Physiological and Metabolic Responses to Water Restriction in Ewes Under Semi-Arid Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Location
2.2. Animals, Management, and Study Design
2.3. Water Treatment
2.4. Environmental Variables
2.5. Physiological Parameters
2.6. Biochemical and Hematological Parameters
2.7. Urine Analysis
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dias e Silva, T.P.; Torreão, J.N.C.; Marques, C.A.T.; Araújo, M.J.; Bezerra, L.R.; Dhanasekaran, D.K.; Sejian, V. Effect of multiple stress factors (thermal, nutritional and pregnancy type) on adaptive capability of native ewes under semi-arid environment. J. Therm. Biol. 2016, 59, 39–46. [Google Scholar] [CrossRef]
- Araújo, G.G.L.; Costa, S.A.P.; Moraes, S.A.; Queiroz, M.A.A.; Gois, G.C.; Santos, N.M.S.S.; Albuquerque, I.R.R.; Moura, J.H.A.; Campos, F.S. Supply of water with salinity levels for Morada Nova sheep. Small Rumin. Res. 2019, 171, 73–76. [Google Scholar] [CrossRef]
- Benatallah, A.; Ghozlane, F.; Marie, M. The effect of water restriction on physiological and blood parameters in lactating dairy cows reared under Mediterranean climate. Asian-Australas. J. Anim. Sci. 2019, 32, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Akinmoladun, O.F.; Muchenje, V.; Fon, F.N.; Mpendulo, C.T. Small ruminants: Farmers’ hope in a world threatened by water scarcity. Animals 2019, 9, 456. [Google Scholar] [CrossRef]
- Araújo, C.A.; Magalhães, A.L.R.; Araújo, G.G.L.; Campos, F.S.; Gois, G.C.; Santos, K.C.; Matos, M.H.T.; Nascimento, D.B.; Santos, N.S. Correlation between mineral profile, physical-chemical characteristics, and proximate composition of meat from Santa Ines ewes under water restriction. Semin. Ciências Agrárias 2023, 44, 529–548. [Google Scholar] [CrossRef]
- Santos, F.M.; Araújo, G.G.L.; Souza, L.L.; Yamamoto, S.M.; Queiroz, M.A.A.; Lanna, D.P.D.; Moraes, S.A. Impact of water restriction periods on carcass traits and meat quality of feedlot lambs in the Brazilian semi-arid region. Meat Sci. 2019, 156, 196–204. [Google Scholar] [CrossRef]
- Souza, L.L.; Araújo, G.G.L.; Turco, S.H.N.; Moraes, S.A.; Voltolini, T.V.; Gois, G.C.; Campos, F.S.; Santos, M.C.R.; Santos, F.M. Water restriction periods affect growth performance and nutritional status of Santa Inês sheep in the Brazilian Semi-arid. Semin. Ciências Agrárias 2022, 43, 1037–1050. [Google Scholar] [CrossRef]
- Nobre, I.S.; Araújo, G.G.L.; Santos, E.M.; Carvalho, G.G.P.; Albuquerque, I.R.R.; Oliveira, J.S.; Ribeiro, O.L.; Turco, S.H.N.; Gois, G.C.; Silva, T.G.F.; et al. Cactus pear silage to mitigate the effects of an intermittent water supply for feedlot lambs: Intake, digestibility, water balance and growth performance. Ruminants 2023, 3, 121–132. [Google Scholar] [CrossRef]
- Albuquerque, I.R.R.; Araújo, G.G.L.; Voltolini, T.V.; Moura, J.H.A.; Costa, R.G.; Gois, G.C.; Costa, S.A.P.; Campos, F.S.; Queiroz, M.A.A.; Santos, N.M.S.S. Saline water intake effects performance, digestibility, nitrogen and water balance of feedlot lambs. Anim. Prod. Sci. 2020, 60, 1591–1597. [Google Scholar] [CrossRef]
- Adeniji, Y.A.; Sanni, M.O.; Abdoun, K.A.; Samara, E.M.; Al-Badwi, M.A.; Bahadi, M.A.; Alhidary, I.A.; Al-Haidary, A.A. Resilience of lambs to limited water availability without compromising their production performance. Animals 2020, 10, 1491. [Google Scholar] [CrossRef]
- Saini, B.S.; Kataria, N.; Kataria, A.K.; Sankhala, L.N. Dehydration stress associated variations in rectal temperature, pulse and respiration rate of Marwari sheep. J. Stress Physiol. Biochem. 2013, 9, 15–20. [Google Scholar]
- IPCC. Summary for policymakers. In Climate change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar] [CrossRef]
- Silva, A.S.S.; Arnan, X.; Medeiros, P.M. Climate change may alter the availability of wild food plants in the Brazilian semiarid. Reg. Environ. Change 2024, 24, e86. [Google Scholar] [CrossRef]
- Al-Ramamneh, D.; Riek, A.; Gerken, M. Effect of water restriction on drinking behaviour and water intake in German black-head mutton sheep and Boer goats. Animal 2012, 6, 173–178. [Google Scholar] [CrossRef]
- Chedid, M.; Jaber, L.S.; Giger-Reverdin, S.; Duvaux-Ponter, C.; Hamadeh, S.K. Review: Water stress in sheep raised under arid conditions. Can. J. Anim. Sci. 2014, 94, 243–257. [Google Scholar] [CrossRef]
- Halfen, J.; Rahal, N.M.; Barbosa, A.A.; Corrêa, M.N.; Del Pino, F.A.B.; Rabassa, V.R.; Brauner, C.C.; Schmitt, E. Influência da restrição alimentar e do estresse térmico sobre parâmetros fisiológicos em ovinos. Arq. Bras. Med. Vet. Zootec. 2020, 72, 1911–1919. [Google Scholar] [CrossRef]
- Mengistu, U.L.; Puchala, R.; Sahlu, T.; Gipson, T.A.; Dawson, L.J.; Goetsch, A.L. Comparison of different levels and lengths of restricted drinking water availability and measurement times with Katahdin sheep and Boer and Spanish goat wethers. Small Rumin. Res. 2016, 144, 320–333. [Google Scholar] [CrossRef]
- Yetisgin, S.O.; Şen, U. Resilience to drought in semi-desert sheep: Effects of water restriction during pregnancy on placental efficiency in the Awassi breed. Anim. Sci. J. 2020, 91, e13494. [Google Scholar] [CrossRef] [PubMed]
- Koppen, W. Grundriss der Klimakunde: Outline of Climate Science, 1st ed.; Walter de Gruyter: Berlin, Germany, 1923; p. 379. [Google Scholar] [CrossRef]
- Lima, P.R.; Araújo, C.A.; Campos, F.S.; Menezes, V.G.; Ribeiro, N.L.; Araújo, G.G.L.; Menezes, D.R.; Matos, M.H.T.; Queiroz, M.A.A.; Santos, E.M. Reductions in the water supply to crossbred Santa Inês ewes in the Brazilian semi-arid: Apparent nutrient digestibility, water and nitrogen balance, and performance. Small Rumin. Res. 2023, 226, e107021. [Google Scholar] [CrossRef]
- Araújo, C.A.; Magalhães, A.L.R.; Araújo, G.G.L.; Campos, F.S.; Gois, G.C.; Matos, M.H.T.; Queiroz, M.A.A.; Menezes, V.G.; Costa, C.J.P.; Santos, K.C.; et al. Effect of reduced of water supply on carcass characteristics, non-carcass components and the volume of digestive compartments of Santa Inês ewes. Livest. Sci. 2021, 245, e104402. [Google Scholar] [CrossRef]
- Araújo, E.J.B.; Pereira, F.D.S.; Nunes, T.S.S.; Cordeiro, A.E.; Silva, H.C.; Queiroz, M.A.A.; Gois, G.C.; Rodrigues, R.T.S.; Menezes, D.R. Nutritional value, feeding behavior, physiological parameters, and performance of crossbred Boer goats kids fed butterfly pea hay and cactus pear meal. Spanish J. Agric. Res. 2022, 20, e0603. [Google Scholar] [CrossRef]
- NRC. National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids, 7th ed.; National Academy Press: Washington, DC, USA, 2007; 384p. [Google Scholar]
- Mader, T.L.; Davis, M.S.; Brown-Brandl, T. Environmental factors influencing heat stress in feedlot cattle. J. Anim. Sci. 2006, 84, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Silanikove, N.; Koluman, N. Impact of climate change on the dairy industry in temperate zones: Predications on the overall negative impact and on the positive role of dairy goats in adaptation to earth warming. Small Rumin. Res. 2015, 123, 27–34. [Google Scholar] [CrossRef]
- Rosa, P.R.; Araújo, G.G.L.; Turco, S.H.N.; Moraes, S.A.; Alves, J.N.; Gois, G.C.; Santos, R.D.; Campos, F.S. Ingestive behavior and physiological parameters of sindhi heifers receiving saline water. J. Agric. Sci. 2019, 11, 381–394. [Google Scholar] [CrossRef]
- Maia, A.S.C.; Silva, R.G.; Loureiro, C.M.B. Sensible and latent heat loss from the body surface of Holstein cows in a tropical environment. Int. J. Biomet. 2005, 50, 17–22. [Google Scholar] [CrossRef]
- Garcia-Navarro, C.E.K. Manual de Urinálise Veterinária, 1st ed.; Varela: São Paulo, Brasil, 2005; 96p. [Google Scholar]
- Santarosa, B.P.; Ferreira, D.O.L.; Rodrigues, M.M.P.; Dantas, G.N.; Sacco, S.R.; Lopes, R.S.; Dias, A.; Gonçalves, R.C. Avaliação clínica, laboratorial e anatomopatológica do sistema urinário de ovinos confinados com ou sem suplementação de cloreto de amônio. Pesq. Vet. Bras. 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Taffarel, L.E.; Costa, P.B.; Pozza, M.S.S.; Wobeto, J.R.; München, E.P. Correlação entre características físicas, pH e contagem bacteriana da urina de ovinos. Synerg. Scyent. 2012, 7, 1–3. [Google Scholar]
- SAS University. Sas/Stat University User Guide; Sas Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- Hoffmann, G.; Herbut, P.; Pinto, S.; Heinicke, J.; Kuhla, B.; Amon, T. Animal-related, non-invasive indicators for determining heat stress in dairy cows. Biosyst Eng. 2020, 199, 83–96. [Google Scholar] [CrossRef]
- Slimen, I.B.; Chniter, M.; Najar, T.; Ghram, A. Meta-analysis of some physiologic, metabolic and oxidative responses of sheep exposed to environmental heat stress. Livest. Sci. 2019, 229, 179–187. [Google Scholar] [CrossRef]
- McKinley, M.; Trevaks, D.; Weissenborn, F.; McAllen, R. Interaction between thermoregulation and osmoregulation in domestic animals. Rev. Bras. Zootec. 2017, 46, 783–790. [Google Scholar] [CrossRef][Green Version]
- West, J.W. Effects of heat-stress on production in dairy Cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Maia, A.S.C.; Nascimento, S.T.; Nascimento, C.C.N.; Gebremedhin, K.G. Thermal equilibrium of goats. J. Therm. Biol. 2016, 58, 43–49. [Google Scholar] [CrossRef]
- Mendes, A.M.P.; Azevedo, M.; Lopes, P.M.O.; Moura, G.B.A. Zoneamento bioclimático para a raça ovina Dorper no Estado de Pernambuco. Pesq. Agropec. Bras. 2014, 49, 986–993. [Google Scholar] [CrossRef][Green Version]
- Silva, E.M.N.; Souza, B.B.; Sousa, O.B.; Silva, G.A.S.; Freitas, M.M.S. Avaliação da adaptabilidade de caprinos ao semiárido através de parâmetros fisiológicos e estruturas do tegumento. Rev. Caat. 2010, 23, 142–148. [Google Scholar][Green Version]
- Reece, W.O. Dukes-Fisiologia dos Animais Domésticos, 13th ed.; Roca: Barueri, SP, Brazil, 2017; 740p. [Google Scholar]
- De, K.; Kumar, D.; Singh, K.A.; Kumar, K.; Sahoo, A.; Naqvi, K.M. Resilience of Malpura ewes on water restriction and rehydration during summer under semi-arid tropical climatic conditions. Small Rumin. Res. 2015, 133, 123–127. [Google Scholar] [CrossRef]
- Vieira, F.M.C.; Pilatti, J.A.; Czekoski, Z.M.W.; Fonsêca, V.F.C.; Herbut, P.; Angrecka, S.; Vismara, E.S.; Macedo, V.P.; Santos, M.C.R.; Pasmionka, I. Effect of the silvopastoral system on the thermal comfort of lambs in a subtropical climate: A preliminary study. Agriculture 2021, 11, 790. [Google Scholar] [CrossRef]
- McManus, C.M.; Lucci, C.M.; Maranhão, A.Q.; Pimentel, D.; Pimentel, F.; Paiva, S.R. Response to heat stress for small ruminants: Physiological and genetic aspects. Livest. Sci. 2022, 263, e105028. [Google Scholar] [CrossRef]
- Habeeb, A.A.; Gad, A.E.; Atta, M.A. Temperature-humidity indices as indicators to heat stress of climatic conditions with relation to production and reproduction of farm animals. Int. J. Biotechnol. Recent Adv. 2018, 1, 35–50. [Google Scholar] [CrossRef]
- Vieira, R.; Louvandini, H.; Barcellos, J.; Martins, C.F.; McManus, C. Path and logistic analysis for heat tolerance in adapted breeds of cattle in Brazil. Livest. Sci. 2022, 258, e104888. [Google Scholar] [CrossRef]
- Jawasreh, K.; Awaedeh, F.; Bani-Ismail, Z.; Al-Rawashdeh, O.; Al-Majali, A. Normal hematology and selected serum biochemical values in different genetic lines of awassi Ewes in Jordan. Int. J. Vet. Med. 2010, 7, 1–5. [Google Scholar]
- Turner, J.C. Osmotic fragility of desert bighorn sheep red blood cells. Comp. Biochem. Physiol. Part A Physiol. 1979, 64, 167–175. [Google Scholar] [CrossRef]
- Getahun, D.; Alemneh, T.; Akeberegn, D.; Getabalew, M.; Zewdie, D. Urea Metabolism and Recycling in Ruminants. Biomed. J. Scient. Techn. Res. 2019, 20, 14790–14796. [Google Scholar] [CrossRef]
- Hailemariam, S.; Zhao, S.; He, Y.; Wang, J. Urea transport and hydrolysis in the rumen: A review. Anim. Nutr. 2021, 7, 989–996. [Google Scholar] [CrossRef]
- Casamassima, D.; Vizzarri, F.; Nardoia, M.; Palazzo, M. The effect of water-restriction on various physiological variables in intensively reared Lacaune ewes. Vet. Med. 2016, 61, 623–634. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 6th ed.; Academic Press: San Diego, CA, USA, 2008; 928p. [Google Scholar]
- Hamadeh, S.K.; Rawda, N.; Jaber, L.S.; Habre, A.; Said, M.A.; Barbour, E.K. Physiological responses to water restriction in dry and lactating Awassi ewes. Livest. Sci. 2006, 101, 101–109. [Google Scholar] [CrossRef]
- Tulu, D.; Gadissa, S.; Hundessa, F. Impact of water stress on adaptation and performance of sheep and goat in dryland regions under climate change scenarios: A systematic review. J. Anim. Behav. Biomet. 2023, 11, e2023012. [Google Scholar] [CrossRef]
- Xue, B.; Hong, Q.; Li, X.; Lu, M.; Zhou, J.; Yue, S.; Wang, Z.; Wang, L.; Peng, Q.; Xue, B. Hepatic injury induced by dietary energy level via lipid accumulation and changed metabolites in growing semi-fine wool sheep. Front. Vet. Sci. 2021, 8, e745078. [Google Scholar] [CrossRef] [PubMed]
- Akinmoladun, O.F.; Fon, F.N.; Mpendulo, C.T.; Okoh, O. Performance, heat tolerance response, and blood metabolites of water-restricted Xhosa goats supplemented with vitamin C. Transl. Anim. Sci. 2020, 4, 1113–1127. [Google Scholar] [CrossRef]
- Noureddine, T.; Mebirouk-boudechiche, L.; Chaker-houd, K.; Aoun, L. Effects of water stress on zootechnical physiological and blood parameters of Ouled Djellal ewes in Algeria. Egypt. J. Vet. Sci. 2022, 53, 293–306. [Google Scholar] [CrossRef]
- Carro, M.D.; Cantalapiedra-Hijar, G.; Ranilla, M.J.; Molina-Alcaide, E. Urinary excretion of purine derivatives, microbial protein synthesis, nitrogen use, and ruminal fermentation in sheep and goats fed diets of different quality. J. Anim. Sci. 2012, 90, 3963–3972. [Google Scholar] [CrossRef]
- Ferreira, F.; Campos, W.E.; Carvalho, A.U.; Pires, M.F.A.; Martinez, M.L.; Silva, M.V.G.B.; Verneque, R.S.; Silva, P.F. Clinical, hematological, biochemical, and hormonal parameters of cattle submitted to heat stress. Arq. Bras. Med. Vet. Zootec. 2009, 61, 769–776. [Google Scholar] [CrossRef]
- Prado, O.R.; Arias, E.I.; Carrillo, M.D.; Hernández, J.R.; García, A.C. Metabolic response to water shortage in an isolated feral sheep population. Austral J. Vet. Sci. 2021, 53, 91–97. [Google Scholar] [CrossRef]
- Casamassima, D.; Pizzo, R.; Palazzo, M.; D’Alessandro, A.G.; Martemucci, G. Effect of water restriction on productive performance and blood parameters in comisana sheep reared under intensive condition. Small Rumin. Res. 2008, 78, 169–175. [Google Scholar] [CrossRef]
- Jaber, L.; Chedid, M.; Hamadeh, S. Water Stress in Small Ruminants. In Responses of Organisms to Water Stress; Akıncı, S., Ed.; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Li, B.T.; Christopherson, R.J.; Cosgrove, S.J. Effect of water restriction and environmental temperatures on metabolic rate and physiological parameters in sheep. Can. J. Anim. Sci. 2000, 80, 97–104. [Google Scholar] [CrossRef]
- Ferreira, D.O.L.; Santarosa, D.P.; Surian, S.R.S.; Takahira, R.K.; Chiacchio, S.B.; Amorim, R.M.; Dias, A.; Gonçalves, R.C. Low performance of vitamin C compared to ammonium chloride as an urinary acidifier in feedlot lambs. Ciênc. Anim. Bras. 2020, 21, e60098. [Google Scholar] [CrossRef]
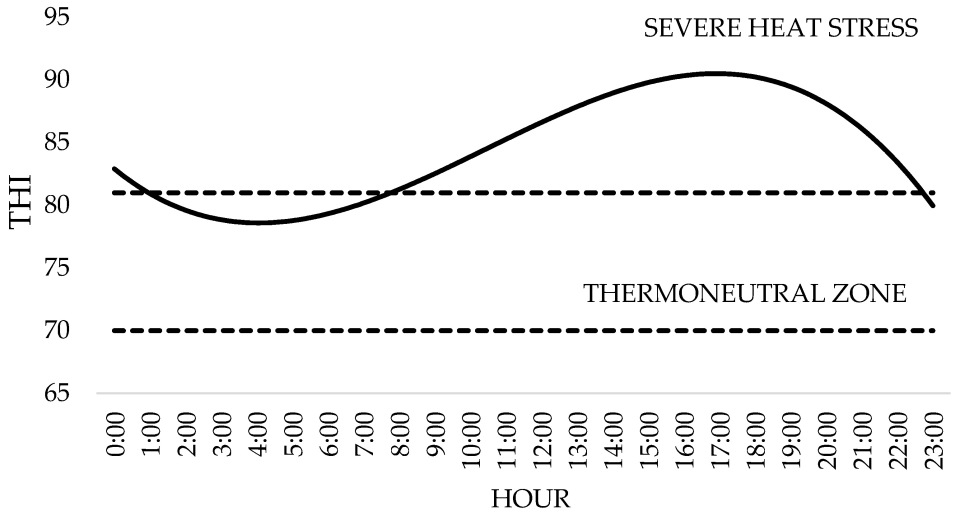
| Ingredients | g/kg Dry Matter | |||
|---|---|---|---|---|
| Elephant grass | 460 | |||
| Corn meal | 381 | |||
| Soybean meal | 132 | |||
| Mineral salt 1 | 20 | |||
| Urea | 7 | |||
| Chemical composition (g/kg dry matter) | ||||
| Elephant grass | Corn meal | Soybean meal | Diet | |
| Dry matter 2 | 261.9 | 889.3 | 886.1 | 576.26 |
| Mineral matter | 105.2 | 12.9 | 64.8 | 61.86 |
| Crude protein | 105.5 | 89.9 | 487.4 | 149.13 |
| Ether extract | 28.7 | 45.1 | 19.0 | 32.89 |
| Neutral detergent fiber | 708.7 | 111.6 | 15.46 | 370.56 |
| Acid detergent fiber | 419.5 | 33.7 | 88.5 | 206.97 |
| Total carbohydrates | 830.5 | 859.9 | 42.8 | 715.30 |
| Non-fiber carbohydrates | 174.0 | 642.0 | 27.85 | 328.31 |
| Total digestible nutrients | 570.1 | 850.0 | 80.48 | 596.71 |
| Variables | Water Supplies (%) | |||
|---|---|---|---|---|
| 100% | 80% | 60% | 40% | |
| ∑ Water supplied (L) | 355.0 | 112.22 | 88.16 | 64.10 |
| Water supplied (L/day) | 5.00 | 1.58 | 1.24 | 0.903 |
| Minimum quantity supplied (L/day) | - | 0.77 | 0.61 | 0.44 |
| Maximum quantity supplied (L/day) | - | 2.46 | 1.97 | 1.48 |
| Variables | Water Supplies (%) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 100 | 80 | 60 | 40 | L | Q | ||
| Respiratory rate (mov/min) 1 | 66.56 | 56.03 | 67.56 | 79.34 | 2.29 | <0.001 | <0.001 |
| Heart rate (beats/min) 2 | 100.7 | 97.5 | 97.06 | 101.12 | 0.93 | 0.844 | <0.001 |
| Rectal temperature (°C) 3 | 39.01 | 38.83 | 38.86 | 38.97 | 0.042 | 0.566 | <0.001 |
| Sweating rate (g/m2/h) | |||||||
| Neck | 74.98 | 77.45 | 78.69 | 128.82 | 35.8 | 0.317 | 0.510 |
| Loin | 56.56 | 63.82 | 83.34 | 64.26 | 8.77 | 0.285 | 0.143 |
| Hour | Respiratory Rate (mov/min) | Heart Rate (Beats/min) | Rectal Temperature (°C) |
|---|---|---|---|
| 09h00 | 67.12 c | 105.21 b | 38.92 ab |
| 12h00 | 85.37 b | 111.03 a | 38.97 ab |
| 15h00 | 93.81 a | 107.28 a | 39.20 a |
| 18h00 | 84.71 b | 106.12 b | 39.07 a |
| 21h00 | 63.62 c | 95.06 c | 38.98 ab |
| 00h00 | 49.68 d | 86.46 d | 38.86 b |
| 03h00 | 46.25 d | 89.78 cd | 38.76 b |
| 06h00 | 48.40 d | 91.81 c | 38.57 b |
| SEM | 3.24 | 1.32 | 0.06 |
| p-value | <0.001 | <0.001 | <0.005 |
| Sweating rate (g/m2/h) | |||
| Hour | Neck | Loin | |
| 09h00 | 116.12 | 62.4 | |
| 15h00 | 65.32 | 71.59 | |
| SEM | 25.31 | 6.20 | |
| p-value | 0.178 | 0.303 | |
| Variables | Water Supplies (%) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 100 | 80 | 60 | 40 | L | Q | ||
| Albumin (g/dL) | 34.3 | 41.08 | 35.89 | 37.71 | 1.66 | 0.502 | 0.147 |
| Creatinine (mg/dL) | 1.1 | 1.13 | 1.17 | 1.25 | 0.07 | 0.155 | 0.690 |
| Glucose (g/dL) | 55.95 | 55.2 | 54.5 | 53.16 | 2.36 | 0.397 | 0.903 |
| Urea (mg/dL) 1 | 70.91 | 82.5 | 82.75 | 93.75 | 4.40 | 0.002 | 0.948 |
| TP (g/dL) | 68.08 | 62.16 | 65.45 | 67.04 | 3.04 | 0.990 | 0.229 |
| Cholesterol (mg/dL) | 58.45 | 64.95 | 57.87 | 54.25 | 3.80 | 0.195 | 0.326 |
| Triglycerides (mg/dL) | 21.83 | 21.79 | 21.87 | 20.08 | 2.15 | 0.597 | 0.688 |
| AST (UI/L) | 47.79 | 62.08 | 50.41 | 64.45 | 5.54 | 0.133 | 0.982 |
| GGT (UI/L) | 17.91 | 16.08 | 20.83 | 18.33 | 1.39 | 0.346 | 0.814 |
| ALT (UI/L) 2 | 14.79 | 14.24 | 14.12 | 9.41 | 1.45 | 0.037 | 0.103 |
| Variables | Water Supplies (%) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 100 | 80 | 60 | 40 | L | Q | ||
| WBC (cells/mm3) | 7.66 | 5.83 | 6.96 | 6.63 | 0.64 | 0.507 | 0.258 |
| RBC (106/µL) 1 | 11.68 | 12.22 | 12.15 | 13.02 | 0.36 | 0.022 | 0.655 |
| HGB (g/dl) | 12.27 | 12.50 | 12.80 | 12.83 | 0.36 | 0.229 | 0.797 |
| HCT (%) | 36.41 | 37.67 | 38.30 | 38.82 | 1.13 | 0.131 | 0.747 |
| MCV (fL) | 31.26 | 31.15 | 31.15 | 30.05 | 0.81 | 0.330 | 0.552 |
| MCH (Pg) | 10.36 | 10.17 | 10.32 | 9.82 | 0.24 | 0.160 | 0.603 |
| MCHC (g/dL) | 33.42 | 33.32 | 33.50 | 32.90 | 0.25 | 0.237 | 0.343 |
| RDWC (%) | 18.01 | 18.73 | 18.28 | 17.85 | 0.48 | 0.670 | 0.320 |
| PC (103/µL) | 577.29 | 698.95 | 660.41 | 634.00 | 73.08 | 0.690 | 0.814 |
| MPV (fL) | 3.63 | 3.72 | 4.98 | 3.65 | 0.61 | 0.638 | 0.253 |
| PDWC (%) | 15.46 | 15.45 | 15.37 | 15.45 | 0.08 | 0.778 | 0.625 |
| PCT (%) | 0.21 | 0.25 | 0.24 | 0.23 | 0.02 | 0.737 | 0.256 |
| Variables | Water Supplies (%) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 100 | 80 | 60 | 40 | L | Q | ||
| Creatinine (mg/dL) 1 | 52.45 | 41.86 | 41.93 | 39.93 | 3.40 | 0.020 | 0.218 |
| Urea (mg/dL) | 43.25 | 43.33 | 46.08 | 42.58 | 6.00 | 0.978 | 0.768 |
| Sedimentoscopy | |||||||
| Leukocytes | 0/8 | 1/8 | 0/8 | 1/8 | - | - | - |
| Crystals | 1/8 | 4/8 | 1/8 | 1/8 | - | - | - |
| Yeast | 0/8 | 1/8 | 0/8 | 0/8 | - | - | - |
| Bacteria | |||||||
| Absent | 3/8 | 0/8 | 0/8 | 1/8 | - | - | - |
| + | 3/8 | 4/8 | 4/8 | 2/8 | - | - | - |
| ++ | 1/8 | 2/8 | 1/8 | 1/8 | - | - | - |
| +++ | 1/8 | 2/8 | 2/8 | 4/8 | - | - | - |
| Cells | |||||||
| Rare | 6/8 | 4/8 | 4/8 | 3/8 | - | - | - |
| Moderate | 0/8 | 3/8 | 2/8 | 2/8 | - | - | - |
| High | 2/8 | 1/8 | 2/8 | 3/8 | - | - | - |
| Variables | Water Supply (%) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 100 | 80 | 60 | 40 | L | Q | ||
| Density | 1.005 | 1.006 | 1.006 | 1.008 | 0.012 | 0.058 | 0.627 |
| pH 1 | 7.62 | 8.68 | 8.75 | 8.31 | 0.33 | 0.169 | 0.034 |
| Protein 2 | 0.00 | 3.75 | 3.75 | 5.62 | 2.21 | 0.039 | 0.675 |
| Urobilinogen 3 | 0.10 | 0.10 | 0.10 | 0.32 | 0.07 | 0.050 | 0.138 |
| Red blood cells | 0.00 | 0.00 | 0.00 | 1.00 | 0.50 | 0.190 | 0.326 |
| Color | |||||||
| Light yellow | 0/8 | 3/8 | 3/8 | 3/8 | - | - | - |
| Straw yellow | 3/8 | 0/8 | 2/8 | 2/8 | - | - | - |
| Citron yellow | 5/8 | 5/8 | 3/8 | 3/8 | - | - | - |
| Appearance | |||||||
| Clear | 4/8 | 3/8 | 3/8 | 4/8 | - | - | - |
| Cloudy | 4/8 | 5/8 | 5/8 | 4/8 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, C.d.J.P.; Araújo, G.G.L.d.; Magalhães, A.L.R.; de Andrade, A.P.; Turco, S.H.N.; Matos, M.H.T.d.; Silva, D.C.N.d.; Araújo, C.d.A.; Valença, R.d.L.; Silva, T.G.F.d.; et al. Physiological and Metabolic Responses to Water Restriction in Ewes Under Semi-Arid Conditions. Vet. Sci. 2025, 12, 790. https://doi.org/10.3390/vetsci12090790
Costa CdJP, Araújo GGLd, Magalhães ALR, de Andrade AP, Turco SHN, Matos MHTd, Silva DCNd, Araújo CdA, Valença RdL, Silva TGFd, et al. Physiological and Metabolic Responses to Water Restriction in Ewes Under Semi-Arid Conditions. Veterinary Sciences. 2025; 12(9):790. https://doi.org/10.3390/vetsci12090790
Chicago/Turabian StyleCosta, Claudenilde de Jesus Pinheiro, Gherman Garcia Leal de Araújo, André Luiz Rodrigues Magalhães, Alberício Pereira de Andrade, Silvia Helena Nogueira Turco, Maria Helena Tavares de Matos, Diego César Nunes da Silva, Cleyton de Almeida Araújo, Roberta de Lima Valença, Thieres George Freire da Silva, and et al. 2025. "Physiological and Metabolic Responses to Water Restriction in Ewes Under Semi-Arid Conditions" Veterinary Sciences 12, no. 9: 790. https://doi.org/10.3390/vetsci12090790
APA StyleCosta, C. d. J. P., Araújo, G. G. L. d., Magalhães, A. L. R., de Andrade, A. P., Turco, S. H. N., Matos, M. H. T. d., Silva, D. C. N. d., Araújo, C. d. A., Valença, R. d. L., Silva, T. G. F. d., Campos, F. S., & Gois, G. C. (2025). Physiological and Metabolic Responses to Water Restriction in Ewes Under Semi-Arid Conditions. Veterinary Sciences, 12(9), 790. https://doi.org/10.3390/vetsci12090790








