour of Pigs Offered Foods with Different Crude Protein Content. Appl. Anim. Behav. Sci. 1993, 37, 211–221. [Google Scholar] [CrossRef]
- Jericho, K.; Church, T. Cannibalism in Pigs. Can. Vet. J. 1972, 3, 156. [Google Scholar]
- Collin, M.; Backberg, M.; Onnestam, K.; Meister, B. 5-HT1A Receptor Immunoreactivity in Hypothalamic Neurons Involved in Body Weight Control. Neuroendocrinology 2002, 13, 945–951. [Google Scholar] [CrossRef]
- Ursinus, W.W.; Van Reenen, C.G.; Reimert, I.; Bolhuis, J.E. Tail biting in pigs: Blood serotonin and fearfulness as pieces of the puzzle? PLoS ONE 2014, 9, e107040. [Google Scholar] [CrossRef]
- Van der Meer, Y.; Gerrits, W.J.J.; Jansman, A.J.M.; Kemp, B.; Bolhuis, J.E. A link between damaging behaviour in pigs, sanitary conditions, dietary protein and amino acid supply. PLoS ONE 2017, 12, e0174688. [Google Scholar] [CrossRef]
- Pastorelli, H.; Le Floc’h, N.; Merlot, E.; Meunier-Salaün, M.C.; van Milgen, J.; Montagne, L. Sanitary Housing Conditions Modify the Performance and Behavioural Response of Weaned Pigs to Feed- and Housing-Related Stressors. Animal 2012, 6, 1811–1820. [Google Scholar] [CrossRef]
- Day, J.; Kyriazakis, I.; Rogers, P. Food Choice and Intake: Towards a Unifying Framework of Learning and Feeding Motivation. Nutr. Res. Rev. 1998, 11, 25–43. [Google Scholar] [CrossRef]
- Wechsler, B.; Lea, S.E.G. Adaptation by learning: Its significance for farm animal husbandry. Appl. Anim. Behav. Sci. 2007, 108, 197–214. [Google Scholar] [CrossRef]
- Remus, A.; Hauschild, L.; Létourneau-Montminy, M.P.; Pomar, C. Estimating Amino Acid Requirements in Real Time for Precision Fed Pigs: The Challenge of Variability Among Individuals. Animals 2021, 11, 3354. [Google Scholar] [CrossRef]
- Wellock, I.J.; Emmans, G.C.; Kyriazakis, I. Modeling the effects of stressors on the performance of populations of pigs. J. Anim. Sci. 2004, 82, 2442–2450. [Google Scholar] [CrossRef]
- Pomar, C.; Pomar, J.; Rivest, J.; Cloutier, L.; Letourneau-Montminy, M.P.; Andretta, I.; Hauschild, L. Estimating Real-Time Individual Amino Acid Requirements in Growing-Finishing Pigs: Toward a New Definition of Nutrient Requirements? In Nutritional Modelling for Pigs and Poultry; Sakomura, N.K., Gous, R., Kyriazakis, I., Hauschild, L., Eds.; CAB International: Wallingford, UK, 2015; pp. 157–174. [Google Scholar]
- Ortiz, M.T.; Arnaut, P.R.; Valini, G.A.C.; França, I.; Silva, C.A.; de Oliveira, M.J.K.; Marçal, D.A.; Melo, A.D.B.; Hauschild, L. A Salmonella Challenge Impacts the Variability of Performance, Body Composition and Lysine Requirements of Growing Pigs Under Poor Housing Conditions. Livest. Sci. 2024, 283, 105462. [Google Scholar] [CrossRef]
- Van der Waaij, E.H. A resource allocation model describing consequences of artificial selection under metabolic stress. J. Anim. Sci. 2004, 82, 973–981. [Google Scholar] [CrossRef]
- Spurlock, M.E. Regulation of metabolism and growth during immune challenge: An overview of cytokine function. J. Anim. Sci. 1997, 75, 1773–1783. [Google Scholar] [CrossRef]
- Aymerich, P.; Soldevila, C.; Bonet, J.; Gasa, J.; Coma, J.; Sola-Oriol, D. Increasing Dietary Lysine Impacts Differently Growth Performance of Growing Pigs Sorted by Body Weight. Animals 2020, 10, 1032. [Google Scholar] [CrossRef]
- Ribeiro, A.M.L.; Farina, G.; Vieira, M.S.; Perales, V.A.; Kessler, A.M. Energy utilization of light and heavy weaned piglets subjected to different dietary energy levels. Rev. Bras. Zootec. 2016, 45, 532–539. [Google Scholar] [CrossRef]
- Laghouaouta, H.; Pena, R.N.; Ros-Freixedes, R.R.; Reixach, J.; Diaz, M.; Estany, J.; Armengol, R.; Bassols, A.; Fraile, L. A Methodology to Quantify Resilience in Growing Pigs. Animals 2021, 11, 2970. [Google Scholar] [CrossRef]
- Njoku, C.P.; Ayasan, T.; Adeyemi, O.A.; Akinola, O.S.; Mobolaji, O.O.; Ayano, T.R.; Arazi, F.M. Effect of Weight Asymmetry on Serological Parameters, Tibia Bone Characteristics and Gut Morphology of Pigs. J. Agric. Fac. Gaziosmanpasa Univ. 2021, 38, 20–27. [Google Scholar] [CrossRef]
- Hauschild, L.; Lovatto, P.A.; Pomar, J.; Pomar, C. Development of Sustainable Precision Farming Systems for Swine: Estimating Real-Time Individual Amino Acid Requirements in Growing-Finishing Pigs. J. Anim. Sci. 2012, 90, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Andretta, I.; Pomar, C.; Rivest, J.; Pomar, J.; Lovatto, P.A.; Radünz Neto, J. The Impact of Feeding Growing–Finishing Pigs with Daily Tailored Diets Using Precision Feeding Techniques on Animal Performance, Nutrient Utilization, and Body and Carcass Composition. J. Anim. Sci. 2014, 92, 3925–3936. [Google Scholar] [CrossRef]
- Andretta, I.; Pomar, C.; Rivest, J.; Pomar, J.; Radünz, J. Precision Feeding Can Significantly Reduce Lysine Intake and Nitrogen Excretion Without Compromising the Performance of Growing Pigs. Animal 2016, 10, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Ajuwon, K.M. Mechanism of endocytic regulation of intestinal tight junction remodeling during nutrient starvation in jejunal IPEC-J2 cells. FASEB J. 2021, 35, e21356. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.A.; Koo, B.; Nyachoti, M.; Columbus, D.A. Formulating diets for improved health status of pigs: Current knowledge and perspectives. Animals 2022, 12, 2877. [Google Scholar] [CrossRef] [PubMed]
| Challenge Model | Advantages | Disadvantages |
|---|---|---|
| Specific pathogen challenge | - Mimics real infections observed under field conditions - Allows investigation of pathogen–host interactions | - Requires high biosafety facilities - Higher biosecurity risk - Lower reproducibility between studies |
| Poor hygiene environment | - Simulates multifactorial challenges similar to commercial settings - Low operational cost | - High variability between trials - Limited control over challenge intensity |
| LPS injection (acute inflammatory model) | - Highly controlled and reproducible - Induces rapid systemic inflammatory response - Useful for mechanistic studies | - Does not mimic natural infections - Transient and artificial effect - Limited application to chronic stress models |
| Author | Phase | Type of Challenge | AA Supplementation | ADG and ADFI Compared to Control | General Observations |
|---|---|---|---|---|---|
| Trevisi et al. [69] | Nursery | ETEC | Trp | ↑ 22% ADG ↑ 8% ADFI | ↓ rectal temperature ↓ ETEC count ↑ [serum IgA] |
| Le Floc’h et al. [99] | Nursery | Poor sanitary condition | Trp | ↑ 9% ADG ↑ 6% ADFI | ↑ 3% feed efficiency ↓ [IFN-α] |
| Mao et al. [110] | Nursery | Pseudorabies live vaccine | Thr | ↑ 7% ADG ↑ 8% ADFI | ↑ 8% feed efficiency ↑ [serum IgA, IgM, IgG] |
| Ren et al. [111] | Nursery | ETEC | Thr | ↑ 7% ADG ↑ 1% ADFI | ↑ Lymphocyte proliferation ↑ [serum IgA, IgG] |
| Capozzalo et al. [112] | Nursery | Escherichia coli | Trp | ↑ 16% ADG ↑ 14% ADFI | ↑ 12% BW ↑ 4% feed efficiency ↓ [urea] |
| Jayaraman et al. [18] | Nursery | Poor sanitary condition | Thr | ↓ 21% ADG ↓ 25% ADFI | ↑ 9% feed efficiency |
| Pinheiro et al. [113] | Nursery | Mycoplasma hyopneumoniae vaccine | Met | ↑ 7% ADG ↑ 1% ADFI | ↑ 6% feed efficiency ↑ 7% protein deposition ↓ 70% fat deposition |
| Xu et al. [114] | Growing | Ovalbumin | Trp and Thr | ↑ 10% ADG ↑ 8% ADFI | ↑ 4% feed efficiency ↑ Lymphocyte proliferation ↓ cellular damage |
| Van der Meer et al. [13] | Growing | Poor sanitary condition | Trp, Thr, and Met | ↑ 4% ADG ↑ 2% ADFI | ↓ [leukocytes] |
| Jayaraman et al. [115] | Nursery | Escherichia coli | Trp | ↑ 18% ADG ↑ 10% ADFI | ↑ 6% feed efficiency ↓ plasma urea |
| Kahindi et al. [116] | Nursery | Poor sanitary condition | Met | ↑ 10% ADG ↑ 8% ADFI | ↑ 2% feed efficiency ↑ 4% BW |
| Kahindi et al. [19] | Nursery | Escherichia coli | Met | ↑ 7% ADG ↑ 7% ADFI | ↑ 6% feed efficiency ↓ plasma TNF- α |
| Wellingtion et al. [15] | Growing | Salmonella Typhimurium | Thr | ↑ 12% ADG ↓ 6% ADFI | ↑ 17% feed efficiency ↓ Salmonella Typhimurium in the cecum and colon |
| Van der Meer et al. [117] | Nursery | Poor sanitary condition | Trp, Thr and Met | ↑ 14% ADG ↓ 5% ADFI | ↑ 11% feed efficiency ↑ [serum IgG] ↓ [haptoglobin] |
| Sterndale et al. [118] | Nursery | ETEC | Trp | ↑ 18% ADG ↑ 6% ADFI | ↑ [cortisol] ↓ [plasma Trp] |
| Rodrigues et al. [14] | Nursery | Salmonella Typhimurium | Trp, Thr, and Met | ↑ 35% ADG ↓ 8% ADFI | ↑ 6% BW ↑ [albumin] ↓ [superoxide dismutase] |
| Rodrigues et al. [119] | Nursery | Salmonella Typhimurium | Trp, Thr, and Met | ↑ 54% ADG ↑ 1% ADFI | ↑ 54% feed efficiency ↓ Salmonella Typhimurium translocation (from intestine to spleen) ↑ [albumin] ↓ [haptoglobin] |
| Valini et al. [24] | Growing | Salmonella Typhimurium + poor sanitary condition + animal group mixing | Trp, Thr, and Met | ↑ 14% ADG ↑ 4% ADFI | ↑ 13% feed efficiency ↑ 7% BW ↑ 14% protein deposition ↑ [albumin] ↓ [urea and creatinine] |
| França et al. [22] | Growing | Salmonella Typhimurium + poor sanitary condition | Trp, Thr, and Met | ↑ 29% ADG ↑ 15% ADFI | ↑ 17% feed efficiency ↑ 12% BW ↑ 25% protein deposition ↑ [albumin and glucose] ↓ [urea] |
| Gonçalves et al. [120] | Nursery | Poor sanitary condition | Trp, Thr, and Met | ↑ 23% ADG ↑ 21% ADFI | ↑ 13% BW ↓ [urea and creatinine] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valini, G.d.C.; Fraga, A.Z.; França, I.; Marçal, D.A.; Arnaut, P.R.; Veira, A.M.; de Oliveira, M.J.K.; Andretta, I.; Hauschild, L. Dietary Supplementation of Methionine, Tryptophan, and Threonine for Pigs Under Sanitary Challenges: Current Knowledge and Future Directions. Vet. Sci. 2025, 12, 794. https://doi.org/10.3390/vetsci12090794
Valini GdC, Fraga AZ, França I, Marçal DA, Arnaut PR, Veira AM, de Oliveira MJK, Andretta I, Hauschild L. Dietary Supplementation of Methionine, Tryptophan, and Threonine for Pigs Under Sanitary Challenges: Current Knowledge and Future Directions. Veterinary Sciences. 2025; 12(9):794. https://doi.org/10.3390/vetsci12090794
Chicago/Turabian StyleValini, Graziela da Cunha, Alícia Zem Fraga, Ismael França, Danilo Alves Marçal, Pedro Righetti Arnaut, Alini Mari Veira, Marllon José Karpeggiane de Oliveira, Ines Andretta, and Luciano Hauschild. 2025. "Dietary Supplementation of Methionine, Tryptophan, and Threonine for Pigs Under Sanitary Challenges: Current Knowledge and Future Directions" Veterinary Sciences 12, no. 9: 794. https://doi.org/10.3390/vetsci12090794
APA StyleValini, G. d. C., Fraga, A. Z., França, I., Marçal, D. A., Arnaut, P. R., Veira, A. M., de Oliveira, M. J. K., Andretta, I., & Hauschild, L. (2025). Dietary Supplementation of Methionine, Tryptophan, and Threonine for Pigs Under Sanitary Challenges: Current Knowledge and Future Directions. Veterinary Sciences, 12(9), 794. https://doi.org/10.3390/vetsci12090794








