Review of Toxoplasmosis: What We Still Need to Do
Simple Summary
Abstract
1. Introduction
2. History
2.1. Etiology
2.2. Historical Background of the Disease in Animals
2.3. Parasite Morphology and Life Cycle
2.4. Notable Outbreaks/Epizootics in Domestic and Wild Species
2.5. Transmission
2.6. Human Toxoplasmosis
2.7. Diagnosis, Treatment, and Control
3. Life Cycle and Immunology/Immunopathogenesis
3.1. Life Cycle in Definitive Host (Felids)
3.1.1. Motility, Invasion, and Egress
3.1.2. Schizogony
3.1.3. Gametogenesis
3.2. Life Cycle in the Environment
Sporulation
3.3. Life Cycle in Intermediate Hosts, Including Humans and Cats
3.4. Pathogenesis in General
| Cytokine | Main Functions | Ref. |
|---|---|---|
| INF α | Induce other inflammatory proteins. | [152] |
| INFγ | Provides protection against T. gondii by activation of MΦ, NO, and GTPase signaling. Also induces cell-autonomous immunity, iNOS, and IDO production. | [153] |
| TNFα | Involved in an acute inflammatory response. | [153] |
| IL1β | Acute phase response mediator; induces other inflammatory proteins. | [154] |
| IL2 | Induces growth of T cells and the release of IFNγ, involved in the lytic activity of MΦ and NK cells. | [155] |
| IL4 | Antagonizes the products of Th1 cells; long exposure leads to chronic toxoplasmosis. | [156] |
| IL5 | Has a counter-protective role in acute toxoplasmosis and a protective role in chronic toxoplasmosis. | [157] |
| IL6 | Has a pleiotropic role in immunity, including creating barriers in early ocular toxoplasmosis, enhancing activities of NK cells, and maturation of T/B cells. | [158] |
| IL7 | Plays a crucial role in the development of memory CD8+ T cells. | [159] |
| IL10 | Suppress inflammation to prevent T. gondii encephalitis, Controls hyper-inflammation, regulates the protective functioning of CD4+ cells, and plays a suppressive microbicidal function for MΦ and Np. | [160] |
| IL12 | Central inducer of IFNγ, activates NK cells, CD4 T cells, and CD8 T cells. | [161] |
| IL15 | Required for optimal role of NK cells, CD8+ cells, and IELs. | [162] |
| IL17A | Mainly involved in innate immunity by the recruitment of Np IL12, IFNγ, and IL6. | [163] |
| IL18 | Involved in the production of IFNγ by NK cells and T cells. | [164] |
| IL23 | Stimulates NK cells and T cells more specifically in the absence of IL-12. | [165] |
| IL27 | Required for resistance to chronic toxoplasmic encephalitis, induces CXCR3, T-bet, Blimp1, and IL10 expression, inhibit Th17 development. | [166] |
| IL33 | induces CCL 2 expression (proinflammatory), induces IL-10 production by M2 macrophages (anti-inflammatory). | [167] |
| TGFβ | Anti-inflammatory role in the brain, eyes, and intestine. | [168] |
4. Epidemiology of Toxoplasmosis
5. Clinical Signs
5.1. Clinical Signs of Toxoplasmosis in Humans
| System Involved | Pregnant Women [7] | Fetus/Infant [199] | Older Children and Adults (Immunocompetent) [200] | Immunocompromised (HIV) [201] | Cardiac/Renal Transplant [202] | Bone Marrow/HSCT Transplantation [203] |
|---|---|---|---|---|---|---|
| Neurological | Same as in older children and adults | Encephalitis, epilepsy, psychomotor retardation, microcephaly, cerebral calcification, hydrocephalus | Encephalitis, meningoencephalitis, meningitis, fatal brain abscess, seizures, poor cognition/motor function | Encephalitis, hemiparesis, altered mental state, seizures, cranial nerve disturbances | Encephalitis (within 3 months post-transplant) | Localized encephalitis, seizures, headache, confusion |
| Ophthalmologic | Retinitis, chorioretinitis, peripheral retinal scars, uveitis | Retinochoroiditis, vitritis, blurred vision, scotoma, photophobia, strabismus, glaucoma, vision loss | Retinitis, retinochoroiditis, branch retinal artery occlusion, risk of permanent vision loss | Ocular involvement | Retinal involvement | - |
| Cardiovascular | Same as in older children and adults | Myocarditis, pericarditis | Myocarditis, pericarditis | - | Myocardial involvement | - |
| Respiratory | Pneumonia | Diffuse interstitial pneumonia | Febrile illness with cough, dyspnea | Pulmonary involvement | ||
| Musculoskeletal | Myositis, dermatomyositis | Polymyositis, dermatomyositis, myositis, myalgias | - | - | - | |
| Gastrointestinal (GI)/Hepatic | Jaundice | Pancreatitis, increased liver enzymes, mesenteric lymphadenopathy, GI pathologies, hepatocellular abnormalities | - | - | - | |
| Hematologic/Systemic | Asymptomatic to illness, cervical lymphadenopathy | Rash, petechiae, anemia, high mortality risk | Sepsis-like syndrome, weight loss | - | Fever | Fever |
| Other | - | - | Guillain–Barre syndrome | Psychosis, dementia, anxiety | - | - |
| Timing/Special Notes | Can show all signs of other groups | Progressive manifestations | - | Brain predominantly infected | Reactivation infection (3 months post) | Often leads to death |
5.2. Clinical Signs of Toxoplasmosis in Animals
5.2.1. Clinical Manifestations Within Wildlife
| Class | Order Family | Species and Clinical Manifestations | Ref. |
|---|---|---|---|
| Mammalia | Carnivora Felidae |
| [128,214] |
| Mephitidae (skunks) | Striped skunk (Mephitis mephitis): asymptomatic. | [205] | |
| Ursidae | Giant panda (Ailuropoda melanoleuca): affects gastrointestinal, and respiratory systems which can lead to death. | [64] | |
| Mustelidae (sea otters) | Southern sea otters (Enhydra lutris nereis): significant mortality with encephalitis. | [212] | |
| Rodentia Sciuridae (squirrels) |
| [68] | |
| Castoridae (beavers) | Beaver (Castor canadensis): fatal systemic toxoplasmosis (lymphohistiocytic encephalitis, myocarditis, interstitial pneumonia with multinucleated cells). | [215] | |
| Sciuridae | Woodchuck (Marmota monax): head tilt, circling, and rapid weight loss. | [204,207] | |
| Caviidae | Capybara (Hydrochoerus hydrochaeris): no clinical toxoplasmosis. | ||
| Echimyidae | Nutria (Myocastor coypus): no clinical toxoplasmosis. | ||
| Lagomorpha Leporidae (rabbits, hares) |
| [216] | |
| Eulipotyphla Talpidae (moles/Insectivores) |
| [217] | |
| Chiroptera Pteropodidae (bats) |
| [206] | |
| Artiodactyla Cervidae (deer) | No transplacental infection unless an acute infection occurs during pregnancy. Acute toxoplasmosis and death in mule deer.
| [218] | |
| Bovidae (Antelope, nilgai) |
| [51,214] | |
| Non-human primates Callitrichidae |
| [214,219] | |
| Cercopithecidae (Macaques) |
| ||
| Atelidae |
| [100] | |
○ Alouatta belzebul: prostration, diarrhea. | [60] | ||
○ Alouatta caraya: prostration, inappetence, abdominal distension and pain, intestinal hypomotility. | [220,221] | ||
| [222] | ||
| [223] | ||
| [98] | ||
| Cebidae |
| [224] | |
| [5,38,225,226] | ||
| Diprotodontia Macropodidae | Acute fatal, respiratory distress, diarrhoea, neurological disturbances, myocardial hemorrhages and pale streaks, lymphadenomegaly, splenomegaly, adrenal enlargement and reddening, gastrointestinal reddening and ulceration, pancreatic swelling, brain malacia.
| [227,228,229,230,231,232] | |
| Phascolarctidae | Koalas (Phascolarctos cinereus): acute fatal, myocardial hemorrhages, and pale streaks. | [233,234] | |
| Vombatidae | Wombats (Vombatus ursinus): respiratory distress, neurological disturbances. | [234] | |
| Phalangeridae (Possums) | Acute fatal, respiratory distress, neurological disturbances, myocardial hemorrhages and pale streaks, splenomegaly, gastrointestinal reddening and ulceration, brain malacia. | [234,235,236] | |
| Peramelemorphia Peramelidae (Bandicoots) | Neurological disturbances, adrenal enlargement and reddening, pancreatic swelling.
| [237,238,239] | |
| Dasyuromorphia Dasyuridae Myrmecobiidae | Acute fatal, neurological disturbances, gastrointestinal reddening, and ulceration | [5] | |
| Peramelemorphia Thylacomyidae | Bilby (Macrotis lagotis): neurological disturbances, adrenal enlargement and reddening, pancreatic swelling. | [240] | |
| Cetacea Delphinidae |
| [68] | |
| Aves |
| [214,241] |
5.2.2. Clinical Manifestations Within Domestic Animals
| Domestic Animals | ||
|---|---|---|
| Pet animals | ||
| Cats (definitive host) | Asymptomatic or polypnea, icterus, uveitis and retinochoroiditis, pericardial and abdominal effusions, diffuse necrotizing hepatitis, transplacental and lactogenically. | [24,177,242] |
| Dogs | Respiratory and hepatic systems, no transplacental infection, resistant to experimental toxoplasmosis. | [243] |
| Ferrets | Congenital toxoplasmosis, acute and chronic forms. | [69] |
| Mink | Can be naturally infected. | [71] |
| Fish, reptiles, amphibians | Not occur in fish, reptiles, or amphibians as natural infection, but they can be experimentally infected. | [4,249] |
| Production Animals | ||
| Horses | Relatively resistant to experimental infection, no clinical disease. | [17,18] |
| Swine | Can be naturally infected, causing sow abortion (sows abort only once). | [245] |
| Cattle | Rare, no report of zoonotic. | [16] |
| Sheep, goats | Abortion. | [244] |
| Buffalos | No clinical disease. | [250] |
| Camels | Acute toxoplasmosis with dyspnea. | [246] |
| Llamas, alpaca, and vicunas | No clinical disease. | [251] |
| Chickens | No clinical signs, no vertical transmission, decreased egg production. | [247] |
| Turkeys | No clinical signs, having tissue cysts in breast and leg muscles. | [248] |
| Ducks and geese | No clinical disease. | [12] |
6. Diagnosis of Toxoplasmosis
6.1. Diagnosis of Toxoplasmosis in Animals
6.2. Diagnosis of Toxoplasmosis in Humans
| Antibody | Life Span | Prediction | Ref. |
|---|---|---|---|
| IgE | Short-term (days to weeks) | Acute toxoplasmosis | [263] |
| IgA | Few weeks | Supports acute/reactivated/congenital infection | [264] |
| IgM | 1 week to months/years | Congenital toxoplasmosis; alone insufficient to establish acute toxoplasmosis | [265,266] |
| IgG | Lifelong (post-infection) | Seroconversion and exposure (timing unclear without avidity testing) | [267] |
| 1 Type | Method Category | Specific Techniques | Target | Detected Analyte (Key Reagent) | |
|---|---|---|---|---|---|
| Direct | Microscopy (MS) | Light MS (Giemsa, H&E, PAS) | Tachyzoites, tissue cysts | Tachyzoites, tissue cysts | |
| Immunohistochemistry (IHC) | T. gondii antigens | Fluorophore-labeled anti-T. gondii antibodies | |||
| Electron microscopy | Ultrastructural parasite features | N/A (morphology only) | |||
| Bioassay | Mouse bioassay | Viable parasites | N/A (relies viable infection) | ||
| Cell culture | Replicating tachyoites | N/A | |||
| Imaging | CT/MRI (CNS lesions) | Brain abscesses, calcifications | N/A | ||
| Ultrasonography (congenital) | Fetal abnormalities | N/A | |||
| Molecular | PCR, qPCR, LAMP, etc. | DNA regions | DNA Region | ||
| Nanoparticle | Piezoelectric immunoagglutination (PIA) | Antigens | IgG | ||
| 1 Plasmonic gold chips (PGC) | Antigens (saliva) | IgG | |||
| Quantum dot-labeled antigen detection | Antigens | ||||
| Immunoassays | Immunofluorescence antigen detection (IFA-D) | Antigens in tissues | Fluorophore-labeled anti-T. gondii antibodies | ||
| Indirect | Serological Assays (Antibody Detection) | Dye test (DT) | Live tachyzoite | IgG, IgA, IgM | |
| Modified agglutination test (MAT) | Formalin-fixed tachyzoite | IgG | |||
| Indirect fluorescent antibody test (IFAT) | Fixed tachyzoites | IgG, IgM | |||
| Indirect hemagglutination (IHA) | Tanned red blood cells sensitized with soluble antigens | IgG | |||
| ELISA |
| Tachyzoite lysate antigens (TLAs) | IgG | ||
| SAG1/GRA7/ROP1 protein | IgG, IgM | |||
| Multiple antigens | IgG, IgM, IgA | |||
| TLA/recombinant antigens | IgG (avidity index) | |||
| Recombinant antigens | IgG, IgM | |||
| Lateral flow strips | IgG, IgM | |||
| Immunosorbent agglutination assay (ISAGA) | Anti-human IgM | IgM | |||
| Latex agglutination test (LAT) | antigen-coated latex particles | IgG, IgM | |||
| Western blotting (WB) | Tachyzoite lysate/recombinant | IgG, IgM | |||
| Immunochromatographic test (ICT) | 2 Conjugate or reagent pad | IgG, 3 ESA | |||
| Avidity test | Tachyzoite lysate antigen, recombinant antigens | IgG (avidity index) | |||
| Antigen Detection | Lateral flow assay | ||||
7. Treatment
7.1. Treatment of Toxoplasmosis in Animals
7.2. Treatment of Toxoplasmosis in Humans
8. Control of Toxoplasmosis
8.1. Vaccines
8.2. Non-Vaccine Prevention Strategies
8.2.1. Food Safety Measures
8.2.2. Personal and Pet Hygiene Practices
8.2.3. Livestock Management Protocols
8.2.4. Feline Management Strategies
8.2.5. One Health Implementation
8.2.6. Environmental Conservation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Splendore, A. Un Nuovo Protozoa Parassita Deconigli Incontrato Nelle Lesioni Anatomiche d’une Malattia Che Ricorda in Molti Punti Il Kala-Azar Dell’uoma. Nota Preliminare Pel. Rev. Soc. Sci. Sao Paulo 1908, 3, 109–112. [Google Scholar]
- Innes, E.A. A Brief History and Overview of Toxoplasma gondii. Zoonoses Public Health 2010, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, T.; Sarvi, S.; Daryani, A. Toxoplasma gondii in Mollusks and Cold-Blooded Animals: A Systematic Review. Parasitology 2021, 148, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.; Kim, K. Toxoplasma gondii: The Model Apicomplexan. Perspectives and Methods, 3rd ed.; Elsevier: London, UK, 2020; ISBN 78-0-12-815041-2. [Google Scholar]
- Dubey, J.P. Toxoplasmosis of Animals and Humans, 3rd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2022; ISBN 978-1-003-19937-3. [Google Scholar]
- Khan, A.; Grigg, M.E. Toxoplasma gondii: Laboratory Maintenance and Growth. Curr. Protoc. Microbiol. 2017, 44, 20C. 1.1–20C. 1.17. [Google Scholar] [CrossRef]
- Rostami, A.; Riahi, S.M.; Contopoulos-Ioannidis, D.G.; Gamble, H.R.; Fakhri, Y.; Shiadeh, M.N.; Foroutan, M.; Behniafar, H.; Taghipour, A.; Maldonado, Y.A. Acute Toxoplasma Infection in Pregnant Women Worldwide: A Systematic Review and Meta-Analysis. PLoS Neglected Trop. Dis. 2019, 13, e0007807. [Google Scholar] [CrossRef]
- Search for Popset Isolate Sequences by Geographic Location. Available online: https://toxodb.org/toxo/app/search/popsetSequence/PopsetByCountry (accessed on 6 June 2025).
- Bouaicha, F.; Amairia, S.; Amdouni, Y.; Elati, K.; Bensmida, B.; Rekik, M.; Gharbi, M. Molecular and Serological Detection of Toxoplasma gondii in Two Species of Rodents: Ctenodactylus Gundi (Rodentia, Ctenodactylidae) and Psammomys Obesus (Rodentia, Muridae) From South Tunisia. Vet. Med. Sci. 2025, 11, e70371. [Google Scholar] [CrossRef]
- Sabin, A.B.; Olitsky, P.K. Toxoplasma and Obligate Intracellular Parasitism. Science 1937, 85, 336–338. [Google Scholar] [CrossRef]
- Wolf, A.; Cowen, D.; Paige, B. Human Toxoplasmosis: Occurrence in Infants as an Encephalomyelitis Verification by Transmission to Animals. Science 1939, 89, 226–227. [Google Scholar] [CrossRef]
- Dubey, J.P. The History of Toxoplasma gondii—The First 100 Years. J. Eukaryot. Microbiol. 2008, 55, 467–475. [Google Scholar] [CrossRef]
- Sokol-Borrelli, S.L.; Reilly, S.M.; Holmes, M.J.; Orchanian, S.B.; Massmann, M.D.; Sharp, K.G.; Cabo, L.F.; Alrubaye, H.S.; Martorelli Di Genova, B.; Lodoen, M.B. A Transcriptional Network Required for Bradyzoite Development in Toxoplasma gondii Is Dispensable for Recrudescent Disease. Nat. Commun. 2023, 14, 6078. [Google Scholar] [CrossRef]
- Hartley, W.J.; Jebson, J.L.; McFarlane, D. New Zealand Type II Abortion in Ewes. Aust. Vet. J. 1954, 30, 216–218. [Google Scholar] [CrossRef]
- Buxton, D. Ovine Toxoplasmosis: A Review. J. R. Soc. Med. 1990, 83, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Julian, A.F. First Report of Acute, Visceral, Fatal Toxoplasmosis in a Naturally Infected Calf (Bos taurus). Vet. Parasitol. 2025, 334, 110373. [Google Scholar] [CrossRef] [PubMed]
- Kimble, K.M.; Gomez, G.; Szule, J.A.; Dubey, J.P.; Buchanan, B.; Porter, B.F. Systemic Toxoplasmosis in a Horse. J. Comp. Pathol. 2021, 182, 27–31. [Google Scholar] [CrossRef]
- Toxoplasma gondii Infection in Horses. A Review—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/18412038/ (accessed on 8 May 2024).
- Jacobs, L.; Remington, J.S.; Melton, M.L. The Resistance of the Encysted Form of Toxoplasma gondii. J. Parasitol. 1960, 46, 11–21. [Google Scholar] [CrossRef]
- Nicolle, C. Sur Une Infection a Corps de Leishman (on Organismes Voisons) Du Gondi. CR Acad. Sci. 1908, 147, 736. [Google Scholar]
- Boothroyd, J.C. Toxoplasma gondii: 25 Years and 25 Major Advances for the Field. Int. J. Parasitol. 2009, 39, 935. [Google Scholar] [CrossRef]
- Goldman, M.; Carver, R.K.; Sulzer, A.J. Reproduction of Toxoplasma gondii by Internal Budding. J. Parasitol. 1958, 44, 161–171. [Google Scholar] [CrossRef]
- Dubey, J.P.; Miller, N.L.; Frenkel, J.K. The Toxoplasma gondii Oocyst from Cat Feces. J. Exp. Med. 1970, 132, 636–662. [Google Scholar] [CrossRef]
- Dubey, J.P.; Frenkel, J.K. Cyst-Induced Toxoplasmosis in Cats. J. Protozool. 1972, 19, 155–177. [Google Scholar] [CrossRef]
- Frenkel, J.K. Toxoplasma in and around Us. BioScience 1973, 23, 343–352. [Google Scholar] [CrossRef]
- Martorelli Di Genova, B.; Wilson, S.K.; Dubey, J.P.; Knoll, L.J. Intestinal Delta-6-Desaturase Activity Determines Host Range for Toxoplasma Sexual Reproduction. PLoS Biol. 2019, 17, e3000364. [Google Scholar] [CrossRef]
- Beverley, J.K.A. Congenital Transmission of Toxoplasmosis through Successive Generations of Mice. Nature 1959, 183, 1348–1349. [Google Scholar] [CrossRef]
- Weinman, D.; Chandler, A.H. Toxoplasmosis in Swine and Rodents; Reciprocal Oral Infection and Potential Human Hazard. Proc. Soc. Exp. Biol. Med. 1954, 87, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, W.M. Experimental Transmission of Toxoplasma gondii. Nature 1965, 206, 961–962. [Google Scholar] [CrossRef] [PubMed]
- Reiter-Owona, I.; Petersen, E.; Joynson, D.; Aspöck, H.; Dardé, M.L.; Disko, R.; Dreazen, O.; Dumon, H.; Grillo, R.; Gross, U.; et al. The Past and Present Role of the Sabin-Feldman Dye Test in the Serodiagnosis of Toxoplasmosis. Bull. World Health Organ. 1999, 77, 929–935. [Google Scholar] [PubMed]
- Eyles, D.E.; Coleman, N. Antibiotics in the Treatment of Toxoplasmosis. Am. J. Trop. Med. Hyg. 1953, 2, 64–69. [Google Scholar] [CrossRef]
- Warren, J.; Sabin, A.B. Effect of Certain Antiprotozoal Drugs on Toxoplasma In Vitro and In Vivo. Proc. Soc. Exp. Biol. Med. 1942, 51, 15–18. Available online: https://journals.sagepub.com/doi/abs/10.3181/00379727-51-13808 (accessed on 7 May 2024). [CrossRef]
- Garin, J.P.; Eyles, D.E. [Spiramycin therapy of experimental toxoplasmosis in mice]. Presse Med. 1958, 66, 957–958. [Google Scholar]
- Araujo, F.G.; Remington, J.S. Effect of Clindamycin on Acute and Chronic Toxoplasmosis in Mice. Antimicrob. Agents Chemother. 1974, 5, 647–651. [Google Scholar] [CrossRef]
- Tabbara, K.F.; O’Connor, G.R. Treatment of Ocular Toxoplasmosis with Clindamycin and Sulfadiazine. Ophthalmology 1980, 87, 129–134. [Google Scholar] [CrossRef]
- Effect on Lambing Percentage of Vaccinating Ewes with Toxoplasma gondii—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/16030917/ (accessed on 7 May 2024).
- Jokelainen, P.; Nylund, M. Acute Fatal Toxoplasmosis in Three Eurasian Red Squirrels (Sciurus Vulgaris) Caused by Genotype II of Toxoplasma gondii. J. Wildl. Dis. 2012, 48, 454–457. [Google Scholar] [CrossRef]
- Anderson, D.C.; McClure, H.M. Acute Disseminated Fatal Toxoplasmosis in a Squirrel Monkey. J. Am. Vet. Med. Assoc. 1982, 181, 1363–1366. [Google Scholar] [CrossRef] [PubMed]
- Riemann, H.P.; Fowler, M.E.; Schulz, T.; Lock, A.; Thilsted, J.; Pulley, L.T.; Henrickson, R.V.; Henness, A.M.; Franti, C.E.; Behymer, D.E. Toxoplasmosis in Pallas Cats. J. Wildl. Dis. 1974, 10, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Howerth, E.W.; Rodenroth, N. Fatal Systemic Toxoplasmosis in a Wild Turkey. J. Wildl. Dis. 1985, 21, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Quinn, W.J.; Weinandy, D. Fatal Neonatal Toxoplasmosis in a Bobcat (Lynx Rufus). J. Wildl. Dis. 1987, 23, 324–327. [Google Scholar] [CrossRef]
- Hartley, W.J.; Dubey, J.P.; Spielman, D.S. Fatal Toxoplasmosis in Koalas (Phascolarctos cinereus). J. Parasitol. 1990, 76, 271–272. [Google Scholar] [CrossRef]
- Migaki, G.; Sawa, T.R.; Dubey, J.P. Fatal Disseminated Toxoplasmosis in a Spinner Dolphin (Stenella longirostris). Vet. Pathol. 1990, 27, 463–464. [Google Scholar] [CrossRef]
- Geisel, O.; Breuer, W.; Minkus, G.; Hermanns, W. [Toxoplasmosis causing death in a mole (Talpa europaea)]. Berl. Munch. Tierarztl. Wochenschr. 1995, 108, 241–243. [Google Scholar]
- Pertz, C.; Dubielzig, R.R.; Lindsay, D.S. Fatal Toxoplasma gondii Infection in Golden Lion Tamarins (Leontopithecus rosalia rosalia). J. Zoo. Wildl. Med. 1997, 28, 491–493. [Google Scholar]
- Mikaelian, I.; Dubey, J.P.; Martineau, D. Severe Hepatitis Resulting from Toxoplasmosis in a Barred Owl (Strix varia) from Québec, Canada. Avian Dis. 1997, 41, 738–740. [Google Scholar] [CrossRef]
- Cole, R.A.; Lindsay, D.S.; Howe, D.K.; Roderick, C.L.; Dubey, J.P.; Thomas, N.J.; Baeten, L.A. Biological and Molecular Characterizations of Toxoplasma gondii Strains Obtained from Southern Sea Otters (Enhydra Lutris Nereis). J. Parasitol. 2000, 86, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Work, T.M.; Massey, J.G.; Rideout, B.A.; Gardiner, C.H.; Ledig, D.B.; Kwok, O.C.; Dubey, J.P. Fatal Toxoplasmosis in Free-Ranging Endangered ’Alala from Hawaii. J. Wildl. Dis. 2000, 36, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Parnell, P.G.; Sreekumar, C.; Vianna, M.C.B.; De Young, R.W.; Dahl, E.; Lehmann, T. Biologic and Molecular Characteristics of Toxoplasma gondii Isolates from Striped Skunk (Mephitis mephitis), Canada Goose (Branta canadensis), Black-Winged Lory (Eos cyanogenia), and Cats (Felis catus). J. Parasitol. 2004, 90, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Forzán, M.J.; Frasca, S. Systemic Toxoplasmosis in a Five-Month-Old Beaver, (Castor canadensis). J. Zoo. Wildl. Med. 2004, 35, 113–115. [Google Scholar] [CrossRef]
- Sedlák, K.; Bártová, E.; Literák, I.; Vodicka, R.; Dubey, J.P. Toxoplasmosis in Nilgais (Boselaphus tragocamelus) and a Saiga Antelope (Saiga tatarica). J. Zoo. Wildl. Med. 2004, 35, 530–533. [Google Scholar] [CrossRef]
- Szabo, K.A.; Mense, M.G.; Lipscomb, T.P.; Felix, K.J.; Dubey, J.P. Fatal Toxoplasmosis in a Bald Eagle (Haliaeetus leucocephalus). J. Parasitol. 2004, 90, 907–908. [Google Scholar] [CrossRef]
- Honnold, S.P.; Braun, R.; Scott, D.P.; Sreekumar, C.; Dubey, J.P. Toxoplasmosis in a Hawaiian Monk Seal (Monachus schauinslandi). J. Parasitol. 2005, 91, 695–697. [Google Scholar] [CrossRef]
- Gerhold, R.W.; Howerth, E.W.; Lindsay, D.S. Sarcocystis Neurona-Associated Meningoencephalitis and Description of Intramuscular Sarcocysts in a Fisher (Martes pennanti). J. Wildl. Dis. 2005, 41, 224–230. [Google Scholar] [CrossRef]
- Lloyd, C.; Stidworthy, M.F. Acute Disseminated Toxoplasmosis in a Juvenile Cheetah (Acinonyx Jubatus). J. Zoo. Wildl. Med. 2007, 38, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Bangari, D.S.; Mouser, P.; Miller, M.A.; Stevenson, G.W.; Vemulapalli, R.; Thacker, H.L. Toxoplasmosis in a Woodchuck (Marmota monax) and Two American Red Squirrels (Tamiasciurus hudsonicus). J. Vet. Diagn. Investig. 2007, 19, 705–709. [Google Scholar] [CrossRef]
- Pas, A.; Dubey, J.P. Fatal Toxoplasmosis in Sand Cats (Felis margarita). J. Zoo. Wildl. Med. 2008, 39, 362–369. [Google Scholar] [CrossRef]
- Pas, A.; Dubey, J.P. Seroprevalence of Antibodies to Toxoplasma gondii in Gordon’s Wildcat (Felis Silvestris Gordoni) in the Middle East. J. Parasitol. 2008, 94, 1169. [Google Scholar] [CrossRef]
- Las, R.D.; Shivaprasad, H.L. An Outbreak of Toxoplasmosis in an Aviary Collection of Nicobar Pigeons (Caloenas Nicobaria). J. S. Afr. Vet. Assoc. 2008, 79, 149–152. [Google Scholar]
- Pena, H.F.J.; Marvulo, M.F.V.; Horta, M.C.; Silva, M.A.; Silva, J.C.R.; Siqueira, D.B.; Lima, P.-A.C.P.; Vitaliano, S.N.; Gennari, S.M. Isolation and Genetic Characterisation of Toxoplasma gondii from a Red-Handed Howler Monkey (Alouatta belzebul), a Jaguarundi (Puma yagouaroundi), and a Black-Eared Opossum (Didelphis aurita) from Brazil. Vet. Parasitol. 2011, 175, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Ploeg, M.; Ultee, T.; Kik, M. Disseminated Toxoplasmosis in Black-Footed Penguins (Spheniscus demersus). Avian Dis. 2011, 55, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Sangster, C.R.; Gordon, A.N.; Hayes, D. Systemic Toxoplasmosis in Captive Flying-Foxes. Aust. Vet. J. 2012, 90, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Mancianti, F.; Nardoni, S.; Mugnaini, L.; Poli, A. Toxoplasma gondii in Waterfowl: The First Detection of This Parasite in Anas Crecca and Anas Clypeata from Italy. J. Parasitol. 2013, 99, 561–563. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Z.; Wang, C.; Li, C.; Wei, F.; Liu, Q. Fatal Toxoplasma gondii Infection in the Giant Panda. Parasite 2015, 22, 30. [Google Scholar] [CrossRef]
- Donahoe, S.L.; Šlapeta, J.; Knowles, G.; Obendorf, D.; Peck, S.; Phalen, D.N. Clinical and Pathological Features of Toxoplasmosis in Free-Ranging Common Wombats (Vombatus ursinus) with Multilocus Genotyping of Toxoplasma gondii Type II-like Strains. Parasitol. Int. 2015, 64, 148–153. [Google Scholar] [CrossRef]
- Fayyad, A.; Kummerfeld, M.; Davina, I.; Wohlsein, P.; Beineke, A.; Baumgärtner, W.; Puff, C. Fatal Systemic Toxoplasma gondii Infection in a Red Squirrel (Sciurus vulgaris), a Swinhoe’s Striped Squirrel (Tamiops swinhoei) and a New World Porcupine (Erethizontidae sp.). J. Comp. Pathol. 2016, 154, 263–267. [Google Scholar] [CrossRef]
- Torres-Castro, M.; Noh-Pech, H.; Puerto-Hernández, R.; Reyes-Hernández, B.; Panti-May, A.; Hernández-Betancourt, S.; Yeh-Gorocica, A.; González-Herrera, L.; Zavala-Castro, J.; Puerto, F. First Molecular Evidence of Toxoplasma gondii in Opossums (Didelphis Virginiana) from Yucatan, Mexico. Open Vet. J. 2016, 6, 57. [Google Scholar] [CrossRef]
- Kumar, A.; Melotti, J.R.; Cooley, T.M.; Fitzgerald, S.D. Mortality Due to Toxoplasmosis in Suburban Eastern Fox Squirrels (Sciurus Niger) in Michigan, USA. J. Wildl. Dis. 2019, 55, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Toxoplasma gondii Infections in Captive Black-Footed Ferrets (Mustela nigripes), 1992–1998: Clinical Signs, Serology, Pathology, and Prevention—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/14733273/ (accessed on 8 May 2024).
- Bonser, G.M. Fatal Intussusception of the Canine Caecum Associated with the Presence of Toxoplasma in the Circular Muscle of the Ileum. J. Pathol. Bacteriol. 1950, 62, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Toxoplasmosis In Mink—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1614573/ (accessed on 8 May 2024).
- Reed, W.M.; Turek, J.J. Concurrent Distemper and Disseminated Toxoplasmosis in a Red Fox. J. Am. Vet. Med. Assoc. 1985, 187, 1264–1265. [Google Scholar] [CrossRef] [PubMed]
- Farrell, R.L.; Docton, F.L.; Chamberlain, D.M.; Cole, C.R. Toxoplasmosis. I. Toxoplasma Isolated from Swine. Am. J. Vet. Res. 1952, 13, 181–185. [Google Scholar]
- Hartley, W.J.; Marshall, S.C. Toxoplasmosis as a Cause of Ovine Perinatal Mortality. N. Z. Vet. J. 1957, 5, 119–124. [Google Scholar] [CrossRef]
- Pande, P.G.; Shukla, R.R.; Sekariah, P.C. Toxoplasma from the Eggs of the Domestic Fowl (Gallus Gallus). Science 1961, 133, 648. [Google Scholar] [CrossRef]
- Munday, B.L.; Mason, R.W. Toxoplasmosis as a Cause of Perinatal Death in Goats. Aust. Vet. J. 1979, 55, 485–487. [Google Scholar] [CrossRef]
- Hagemoser, W.A.; Dubey, J.P.; Thompson, J.R. Acute Toxoplasmosis in a Camel. J. Am. Vet. Med. Assoc. 1990, 196, 347. [Google Scholar] [CrossRef]
- Dubey, J.P.; Graham, D.H.; Dahl, E.; Hilali, M.; El-Ghaysh, A.; Sreekumar, C.; Kwok, O.C.H.; Shen, S.K.; Lehmann, T. Isolation and Molecular Characterization of Toxoplasma gondii from Chickens and Ducks from Egypt. Vet. Parasitol. 2003, 114, 89–95. [Google Scholar] [CrossRef]
- Dubey, J.P.; Casey, S.J.; Zajac, A.M.; Wildeus, S.A.; Lindsay, D.S.; Verma, S.K.; Oliveira, S.; Kwok, O.C.H.; Su, C. Isolation and Genetic Characterization of Toxoplasma gondii from Alpaca (Vicugna Pacos) and Sheep (Ovis Aries). Trop. Anim. Health Prod. 2014, 46, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Sarkari, B.; Asgari, Q.; Bagherian, N.; Ashkani Esfahani, S.; Kalantari, M.; Mohammadpour, I.; Ashrafmansori, M.; Amerinia, M.; Sabet Sarvestani, F. Molecular and Serological Evaluation of Toxoplasma gondii Infection in Reared Turkeys in Fars Province, Iran. Jundishapur J. Microbiol. 2014, 7, e11598. [Google Scholar] [CrossRef]
- Rong, G.; Zhou, H.-L.; Hou, G.-Y.; Zhao, J.-M.; Xu, T.-S.; Guan, S. Seroprevalence, Risk Factors and Genotyping of Toxoplasma gondii in Domestic Geese (Anser Domestica) in Tropical China. Parasit. Vectors 2014, 7, 459. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, J.; Hajipour, N.; Hassanzadeh, P.; Ketzisc, J. Detection of Toxoplasma gondii Infection in Buffaloes (Bubalus Bubalis) and Cattle (Bos taurus) at the Tabriz Abattoir, Iran. Vet. Med. Sci. 2024, 10, e1511. [Google Scholar] [CrossRef]
- Edwards, J.F.; Dubey, J.P. Toxoplasma gondii Abortion Storm in Sheep on a Texas Farm and Isolation of Mouse Virulent Atypical Genotype T. Gondii from an Aborted Lamb from a Chronically Infected Ewe. Vet. Parasitol. 2013, 192, 129–136. [Google Scholar] [CrossRef]
- Fernández-Escobar, M.; Calero-Bernal, R.; Benavides, J.; Regidor-Cerrillo, J.; Guerrero-Molina, M.C.; Gutiérrez-Expósito, D.; Collantes-Fernández, E.; Ortega-Mora, L.M. Isolation and Genetic Characterization of Toxoplasma gondii in Spanish Sheep Flocks. Parasites Vectors 2020, 13, 396. [Google Scholar] [CrossRef]
- Gabardo, M.; Oliveira, J.; Ecco, R.; Guedes, R. Outbreak of Ovine Abortion by Toxoplasmosis in Southeastern Brazil. Braz. J. Vet. Pathol. 2013, 6, 37–41. [Google Scholar]
- Gutiérrez-Expósito, D.; Tejerina, F.; Gutiérrez, J.; Fernández-Escobar, M.; Ortega-Mora, L.M.; Mantecón, A.R.; Dagleish, M.P.; Pérez, V.; Benavides, J. Direct Economic Losses of Toxoplasma gondii Abortion Outbreaks in Two Spanish Sheep Flocks. Vet. Parasitol. Reg. Stud. Rep. 2021, 26, 100623. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Yu, F.; Li, T.; Zhang, D. An Outbreak of Lethal Toxoplasmosis in Pigs in the Gansu Province of China. J. Vet. Diagn. Investig. 2010, 22, 442–444. [Google Scholar] [CrossRef]
- Gelmetti, D.; Sironi, G.; Finazzi, M.; Gelmini, L.; Rosignoli, C.; Cordioli, P.; Lavazza, A. Diagnostic Investigations of Toxoplasmosis in Four Swine Herds. J. Vet. Diagn. Investig. 1999, 11, 87–90. [Google Scholar] [CrossRef]
- Roe, W.D.; Howe, L.; Baker, E.J.; Burrows, L.; Hunter, S.A. An Atypical Genotype of Toxoplasma gondii as a Cause of Mortality in Hector’s Dolphins (Cephalorhynchus hectori). Vet. Parasitol. 2013, 192, 67–74. [Google Scholar] [CrossRef]
- Dubey, J.P.; Mergl, J.; Gehring, E.; Sundar, N.; Velmurugan, G.V.; Kwok, O.C.H.; Grigg, M.E.; Su, C.; Martineau, D. Toxoplasmosis in Captive Dolphins (Tursiops truncatus) and Walrus (Odobenus rosmarus). J. Parasitol. 2009, 95, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.M.; Kashinsky, L.; Rotstein, D.S.; Colegrove, K.M.; Haman, K.H.; Magargal, S.L.; Sweeny, A.R.; Kaufman, A.C.; Grigg, M.E.; Littnan, C.L. Protozoal-Related Mortalities in Endangered Hawaiian Monk Seals Neomonachus Schauinslandi. Dis. Aquat. Organ. 2016, 121, 85–95. [Google Scholar] [CrossRef]
- McAllister, R.A. An Outbreak of Toxoplasmosis in an Ontario Chinchilla Herd. Can. J. Comp. Med. Vet. Sci. 1964, 28, 53–56. [Google Scholar] [PubMed]
- Burns, R.; Williams, E.S.; O’Toole, D.; Dubey, J.P. Toxoplasma gondii Infection in Captive Black-Footed Ferrets (Mustela nigripes), 1992-1998: Clinical Signs, Serology, Pathology, and Pevention. J. Wildl. Dis. 2003, 39, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Sós, E.; Szigeti, A.; Fok, E.; Molnár, V.; Erdélyi, K.; Perge, E.; Biksi, I.; Gál, J. Toxoplasmosis in Tammar Wallabies (Macropus Eugenii) in the Budapest Zoo and Botanical Garden (2006–2010). Acta Vet. Hung. 2012, 60, 361–370. [Google Scholar] [CrossRef]
- Guthrie, A.; Rooker, L.; Tan, R.; Gerhold, R.; Trainor, K.; Jiang, T.; Su, C. Newly Described Toxoplasma gondii Strain Causes High Mortality in Red Necked Wallabies (Macropus rufogriseus) in a Zoo. J. Zoo. Wildl. Med. 2017, 48, 694–702. [Google Scholar] [CrossRef]
- Verma, S.K.; Knowles, S.; Cerqueira-Cézar, C.K.; Kwok, O.C.; Jiang, T.; Su, C.; Dubey, J.P. An Update on Toxoplasma gondii Infections in Northern Sea Otters (Enhydra Lutris Kenyoni) from Washington State, USA. Vet. Parasitol. 2018, 258, 133–137. [Google Scholar] [CrossRef]
- Frank, R.K. An Outbreak of Toxoplasmosis in Farmed Mink (Mustela Vison S.). J. Vet. Diagn. Investig. 2001, 13, 245–249. [Google Scholar] [CrossRef]
- Bouer, A.; Werther, K.; Catão-Dias, J.L.; Nunes, A.L.V. Outbreak of Toxoplasmosis in Lagothrix Lagotricha. IJFP 1999, 70, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Carme, B.; Ajzenberg, D.; Demar, M.; Simon, S.; Dardé, M.L.; Maubert, B.; de Thoisy, B. Outbreaks of Toxoplasmosis in a Captive Breeding Colony of Squirrel Monkeys. Vet. Parasitol. 2009, 163, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Santana, C.H.; de Oliveira, A.R.; Dos Santos, D.O.; Pimentel, S.P.; de Souza, L.D.R.; Moreira, L.G.A.; Braz, H.M.B.; de Carvalho, T.P.; Lopes, C.E.B.; Oliveira, J.B.S.; et al. Genotyping of Toxoplasma gondii in a Lethal Toxoplasmosis Outbreak Affecting Captive Howler Monkeys (Alouatta Sp.). J. Med. Primatol. 2021, 50, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.C.; Hartley, W.J.; Mason, R.W.; Dubey, J.P.; Schollam, L. Blindness Associated with Toxoplasmosis in Canaries. J. Am. Vet. Med. Assoc. 1992, 200, 1723–1725. [Google Scholar] [CrossRef]
- Vielmo, A.; Pena, H.F.J.; Panziera, W.; Bianchi, R.M.; De Lorenzo, C.; Oliveira, S.; Alves, B.F.; Gennari, S.M.; Pavarini, S.P.; de Barros, C.S.L.; et al. Outbreak of Toxoplasmosis in a Flock of Domestic Chickens (Gallus Gallus Domesticus) and Guinea Fowl (Numida Meleagris). Parasitol. Res. 2019, 118, 991–997. [Google Scholar] [CrossRef]
- Erichsen, S.; Harboe, A. Toxoplasmosis in Chickens: I. An Epidemic Outbreak of Toxoplasmosis in a Chicken Flock in South-Eastern Norway. Acta Pathol. Microbiol. Scand. 1953, 33, 56–71. [Google Scholar] [CrossRef]
- Shimizu, K. Studies on Toxoplasmosis I: An Outbreak of Toxoplasmosis among Hares (Lepus Timidus Ainu) in Sapporo. Jpn. J. Vet. Res. 1958, 6, 157–166. [Google Scholar] [CrossRef]
- Oh, H.; Eo, K.; Gumber, S.; Hong, J.J.; Kim, C.; Lee, H.; Jung, Y.; Kim, J.; Whang, G.; Lee, J.; et al. An Outbreak of Toxoplasmosis in Squirrel Monkeys (Saimiri sciureus) in South Korea. J. Med. Primatol. 2018, 47, 238–246. [Google Scholar] [CrossRef]
- Nishimura, M.; Goyama, T.; Tomikawa, S.; Fereig, R.M.; El-Alfy, E.-S.N.; Nagamune, K.; Kobayashi, Y.; Nishikawa, Y. Outbreak of Toxoplasmosis in Four Squirrel Monkeys (Saimiri sciureus) in Japan. Parasitol. Int. 2019, 68, 79–86. [Google Scholar] [CrossRef]
- English, E.D.; Striepen, B. The Cat Is out of the Bag: How Parasites Know Their Hosts. PLoS Biol. 2019, 17, e3000446. [Google Scholar] [CrossRef]
- Sasai, M.; Yamamoto, M. Innate, Adaptive, and Cell-Autonomous Immunity against Toxoplasma gondii Infection. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Kumar, P.; Tomita, T.; Gerken, T.A.; Ballard, C.J.; Lee, Y.S.; Weiss, L.M.; Samara, N.L. A Toxoplasma gondii O-Glycosyltransferase That Modulates Bradyzoite Cyst Wall Rigidity Is Distinct from Host Homologues. Nat. Commun. 2024, 15, 3792. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.E.; McLeod, R.; Roberts, C.W. Toxoplasma gondii Tachyzoite-Bradyzoite Interconversion. Trends Parasitol. 2002, 18, 198–201. [Google Scholar] [CrossRef]
- Dubey, J.P. Schizogony and Gametogony of Oocyst-Deficient T-263 Strain of Toxoplasma gondii. Vet. Parasitol. 2017, 245, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Benavides, J.; Maley, S.; Pang, Y.; Palarea, J.; Eaton, S.; Katzer, F.; Innes, E.A.; Buxton, D.; Chianini, F. Development of Lesions and Tissue Distribution of Parasite in Lambs Orally Infected with Sporulated Oocysts of Toxoplasma gondii. Vet. Parasitol. 2011, 179, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental Transmission of Toxoplasma gondii: Oocysts in Water, Soil and Food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef]
- Attias, M.; Teixeira, D.E.; Benchimol, M.; Vommaro, R.C.; Crepaldi, P.H.; De Souza, W. The Life-Cycle of Toxoplasma gondii Reviewed Using Animations. Parasites Vectors 2020, 13, 588. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lindsay, D.S.; Speer, C. Structures of Toxoplasma gondii Tachyzoites, Bradyzoites, and Sporozoites and Biology and Development of Tissue Cysts. Clin. Microbiol. Rev. 1998, 11, 267–299. [Google Scholar] [CrossRef]
- Boucher, L.E.; Bosch, J. The Apicomplexan Glideosome and Adhesins—Structures and Function. J. Struct. Biol. 2015, 190, 93–114. [Google Scholar] [CrossRef]
- Kumar, A.; Vadas, O.; Dos Santos Pacheco, N.; Zhang, X.; Chao, K.; Darvill, N.; Rasmussen, H.Ø.; Xu, Y.; Lin, G.M.-H.; Stylianou, F.A.; et al. Structural and Regulatory Insights into the Glideosome-Associated Connector from Toxoplasma gondii. eLife 2023, 12, e86049. [Google Scholar] [CrossRef]
- Jacot, D.; Frénal, K.; Marq, J.-B.; Sharma, P.; Soldati-Favre, D. Assessment of Phosphorylation in Toxoplasma Glideosome Assembly and Function. Cell Microbiol. 2014, 16, 1518–1532. [Google Scholar] [CrossRef]
- Opitz, C.; Soldati, D. “The Glideosome”: A Dynamic Complex Powering Gliding Motion and Host Cell Invasion by Toxoplasma gondii. Mol. Microbiol. 2002, 45, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Keeley, A.; Soldati, D. The Glideosome: A Molecular Machine Powering Motility and Host-Cell Invasion by Apicomplexa. Trends Cell Biol. 2004, 14, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Heintzelman, M.B. Gliding Motility in Apicomplexan Parasites. Semin. Cell Dev. Biol. 2015, 46, 135–142. [Google Scholar] [CrossRef]
- Kato, K. How Does Toxoplama Gondii Invade Host Cells? J. Vet. Med. Sci. 2018, 80, 1702–1706. [Google Scholar] [CrossRef]
- Nasiru Wana, M.; Mohd Moklas, M.A.; Watanabe, M.; Nordin, N.; Zasmy Unyah, N.; Alhassan Abdullahi, S.; Ahmad Issa Alapid, A.; Mustapha, T.; Basir, R.; Roslaini, A.M. A Review on the Prevalence of Toxoplasma gondii in Humans and Animals Reported in Malaysia from 2008–2018. Int. J. Environ. Res. Public Health 2020, 17, 4809. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shi, Y.; Xue, Y.; Hu, D.; Song, X. AP2XII-1 and AP2XI-2 Suppress Schizogony Gene Expression in Toxoplasma gondii. Int. J. Mol. Sci. 2024, 25, 5527. [Google Scholar] [CrossRef]
- Tomasina, R.; Francia, M.E. The Structural and Molecular Underpinnings of Gametogenesis in Toxoplasma gondii. Front. Cell Infect. Microbiol. 2020, 10, 608291. [Google Scholar] [CrossRef]
- Ferguson, D.J.; Hutchison, W.M.; Siim, J.C. The Ultrastructural Development of the Macrogamete and Formation of the Oocyst Wall of Toxoplasma gondii. Acta Pathol. Microbiol. Scand. B 1975, 83, 491–505. [Google Scholar] [CrossRef]
- Dubey, J.P.; Cerqueira-Cézar, C.K.; Murata, F.H.A.; Kwok, O.C.H.; Yang, Y.R.; Su, C. All about Toxoplasmosis in Cats: The Last Decade. Vet. Parasitol. 2020, 283, 109145. [Google Scholar] [CrossRef]
- Hatam-Nahavandi, K.; Calero-Bernal, R.; Rahimi, M.T.; Pagheh, A.S.; Zarean, M.; Dezhkam, A.; Ahmadpour, E. Toxoplasma gondii Infection in Domestic and Wild Felids as Public Health Concerns: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 9509. [Google Scholar] [CrossRef]
- Dubey, J.P.; Ferreira, L.R.; Martins, J.; Jones, J.L. Sporulation and Survival of Toxoplasma gondii Oocysts in Different Types of Commercial Cat Litter. J. Parasitol. 2011, 97, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.J.; Birch-Andersen, A.; Siim, J.C.; Hutchison, W.M. Ultrastructural Studies on the Sporulation of Oocysts of Toxoplasma gondii. I. Development of the Zygote and Formation of the Sporoblasts. Acta Pathol. Microbiol. Scand. B 1979, 87B, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Dubey, J.P. Toxoplasma gondii: Transmission, Diagnosis and Prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Deganich, M.; Boudreaux, C.; Benmerzouga, I. Toxoplasmosis Infection during Pregnancy. Trop. Med. Infect. Dis. 2022, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Holec-Gąsior, L.; Sołowińska, K. Detection of Toxoplasma gondii Infection in Small Ruminants: Old Problems, and Current Solutions. Animals 2023, 13, 2696. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M.; Zhang, Y. Toxoplasma gondii Secretory Proteins and Their Role in Invasion and Pathogenesis. Microbiol. Res. 2019, 227, 126293. [Google Scholar] [CrossRef]
- Malkwitz, I.; Berndt, A.; Zhang, R.; Daugschies, A.; Bangoura, B. Replication of Toxoplasma gondii in Chicken Erythrocytes and Thrombocytes Compared to Macrophages. Parasitol. Res. 2017, 116, 123–131. [Google Scholar] [CrossRef]
- Clough, B.; Frickel, E.-M. The Toxoplasma Parasitophorous Vacuole: An Evolving Host-Parasite Frontier. Trends Parasitol. 2017, 33, 473–488. [Google Scholar] [CrossRef]
- Zhang, N.-Z.; Chen, J.; Wang, M.; Petersen, E.; Zhu, X.-Q. Vaccines against Toxoplasma gondii: New Developments and Perspectives. Expert Rev. Vaccines 2013, 12, 1287–1299. [Google Scholar] [CrossRef]
- Tomita, T.; Guevara, R.B.; Shah, L.M.; Afrifa, A.Y.; Weiss, L.M. Secreted Effectors Modulating Immune Responses to Toxoplasma gondii. Life 2021, 11, 988. [Google Scholar] [CrossRef]
- Castaño, P.; Fernández, M.; Regidor-Cerrillo, J.; Fuertes, M.; Horcajo, P.; Ferre, I.; Ferreras, M.C.; Ortega-Mora, L.M.; Pérez, V.; Benavides, J. Peripheral and Placental Immune Responses in Sheep after Experimental Infection with Toxoplasma gondii at the Three Terms of Gestation. Vet. Res. 2019, 50, 66. [Google Scholar] [CrossRef]
- Sana, M.; Rashid, M.; Rashid, I.; Akbar, H.; Gomez-Marin, J.E.; Dimier-Poisson, I. Immune Response against Toxoplasmosis—Some Recent Updates RH: Toxoplasma gondii Immune Response. Int. J. Immunopathol. Pharmacol. 2022, 36, 03946320221078436. [Google Scholar] [CrossRef]
- Lima, T.S.; Lodoen, M.B. Mechanisms of Human Innate Immune Evasion by Toxoplasma gondii. Front. Cell Infect. Microbiol. 2019, 9, 103. [Google Scholar] [CrossRef]
- Sasai, M.; Pradipta, A.; Yamamoto, M. Host Immune Responses to Toxoplasma gondii. Int. Immunol. 2018, 30, 113–119. [Google Scholar] [CrossRef]
- Sanchez, S.G.; Besteiro, S. The Pathogenicity and Virulence of Toxoplasma gondii. Virulence 2021, 12, 3095–3114. [Google Scholar] [CrossRef] [PubMed]
- Bando, H.; Murata, Y.; Han, Y.; Sugi, T.; Fukuda, Y.; Bzik, D.J.; Fox, B.A.; Kato, K. Toxoplasma gondii Chitinase-like Protein TgCLP1 Regulates the Parasite Cyst Burden. Front. Cell Infect. Microbiol. 2024, 14, 1359888. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xin, S.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Su, C.; Dubey, J.P. Recent Epidemiologic, Clinical, Subclinical and Genetic Diversity of Toxoplasma gondii Infections in Bats. Res. Vet. Sci. 2021, 140, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.; Webster, J.P.; Walker, M. Toxoplasma gondii: AnUnderestimated Threat? Trends Parasitol. 2020, 36, 959–969. [Google Scholar] [CrossRef]
- Henriquez, S.A.; Brett, R.; Alexander, J.; Pratt, J.; Roberts, C.W. Neuropsychiatric Disease and Toxoplasma gondii Infection. Neuroimmunomodulation 2009, 16, 122–133. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, J.; Zhang, C.; Peng, H. Pathological Mechanisms of Glial Cell Activation and Neurodegenerative and Neuropsychiatric Disorders Caused by Toxoplasma gondii Infection. Front. Microbiol. 2024, 15, 1512233. [Google Scholar] [CrossRef]
- Tyebji, S.; Seizova, S.; Hannan, A.J.; Tonkin, C.J. Toxoplasmosis: A Pathway to Neuropsychiatric Disorders. Neurosci. Biobehav. Rev. 2019, 96, 72–92. [Google Scholar] [CrossRef]
- Tong, W.H.; Pavey, C.; O’Handley, R.; Vyas, A. Behavioral Biology of Toxoplasma gondii Infection. Parasit. Vectors 2021, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A. Mechanisms of Host Behavioral Change in Toxoplasma gondii Rodent Association. PLoS Pathog. 2015, 11, e1004935. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Xia, N.; Zhou, T.; Shen, B. Antitumor Effects of a Toxoplasma Mutant Lacking Lactate Dehydrogenases. Parasitol. Res. 2021, 120, 3335–3339. [Google Scholar] [CrossRef] [PubMed]
- Involvement of MyD88 in Host Defense and the Down-Regulation of Anti-Heat Shock Protein 70 Autoantibody Formation by MyD88 in Toxoplasma gondii-Infected Mice—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12435148/ (accessed on 7 May 2024).
- T Cell Suppression in Vitro During Toxoplasma gondii Infection Is the Result of IL-2 Competition Between Tregs and T Cells Leading to Death of Proliferating T Cells—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/24117537/ (accessed on 7 May 2024).
- The Murine C-Rel Proto-Oncogene Encodes Two mRNAs the Expression of Which Is Modulated by Lymphoid Stimuli—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/2204017/ (accessed on 7 May 2024).
- Akbar, H.; Dimier-Poisson, I.; Moiré, N. Role of CD4+ Foxp3+ Regulatory T Cells in Protection Induced by a Live Attenuated, Replicating Type I Vaccine Strain of Toxoplasma gondii. Infect. Immun. 2015, 83, 3601–3611. [Google Scholar] [CrossRef]
- Mitchell, D.M.; Ravkov, E.V.; Williams, M.A. Distinct Roles for IL-2 and IL-15 in the Differentiation and Survival of CD8+ Effector and Memory T Cells. J. Immunol. 2010, 184, 6719–6730. [Google Scholar] [CrossRef]
- Benedetto, N.; Auriault, C.; Darcy, F.; Lando, D.; Watier, H.; Capron, A. Effect of rIFN-Gamma and IL-2 Treatments in Mouse and Nude Rat Infections with Toxoplasma gondii. Eur. Cytokine Netw. 1991, 2, 107–114. [Google Scholar]
- A Single Polymorphic Amino Acid on Toxoplasma gondii Kinase ROP16 Determines the Direct and Strain-Specific Activation of Stat3—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/19901082/ (accessed on 7 May 2024).
- Possibilities for Immunomodulation in Congenital Toxoplasmosis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27357341/ (accessed on 7 May 2024).
- TGF-Β1 Levels and Intraocular Tissue Alterations in Mice Infected with a Virulent Type I RH Toxoplasma gondii Strain—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26773166/ (accessed on 7 May 2024).
- The Role of IL-27 in the Development of T-Cell Responses during Parasitic Infections—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/15546389/ (accessed on 7 May 2024).
- IL-6-Dependent Spontaneous Proliferation Is Required for the Induction of Colitogenic IL-17-Producing CD8+ T Cells—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/18426983/ (accessed on 7 May 2024).
- Transforming Growth Factor-Beta Inhibits Interleukin-12-Induced Production of Interferon-Gamma by Natural Killer Cells: A Role for Transforming Growth Factor-Beta in the Regulation of T Cell-Independent Resistance to Toxoplasma gondii—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/7737303/ (accessed on 7 May 2024).
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 Drives a Pathogenic T Cell Population That Induces Autoimmune Inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef]
- Tong, X.; Chen, S.; Zheng, H.; Huang, S.; Lu, F. Increased IL-27/IL-27R Expression in Association with the Immunopathology of Murine Ocular Toxoplasmosis. Parasitol. Res. 2018, 117, 2255–2263. [Google Scholar] [CrossRef]
- Clark, J.T.; Christian, D.A.; Gullicksrud, J.A.; Perry, J.A.; Park, J.; Jacquet, M.; Tarrant, J.C.; Radaelli, E.; Silver, J.; Hunter, C.A. IL-33 Promotes Innate Lymphoid Cell-Dependent IFN-γ Production Required for Innate Immunity to Toxoplasma gondii. Elife 2021, 10, e65614. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, H.; Liu, X.; Jiang, Y.; Ren, L.; Hu, X. The Effect of TGF-β on Treg Cells in Adverse Pregnancy Outcome upon Toxoplasma gondii Infection. Front. Microbiol. 2017, 8, 901. [Google Scholar] [CrossRef] [PubMed]
- Ayub, I.; Sani, S.S.; Zaman, A.; Shahid, M.R.; Bashir, W.; Ijaz, M.; Zafar, S.; Rafique, A. Prevalence of Toxoplasma gondii in Human and Animals and Its Economic Impacts: A Review. Pak. Veter-J. 2024, 45, 1–9. [Google Scholar] [CrossRef]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From Animals to Humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef]
- Döşkaya, M.; Caner, A.; Ajzenberg, D.; Değirmenci, A.; Dardé, M.-L.; Can, H.; Erdoğan, D.D.; Korkmaz, M.; Üner, A.; Güngör, Ç. Isolation of Toxoplasma gondii Strains Similar to Africa 1 Genotype in Turkey. Parasitol. Int. 2013, 62, 471–474. [Google Scholar] [CrossRef]
- Molan, A.; Nosaka, K.; Hunter, M.; Wang, W. Global Status of Toxoplasma gondii Infection: Systematic Review and Prevalence Snapshots. Trop. Biomed. 2019, 36, 898–925. [Google Scholar]
- Ceylan, C.; Sevinc, F.; Ceylan, O. Serostatus of Small Ruminant Toxoplasmosis and Neosporosis throughout the Southeastern Anatolia Region of Türkiye. Pak. Vet. J. 2024, 44, 917–923. [Google Scholar] [CrossRef]
- Jokelainen, P.; Murat, J.B.; Nielsen, H.V. Direct Genetic Characterization of Toxoplasma gondii from Clinical Samples from Denmark: Not Only Genotypes II and III. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 579–586. [Google Scholar] [CrossRef]
- Kalogeropoulos, D.; Sakkas, H.; Mohammed, B.; Vartholomatos, G.; Malamos, K.; Sreekantam, S.; Kanavaros, P.; Kalogeropoulos, C. Ocular Toxoplasmosis: A Review of the Current Diagnostic and Therapeutic Approaches. Int. Ophthalmol. 2022, 42, 295–321. [Google Scholar] [CrossRef]
- Fabiani, S.; Caroselli, C.; Menchini, M.; Gabbriellini, G.; Falcone, M.; Bruschi, F. Ocular Toxoplasmosis, an Overview Focusing on Clinical Aspects. Acta Trop. 2022, 225, 106180. [Google Scholar] [CrossRef]
- Calero-Bernal, R.; Gennari, S.M. Clinical Toxoplasmosis in Dogs and Cats: An Update. Front. Vet. Sci. 2019, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.Q.; Casadevall, A. On the Relationship between Pathogenic Potential and Infective Inoculum. PLoS Pathog. 2022, 18, e1010484. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Horáček, J. Negative Effects of Latent Toxoplasmosis on Mental Health. Front. Psychiatry 2020, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Al Malki, J.S.; Hussien, N.A.; Al Malki, F. Maternal Toxoplasmosis and the Risk of Childhood Autism: Serological a Nd Molecular Small-Scale Studies. BMC Pediatr. 2021, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.A.; Gad, N.; Koren, G. Toxoplasmosis and Pregnancy. Can. Fam. Physician 2014, 60, 334–336. [Google Scholar]
- Hampton, M.M. Congenital Toxoplasmosis: A Review. Neonatal Netw. 2015, 34, 274–278. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Dardé, M.-L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef]
- Bollani, L.; Auriti, C.; Achille, C.; Garofoli, F.; De Rose, D.U.; Meroni, V.; Salvatori, G.; Tzialla, C. Congenital Toxoplasmosis: The State of the Art. Front. Pediatr. 2022, 10, 894573. [Google Scholar] [CrossRef]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Villena, I. Congenital Toxoplasmosis in Humans: An Update of Worldwide Rate of Congenital Infections. Parasitology 2021, 148, 1406–1416. [Google Scholar] [CrossRef]
- Blanchard, N.; Dunay, I.R.; Schlüter, D. Persistence of Toxoplasma gondii in the Central Nervous System: A Fine-tuned Balance between the Parasite, the Brain and the Immune System. Parasite Immunol. 2015, 37, 150–158. [Google Scholar] [CrossRef]
- Kong, P.; Gupta, N. Phospholipid Biogenesis in the Apicomplexan Parasites Eimeria Falciformis and Toxoplasma gondii. Ph.D. Thesis, Humboldt-Universität zu Berlin, Berlin, Germany, 2017. [Google Scholar]
- Nitzsche, R.; Günay-Esiyok, Ö.; Tischer, M.; Zagoriy, V.; Gupta, N. A Plant/Fungal-Type Phosphoenolpyruvate Carboxykinase Located in the Parasite Mitochondrion Ensures Glucose-Independent Survival of Toxoplasma gondii. J. Biol. Chem. 2017, 292, 15225–15239. [Google Scholar] [CrossRef] [PubMed]
- Wallon, M.; Liou, C.; Garner, P.; Peyron, F. Congenital Toxoplasmosis: Systematic Review of Evidence of Efficacy of Treatment in Pregnancy. Bmj 1999, 318, 1511–1514. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, M.; Zong, Y.; Yang, M.; Zhang, J.; Zou, Y.; Ohno-Matsui, K.; Kamoi, K. Ocular Toxoplasmosis: Advances in Toxoplasma gondii Biology, Clinical Manifestations, Diagnostics, and Therapy. Pathogens 2024, 13, 898. [Google Scholar] [CrossRef]
- Smith, J.R.; Ashander, L.M.; Arruda, S.L.; Cordeiro, C.A.; Lie, S.; Rochet, E.; Belfort, R.J.; Furtado, J.M. Pathogenesis of Ocular Toxoplasmosis. Prog. Retin. Eye Res. 2021, 81, 100882. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Jaimes, J.; Ortiz, M.C.; Ramírez, J.D. Host and Toxoplasma gondii Genetic and Non-Genetic Factors Influencing the Development of Ocular Toxoplasmosis: A Systematic Review. Infect. Genet. Evol. 2016, 44, 199–209. [Google Scholar] [CrossRef]
- Grigg, M.E.; Dubey, J.P.; Nussenblatt, R.B. Ocular Toxoplasmosis: Lessons from Brazil. Am. J. Ophthalmol. 2015, 159, 999–1001. [Google Scholar] [CrossRef]
- Ventura, L.; Zanelli, M.; Zizzo, M.; Sanguedolce, F.; Martino, G.; Castro Ruiz, C.; Annessi, V.; Ascani, S. Toxoplasma Cyst Detection in Piringer-Kuchinka Lymphadenitis. Report of Two Cases and Literature Review. Pathologica 2021, 113, 126–130. [Google Scholar] [CrossRef]
- Derouin, F.; Pelloux, H.; ESCMID Study Group on Clinical Parasitology. Prevention of Toxoplasmosis in Transplant Patients. Clin. Microbiol. Infect. 2008, 14, 1089–1101. [Google Scholar] [CrossRef]
- Bledsoe, J.R.; Hasserjian, R.P. Toxoplasma Lymphadenitis. In Hematopathology; Springer: Cham, Switzerland, 2020; pp. 495–500. ISBN 3-319-95308-7. [Google Scholar]
- Mele, A.; Paterson, P.; Prentice, H.; Leoni, P.; Kibbler, C. Toxoplasmosis in Bone Marrow Transplantation: A Report of Two Cases and Systematic Review of the Literature. Bone Marrow Transplant. 2002, 29, 691–698. [Google Scholar] [CrossRef]
- Loscalzo, J.; Fauci, A.; Kasper, D.; Hauser, S.; Longo, D.; Jameson, J.L. Harrison’s Principles of Internal Medicine, 21st ed.; McGraw-Hill: New York, NY, USA; Health Professions Division: Fort Lauderdale, FL, USA, 2022. [Google Scholar]
- Moncada, P.A.; Montoya, J.G. Toxoplasmosis in the Fetus and Newborn: An Update on Prevalence, Diagnosis and Treatment. Expert Rev. Anti-Infect. Ther. 2012, 10, 815–828. [Google Scholar] [CrossRef]
- Weiss, L.M.; Dubey, J.P. Toxoplasmosis: A History of Clinical Observations. Int. J. Parasitol. 2009, 39, 895–901. [Google Scholar] [CrossRef]
- Basavaraju, A. Toxoplasmosis in HIV Infection: An Overview. Trop. Parasitol. 2016, 6, 129–135. [Google Scholar] [CrossRef]
- Khurana, S.; Batra, N. Toxoplasmosis in Organ Transplant Recipients: Evaluation, Implication, and Prevention. Trop. Parasitol. 2016, 6, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Martino, R.; Maertens, J.; Bretagne, S.; Rovira, M.; Deconinck, E.; Ullmann, A.J.; Held, T.; Cordonnier, C.; European Group for Blood and Marrow Transplantation Infectious Diseases Working Party. Toxoplasmosis after Hematopoietic Stem Cell Transplantation. Clin. Infect. Dis. 2000, 31, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Truppel, J.H.; Reifur, L.; Montiani-Ferreira, F.; Lange, R.R.; de Castro Vilani, R.G.D.; Gennari, S.M.; Thomaz-Soccol, V. Toxoplasma gondii in Capybara (Hydrochaeris Hydrochaeris) Antibodies and DNA Detected by IFAT and PCR. Parasitol. Res. 2010, 107, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Quirk, T.; Dubey, J.P. Experimental Toxoplasma gondii Infection in Striped Skunk (Mephitis mephitis). J. Parasitol. 2008, 94, 761–763. [Google Scholar] [CrossRef]
- Jensen, S.K.; Aars, J.; Lydersen, C.; Kovacs, K.M.; Åsbakk, K. The Prevalence of Toxoplasma gondii in Polar Bears and Their Marine Mammal Prey: Evidence for a Marine Transmission Pathway? Polar Biol. 2010, 33, 599–606. [Google Scholar] [CrossRef]
- Galeh, T.M.; Sarvi, S.; Montazeri, M.; Moosazadeh, M.; Nakhaei, M.; Shariatzadeh, S.A.; Daryani, A. Global Status of Toxoplasma gondii Seroprevalence in Rodents: A Systematic Review and Meta-Analysis. Front. Vet. Sci. 2020, 7, 461. [Google Scholar] [CrossRef]
- Nava, M.; Corlatti, L.; Formenti, N.; Trogu, T.; Pedrotti, L.; Gugiatti, A.; Lanfranchi, P.; Luzzago, C.; Ferrari, N. Parasite-Mediated Manipulation? Toxoplasma gondii Infection Increases Risk Behaviour towards Culling in Red Deer. Biol. Lett. 2023, 19, 20230292. [Google Scholar] [CrossRef]
- French, S.K.; Pearl, D.L.; Stevens, B.; Peregrine, A.S.; Jardine, C.M. Morbidity and Mortality in Ontario Rodents and Lagomorphs: A 30-Year Retrospective Review. J. Wildl. Dis. 2021, 57, 874–883. [Google Scholar] [CrossRef]
- Karadjian, G.; Laboutière, L.; Chevillot, A.; Voisinot, A.; Blaizot, A.; Puech, M.-P.; Aubert, D.; Risco-Castillo, V.; Blaga, R.; Vallée, I. Toxocara Cati and Toxoplasma gondii in French Birds of Prey. J. Wildl. Dis. 2022, 58, 373–379. [Google Scholar] [CrossRef]
- Canfield, P.J.; Hartley, W.J.; Dubey, J.P. Lesions of Toxoplasmosis in Australian Marsupials. J. Comp. Pathol. 1990, 103, 159–167. [Google Scholar] [CrossRef]
- Shapiro, K.; VanWormer, E.; Packham, A.; Dodd, E.; Conrad, P.A.; Miller, M. Type X Strains of Toxoplasma gondii Are Virulent for Southern Sea Otters (Enhydra Lutris Nereis) and Present in Felids from Nearby Watersheds. Proc. Biol. Sci. 2019, 286, 20191334. [Google Scholar] [CrossRef]
- Epiphanio, S.; Sinhorini, I.L.; Catão-Dias, J.L. Pathology of Toxoplasmosis in Captive New World Primates. J. Comp. Pathol. 2003, 129, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Denk, D.; De Neck, S.; Khaliq, S.; Stidworthy, M.F. Toxoplasmosis in Zoo Animals: A Retrospective Pathology Review of 126 Cases. Animals 2022, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.N.; Kaur, T.; Koenen, K.; DeStefano, S.; Zajac, A.M.; Lindsay, D.S. Prevalence of Agglutinating Antibodies to Toxoplasma gondii and Sarcocystis Neurona in Beavers (Castor Canadensis) from Massachusetts. J. Parasitol. 2005, 91, 1228–1229. [Google Scholar] [CrossRef] [PubMed]
- Almeria, S.; Murata, F.H.; Cerqueira-Cézar, C.K.; Kwok, O.C.; Shipley, A.; Dubey, J.P. Epidemiological and Public Health Significance of Toxoplasma gondii Infection in Wild Rabbits and Hares: 2010–2020. Microorganisms 2021, 9, 597. [Google Scholar] [CrossRef]
- Wilson, A.G.; Lapen, D.R.; Mitchell, G.W.; Provencher, J.F.; Wilson, S. Interaction of Diet and Habitat Predicts Toxoplasma gondii Infection Rates in Wild Birds at a Global Scale. Glob. Ecol. Biogeogr. 2020, 29, 1189–1198. [Google Scholar] [CrossRef]
- Bouchard, É.; Sharma, R.; Bachand, N.; Gajadhar, A.A.; Jenkins, E.J. Pathology, Clinical Signs, and Tissue Distribution of Toxoplasma gondii in Experimentally Infected Reindeer (Rangifer Tarandus). Int. J. Parasitol.: Parasites Wildl. 2017, 6, 234–240. [Google Scholar] [CrossRef]
- Fatal Acute Toxoplasmosis in Three Golden Lion Tamarins (Leontopithecus Rosalia)—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/9638627/ (accessed on 8 May 2024).
- Reis Amendoeira, M.R.; Arruda, I.F.; Moreira, S.B.; Ubiali, D.G.; da Silva Barbosa, A.; Jesus Pena, H.F.; Barbosa Pereira, A.H.; Nascimento da Silveira, C.; Bonifácio, T.F.; Clemes, Y.S.; et al. Isolation and Genetic Characterization of Toxoplasma gondii from a Captive Black-and-Gold Howler Monkey (Alouatta Caraya Humboldt, 1812) in Brazil. Int. J. Parasitol. Parasites Wildl. 2022, 19, 187–190. [Google Scholar] [CrossRef]
- Moreira, S.B.; Pereira, A.H.B.; Pissinatti, T.D.A.; Arruda, I.F.; de Azevedo, R.R.M.; Schiffler, F.B.; Amendoeira, M.R.R.; Dos Santos, A.F.A.; Pissinatti, A.; Ubiali, D.G. Subacute Multisystemic Toxoplasmosis in a Captive Black-and-Gold Howler Monkey (Alouatta caraya) Indicates Therapy Challenging. J. Med. Primatol. 2022, 51, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.V.; Pena, H.F.J.; Talebi, M.G.; Teixeira, R.H.F.; Kanamura, C.T.; Diaz-Delgado, J.; Gennari, S.M.; Catão-Dias, J.L. Fatal Toxoplasmosis in a Southern Muriqui (Brachyteles arachnoides) from São Paulo State, Brazil: Pathological, Immunohistochemical, and Molecular Characterization. J. Med. Primatol. 2018, 47, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Grandi, F.; Monteiro, L.N.; Salgado, B.S.; Rocha, R.M.; Nonogaki, S.; Rocha, N.S. What Is Your Diagnosis? Hepatosplenomegaly in a Howler Monkey (Alouatta fusca). Vet. Clin. Pathol. 2012, 41, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Fiorello, C.V.; Heard, D.J.; Heller, H.L.B.; Russell, K. Medical Management of Toxoplasma Meningitis in a White-Throated Capuchin (Cebus capucinus). J. Zoo. Wildl. Med. 2006, 37, 409–412. [Google Scholar] [CrossRef]
- Cedillo-Peláez, C.; Rico-Torres, C.P.; Salas-Garrido, C.G.; Correa, D. Acute Toxoplasmosis in Squirrel Monkeys (Saimiri sciureus) in Mexico. Vet. Parasitol. 2011, 180, 368–371. [Google Scholar] [CrossRef]
- Inoue, M. Acute Toxoplasmosis in Squirrel Monkeys. J. Vet. Med. Sci. 1997, 59, 593–595. [Google Scholar] [CrossRef]
- (PDF) Toxoplasmosis in Wild Eastern Barred Bandicoots, Perameles gunnii. Available online: https://www.researchgate.net/publication/236942612_Toxoplasmosis_in_wild_eastern_barred_bandicoots_Perameles_gunnii (accessed on 8 May 2024).
- Attwood, H.D.; Woolley, P.A.; Rickard, M.D. Toxoplasmosis in Dasyurid Marsupials. J. Wildl. Dis. 1975, 11, 543–551. [Google Scholar] [CrossRef]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Su, C.; Grigg, M.E. Recent Aspects on Epidemiology, Clinical Disease, and Genetic Diversity of Toxoplasma gondii Infections in Australasian Marsupials. Parasites Vectors 2021, 14, 301. [Google Scholar] [CrossRef]
- Portas, T.J. Toxoplasmosis in Macropodids: A Review. J. Zoo Wildl. Med. 2010, 41, 1–6. [Google Scholar] [CrossRef]
- Hartley, M.P. Toxoplasma gondii Infection in Two Common Wombats (Vombatus ursinus). Aust. Vet. J. 2006, 84, 107–109. [Google Scholar] [CrossRef]
- Hill, N.J.; Dubey, J.P.; Vogelnest, L.; Power, M.L.; Deane, E.M. Do Free-Ranging Common Brushtail Possums (Trichosurus Vulpecula) Play a Role in the Transmission of Toxoplasma gondii within a Zoo Environment? Vet. Parasitol. 2008, 152, 202–209. [Google Scholar] [CrossRef]
- Taggart, P.L.; Fancourt, B.A.; Boardman, W.S.J.; Peacock, D.E.; Caraguel, C.G.B. Infection Pressure is necessary, but not sufficient by Itself, to Explain Toxoplasma gondii Seroprevalence in Intermediate Host Species. J. Parasitol. 2021, 107, 554–561. [Google Scholar] [CrossRef]
- Taggart, P.L.; Fancourt, B.A.; Fabijan, J.; Peacock, D.E.; Speight, K.N.; Caraguel, C.G.B.; McAllister, M.M. No Evidence of Toxoplasma gondii Exposure in South Australian Koalas (Phascolarctos cinereus). J. Parasitol. 2019, 105, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.Y.; Hartigan, A.; Reppas, G.; Higgins, D.P.; Canfield, P.J.; Slapeta, J. Looks Can Deceive: Molecular Identity of an Intraerythrocytic Apicomplexan Parasite in Australian Gliders. Vet. Parasitol. 2009, 159, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hillman, A.E.; Ash, A.L.; Kristancic, A.R.; Elliot, A.D.; Lymbery, A.J.; Robertson, I.D.; Thompson, R.C.A. Validation of Various Parasite Detection Tests for Use in the Australian Marsupials Quenda (Isoodon obesulus) and Brushtail Possums (Trichosurus vulpecula). J. Vet. Diagn. Investig. 2017, 29, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Adriaanse, K.; Lynch, M.; Sutherland, D.; Traub, R.; Lowe, J.; Hufschmid, J. Toxoplasma gondii Does Not Inhibit the Assisted Colonization of Eastern Barred Bandicoots (Perameles gunnii) to Phillip Island, Victoria, Australia. J. Wildl. Dis. 2024, 60, 116–125. [Google Scholar] [CrossRef]
- Lynch, M.; Liyanage, K.L.D.T.D.; Stent, A.; Sutherland, D.R.; Coetsee, A.; Adriaanse, K.; Jabbar, A.; Hufschmid, J. Assessing the Impact of Toxoplasma gondii in Endangered Eastern Barred Bandicoots (Perameles gunnii) on Phillip and French Islands. J. Wildl. Dis. 2025, 61, 654–662. [Google Scholar] [CrossRef]
- Liyanage, K.L.D.T.D.; Lynch, M.; Omotainse, O.S.; Su, C.; Hufschmid, J.; Jabbar, A. Molecular Detection and Characterisation of Toxoplasma gondii in Eastern Barred Bandicoots (Perameles gunnii) in Victoria, Australia. Int. J. Parasitol. Parasites Wildl. 2025, 27, 101071. [Google Scholar] [CrossRef]
- Randel, C.J.; Vanherweg, W.J. Survey for Select Pathogens in the Desert Kit Fox (Vulpes Macrotis Arsipus) in California, USA. J. Wildl. Dis. 2022, 58, 631–635. [Google Scholar] [CrossRef]
- Morozińska-Gogol, J. The Presence of Toxoplasma gondii in the Terrestrial and Marine Environments and Its Importance for Public Health. Ann. Parasitol. 2021, 67, 137–149. [Google Scholar] [CrossRef]
- Rahimi, M.T.; Daryani, A.; Sarvi, S.; Shokri, A.; Ahmadpour, E.; Teshnizi, S.H.; Mizani, A.; Sharif, M. Cats and Toxoplasma gondii: A Systematic Review and Meta-Analysis in Iran. Onderstepoort J. Vet. Res. 2015, 82, e1–e10. [Google Scholar] [CrossRef]
- Toxoplasma gondii Infections in Dogs: 2009-2020—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/33160144/ (accessed on 8 May 2024).
- Mumtaz, T.; Awan, U.A.; Mushtaq, A.; Afzal, M.S.; Mahmood, T.; Wasif, S.; Ali, A.; Ajmal, K.; Mohamed, T.; Muhammad, A.; et al. Prevalence of Toxoplasmosis in Sheep and Goats in Pakistan: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 1331. [Google Scholar] [CrossRef]
- A Review of Toxoplasmosis in Pigs—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/3518210/ (accessed on 8 May 2024).
- Agriculture|Free Full-Text|Seroprevalence and Potential Risk Factors of Toxoplasma gondii in Dromedary Camels. Available online: https://www.mdpi.com/2077-0472/13/1/129 (accessed on 8 May 2024).
- Toxoplasma gondii Infections in Chickens (Gallus Domesticus): Prevalence, Clinical Disease, Diagnosis and Public Health Significance—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/19744305/ (accessed on 8 May 2024).
- Zaki, L.; Olfatifar, M.; Ghaffarifar, F.; Eslahi, A.V.; KarimiPourSaryazdi, A.; Taghipour, A.; Hamidianfar, N.; Badri, M.; Jokelainen, P. Global Prevalence of Toxoplasma gondii in Birds: A Systematic Review and Meta-Analysis. Parasite Epidemiol. Control 2024, 25, e00350. [Google Scholar] [CrossRef]
- Marino, A.M.F.; Giunta, R.P.; Salvaggio, A.; Castello, A.; Alfonzetti, T.; Barbagallo, A.; Aparo, A.; Scalzo, F.; Reale, S.; Buffolano, W.; et al. Toxoplasma gondii in Edible Fishes Captured in the Mediterranean Basin. Zoonoses Public Health 2019, 66, 826–834. [Google Scholar] [CrossRef]
- de Barros, L.D.; Garcia, J.L.; Bresciani, K.D.S.; Cardim, S.T.; Storte, V.S.; Headley, S.A. A Review of Toxoplasmosis and Neosporosis in Water Buffalo (Bubalus Bubalis). Front. Vet. Sci. 2020, 7, 455. [Google Scholar] [CrossRef]
- Basso, W.; Sollberger, E.; Schares, G.; Küker, S.; Ardüser, F.; Moore-Jones, G.; Zanolari, P. Toxoplasma gondii and Neospora Caninum Infections in South American Camelids in Switzerland and Assessment of Serological Tests for Diagnosis. Parasit. Vectors 2020, 13, 256. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.-D.; Huang, S.-Y.; Zhu, X.-Q. Diagnosis of Toxoplasmosis and Typing of Toxoplasma gondii. Parasites Vectors 2015, 8, 292. [Google Scholar] [CrossRef]
- da Silva, Y.H.; Campos, D.R.; Lima, G.A.C.; Quintal, J.P.; Guimarães, B.G.; do Rêgo, G.M.M.; de Avelar, B.R.; Intrieri, J.d.M.; Correia, T.R.; Scott, F.B. Prevalence of Gastrointestinal Parasites in Domestic Cats (Felis catus) Diagnosed by Different Coproparasitological Techniques in the Municipality of Seropédica, Rio de Janeiro. Rev. Bras. Parasitol. Vet. 2023, 32, e006223. [Google Scholar] [CrossRef] [PubMed]
- Mikita, K.; Maeda, T.; Ono, T.; Miyahira, Y.; Asai, T.; Kawana, A. The Utility of Cerebrospinal Fluid for the Molecular Diagnosis of Toxoplasmic Encephalitis. Diagn. Microbiol. Infect. Dis. 2013, 75, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, J.J.; Kirchgessner, M.S.; Whipps, C.M.; Mohammed, H.O.; Bunting, E.M.; Wade, S.E. Prevalence of Antibodies to Toxoplasma gondii in White-Tailed Deer (Odocoileus virginianus) in New York State, USA. J. Wildl. Dis. 2013, 49, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Carpenter, J.L. Histologically Confirmed Clinical Toxoplasmosis in Cats: 100 Cases (1952–1990). J. Am. Vet. Med. Assoc. 1993, 203, 1556–1566. [Google Scholar] [CrossRef]
- Dubey, J.P.; Darrington, C.; Tiao, N.; Ferreira, L.R.; Choudhary, S.; Molla, B.; Saville, W.J.A.; Tilahun, G.; Kwok, O.C.H.; Gebreyes, W.A. Isolation of Viable Toxoplasma gondii from Tissues and Feces of Cats from Addis Ababa, Ethiopia. J. Parasitol. 2013, 99, 56–58. [Google Scholar] [CrossRef]
- Ybañez, R.H.D.; Ybañez, A.P.; Nishikawa, Y. Review on the Current Trends of Toxoplasmosis Serodiagnosis in Humans. Front. Cell Infect. Microbiol. 2020, 10, 204. [Google Scholar] [CrossRef]
- Mcauley, J.B.; Jones, J.L.; Singh, K. Toxoplasma. In Manual of Clinical Microbiology; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 2373–2386. [Google Scholar]
- Carvalho, N.O.R.; Gaspar, R.C.; Lima, S.G.; da Silva Zanini, D.; Rodrigues, N.J.L.; Guimarães, V.Y.; da Silva, A.V.; Rahal, S.C.; Henrique, A.L.P.; Fornazari, F.; et al. Comparison Between Indirect Fluorescent Antibody Test and Modified Agglutination Test for Detecting Anti-Toxoplasma gondii IgG Antibodies in Neotropical Primates. J. Med. Primatol. 2025, 54, e70002. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, J.; Wang, Q.; Shen, Y.; Zhou, B.; Dai, L.; Zhu, W.; Sun, H.; Xie, X.; Xie, H.; et al. Development of an Indirect ELISA for Detecting Toxoplasma gondii IgG Antibodies Based on a Recombinant TgIMP1 Protein. PLoS Negl. Trop. Dis. 2024, 18, e0012421. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Khalid, N. Detection of Acute Toxoplasma gondii Infection in Early Pregnancy by IgG Avidity and PCR Analysis. J. Med. Microbiol. 2007, 56, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.; Hajdu, M.P.; Ramirez, R.; Thulliez, P.; McLeod, R.; Remington, J.S. Role of Specific Immunoglobulin E in Diagnosis of Acute Toxoplasma Infection and Toxoplasmosis. J. Clin. Microbiol. 1993, 31, 2952–2959. [Google Scholar] [CrossRef]
- Olariu, T.R.; Blackburn, B.G.; Press, C.; Talucod, J.; Remington, J.S.; Montoya, J.G. Role of Toxoplasma IgA as Part of a Reference Panel for the Diagnosis of Acute Toxoplasmosis during Pregnancy. J. Clin. Microbiol. 2019, 57, e01357-18. [Google Scholar] [CrossRef]
- Naot, Y.; Remington, J.S. An Enzyme-Linked Immunosorbent Assay for Detection of IgM Antibodies to Toxoplasma gondii: Use for Diagnosis of Acute Acquired Toxoplasmosis. J. Infect. Dis. 1980, 142, 757–766. [Google Scholar] [CrossRef]
- Dhakal, R.; Gajurel, K.; Pomares, C.; Talucod, J.; Press, C.J.; Montoya, J.G. Significance of a Positive Toxoplasma Immunoglobulin M Test Result in the United States. J. Clin. Microbiol. 2015, 53, 3601–3605. [Google Scholar] [CrossRef]
- Teimouri, A.; Mohtasebi, S.; Kazemirad, E.; Keshavarz, H. Role of Toxoplasma gondii IgG Avidity Testing in Discriminating between Acute and Chronic Toxoplasmosis in Pregnancy. J. Clin. Microbiol. 2020, 58, e00505-20. [Google Scholar] [CrossRef]
- Brenier-Pinchart, M.P.; Morand-Bui, V.; Fricker-Hidalgo, H.; Equy, V.; Marlu, R.; Pelloux, H. Adapting a Conventional PCR Assay for Toxoplasma gondii Detection to Real-Time Quantitative PCR Including a Competitive Internal Control. Parasite 2007, 14, 149–154. [Google Scholar] [CrossRef]
- Lin, M.-H.; Chen, T.-C.; Kuo, T.; Tseng, C.-C.; Tseng, C.-P. Real-Time PCR for Quantitative Detection of Toxoplasma gondii. J. Clin. Microbiol. 2000, 38, 4121–4125. [Google Scholar] [CrossRef]
- Kong, Q.-M.; Lu, S.-H.; Tong, Q.-B.; Lou, D.; Chen, R.; Zheng, B.; Kumagai, T.; Wen, L.-Y.; Ohta, N.; Zhou, X.-N. Loop-Mediated Isothermal Amplification (LAMP): Early Detection of Toxoplasma gondii Infection in Mice. Parasites Vectors 2012, 5, 2. [Google Scholar] [CrossRef]
- Ajzenberg, D.; Bañuls, A.-L.; Tibayrenc, M.; Dardé, M.L. Microsatellite Analysis of Toxoplasma gondii Shows Considerable Polymorphism Structured into Two Main Clonal Groups. Int. J. Parasitol. 2002, 32, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Shwab, E.K.; Zhu, X.-Q.; Majumdar, D.; Pena, H.F.; Gennari, S.M.; Dubey, J.P.; Su, C. Geographical Patterns of Toxoplasma gondii Genetic Diversity Revealed by Multilocus PCR-RFLP Genotyping. Parasitology 2014, 141, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-G.; Johnson, A.M. DNA Polymorphisms Associated with Murine Virulence of Toxoplasma gondii Identified by RAPD-PCR. In Toxoplasma gondii; Springer: Berlin/Heidelberg, Germany, 1996; pp. 17–26. [Google Scholar]
- Costa, J.-M.; Cabaret, O.; Moukoury, S.; Bretagne, S. Genotyping of the Protozoan Pathogen Toxoplasma gondii Using High-Resolution Melting Analysis of the Repeated B1 Gene. J. Microbiol. Methods 2011, 86, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Sugi, T.; Weiss, L.M.; Kato, K. Identification of Compounds That Suppress Toxoplasma gondii Tachyzoites and Bradyzoites. PLoS ONE 2017, 12, e0178203. [Google Scholar] [CrossRef]
- Moré, G.; Venturini, M.C.; Pardini, L.; Unzaga, J.M. Toxoplasma. In Parasitic Protozoa of Farm Animals and Pets; Springer: Berlin/Heidelberg, Germany, 2018; pp. 149–168. [Google Scholar]
- WOAH. WOAH Terrestrial Manual; WOAH: Paris, France, 2018. [Google Scholar]
- Arafa, F.M.; Osman, D.H.; Tolba, M.M.; Rezki, N.; Aouad, M.R.; Hagar, M.; Osman, M.; Said, H. Sulfadiazine Analogs: Anti-Toxoplasma in Vitro Study of Sulfonamide Triazoles. Parasitol. Res. 2023, 122, 2353–2365. [Google Scholar] [CrossRef]
- Toxoplasmosis in Animals—Generalized Conditions. Available online: https://www.msdvetmanual.com/generalized-conditions/toxoplasmosis/toxoplasmosis-in-animals (accessed on 7 May 2024).
- Halonen, S.K.; Weiss, L.M. Toxoplasmosis. Handb. Clin. Neurol. 2013, 114, 125–145. [Google Scholar] [CrossRef]
- Greene, C.E.; Cook, J.R.; Mahaffey, E.A. Clindamycin for Treatment of Toxoplasma Polymyositis in a Dog. J. Am. Vet. Med. Assoc. 1985, 187, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Schmidt, D.R. Sulfadiazine and Pyrimethamine in the Postnatal Treatment of Congenital Toxoplasmosis: What Are the Options? Expert Rev. Anti-Infect. Ther. 2003, 1, 175–182. [Google Scholar] [CrossRef] [PubMed]
- De-La-Torre, A.; Stanford, M.; Curi, A.; Jaffe, G.J.; Gomez-Marin, J.E. Therapy for Ocular Toxoplasmosis. Ocul. Immunol. Inflamm. 2011, 19, 314–320. [Google Scholar] [CrossRef]
- Alday, P.H.; Doggett, J.S. Drugs in Development for Toxoplasmosis: Advances, Challenges, and Current Status. Drug Des. Dev. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef]
- Montoya, J.G.; Remington, J.S. Clinical Practice: Management of Toxoplasma gondii Infection during Pregnancy. Clin. Infect. Dis. 2008, 47, 554–566. [Google Scholar] [CrossRef]
- Wang, Z.-D.; Liu, H.-H.; Ma, Z.-X.; Ma, H.-Y.; Li, Z.-Y.; Yang, Z.-B.; Zhu, X.-Q.; Xu, B.; Wei, F.; Liu, Q. Toxoplasma gondii Infection in Immunocompromised Patients: A Systematic Review and Meta-Analysis. Front. Microbiol. 2017, 8, 389. [Google Scholar] [CrossRef]
- Kavanagh, O.N. Alkalising Agents in Urinary Tract Infections: Theoretical Contraindications, Interactions and Synergy. Ther. Adv. Drug Saf. 2022, 13, 20420986221080794. [Google Scholar] [CrossRef]
- Brunton, L.; Lazo, J.; Parker, K. Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 11th ed.; McGraw Hill Professional: New York, NY, USA, 2005; ISBN 978-0-07-160891-6. [Google Scholar]
- Zuther, E.; Johnson, J.J.; Haselkorn, R.; McLeod, R.; Gornicki, P. Growth of Toxoplasma gondii Is Inhibited by Aryloxyphenoxypropionate Herbicides Targeting Acetyl-CoA Carboxylase. Proc. Natl. Acad. Sci. USA 1999, 96, 13387–13392. [Google Scholar] [CrossRef]
- Tilley, M.; Fichera, M.E.; Jerome, M.E.; Roos, D.S.; White, M.W. Toxoplasma gondii Sporozoites Form a Transient Parasitophorous Vacuole That Is Impermeable and Contains Only a Subset of Dense-Granule Proteins. Infect. Immun. 1997, 65, 4598–4605. [Google Scholar] [CrossRef]
- Malik, M.A.; Sajid, M.S.; Abbas, R.Z.; Aleem, M.T.; Anjum, F.R.; Khan, A.; Farhab, M.; Maqbool, M.; Zeeshan, M.; Hussain, K. Anthelmintic Drug Resistance in Livestock: Current Understanding and Future Trends. In Parasitic Helminths and Zoonoses-From Basic to Applied Research; IntechOpen: London, UK, 2022; ISBN 1-80355-568-8. [Google Scholar]
- Zhang, Y.; Li, D.; Lu, S.; Zheng, B. Toxoplasmosis Vaccines: What We Have and Where to Go? NPJ Vaccines 2022, 7, 131. [Google Scholar] [CrossRef]
- Innes, E.A.; Bartley, P.M.; Rocchi, M.; Benavidas-Silvan, J.; Burrells, A.; Hotchkiss, E.; Chianini, F.; Canton, G.; Katzer, F. Developing Vaccines to Control Protozoan Parasites in Ruminants: Dead or Alive? Vet. Parasitol. 2011, 180, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Farhab, M.; Kyaw, P.O.; Xia, X.-X.; Cai, H.-Q.; Zhang, T.; Cao, M.-X.; Li, J.-G.; Yuan, Y.-G. Targeted Gene Knock-Out of Fel D1 in Fetal Fibroblasts Using CRISPR–Cas9: Implications for Cat Allergies. Animals 2025, 15, 927. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-G.; Liu, S.-Z.; Farhab, M.; Lv, M.-Y.; Zhang, T.; Cao, S.-X. Genome Editing: An Insight into Disease Resistance, Production Efficiency, and Biomedical Applications in Livestock. Funct. Integr. Genom. 2024, 24, 81. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, T.; Farhab, M.; Xia, X.-X.; Reza, A.M.M.T.; Kyaw, P.O.; Chen, F.; Aly Sayed Ismail, E.; Xue, G.; Zhong, P.; et al. Comprehensive Analysis of circRNAs and lncRNAs Involvement in the Development of Skeletal Muscle in Myostatin-Deficient Rabbits. Anim. Biotechnol. 2025, 36, 2465624. [Google Scholar] [CrossRef]
- Innes, E.A.; Hamilton, C.; Garcia, J.L.; Chryssafidis, A.; Smith, D. A One Health Approach to Vaccines against Toxoplasma gondii. Food Waterborne Parasitol. 2019, 15, e00053. [Google Scholar] [CrossRef]
- Khan, M.S.; Buzdar, S.A.; Hussain, R.; Alouffi, A.; Aleem, M.T.; Farhab, M.; Javid, M.A.; Akhtar, R.W.; Khan, I.; Almutairi, M.M. Cobalt Iron Oxide (CoFe2O4) Nanoparticles Induced Toxicity in Rabbits. Vet. Sci. 2023, 10, 514. [Google Scholar] [CrossRef]
- Chu, K.-B.; Quan, F.-S. Advances in Toxoplasma gondii Vaccines: Current Strategies and Challenges for Vaccine Development. Vaccines 2021, 9, 413. [Google Scholar] [CrossRef]
- Innes, E.A.; Bartley, P.M.; Maley, S.; Katzer, F.; Buxton, D. Veterinary Vaccines against Toxoplasma gondii. Memórias Do Inst. Oswaldo Cruz 2009, 104, 246–251. [Google Scholar] [CrossRef]
- Jongert, E.; Roberts, C.W.; Gargano, N.; Förster-Waldl, E.; Petersen, E. Vaccines against Toxoplasma gondii: Challenges and Opportunities. Memórias Do Inst. Oswaldo Cruz 2009, 104, 252–266. [Google Scholar] [CrossRef]
- Liu, Q.; Singla, L.D.; Zhou, H. Vaccines against Toxoplasma gondii: Status, Challenges and Future Directions. Hum. Vaccines Immunother. 2012, 8, 1305–1308. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis–a Waterborne Zoonosis. Vet. Parasitol. 2004, 126, 57–72. [Google Scholar] [CrossRef]
- Stelzer, S.; Basso, W.; Benavides Silván, J.; Ortega-Mora, L.M.; Maksimov, P.; Gethmann, J.; Conraths, F.J.; Schares, G. Toxoplasma gondii Infection and Toxoplasmosis in Farm Animals: Risk Factors and Economic Impact. Food Waterborne Parasitol. 2019, 15, e00037. [Google Scholar] [CrossRef]
- Kuruca, L.; Belluco, S.; Vieira-Pinto, M.; Antic, D.; Blagojevic, B. Current Control Options and a Way towards Risk-Based Control of Toxoplasma gondii in the Meat Chain. Food Control 2023, 146, 109556. [Google Scholar] [CrossRef]
- Teweldemedhin, M.; Gebremichael, A.; Geberkirstos, G.; Hadush, H.; Gebrewahid, T.; Asgedom, S.W.; Gidey, B.; Asres, N.; Gebreyesus, H. Seroprevalence and Risk Factors of Toxoplasma gondii among Pregnant Women in Adwa District, Northern Ethiopia. BMC Infect. Dis. 2019, 19, 327. [Google Scholar] [CrossRef] [PubMed]
- Kurth, K.; Jiang, T.; Muller, L.; Su, C.; Gerhold, R.W. Toxoplasma gondii Contamination at an Animal Agriculture Facility: Environmental, Agricultural Animal, and Wildlife Contamination Indicator Evaluation. Int. J. Parasitol. Parasites Wildl. 2021, 16, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Peris, M.P.; Planas, S.; Langa, J.; Laborda, A.; Castillo, J.A.; Gracia, M.J. Seroprevalence of Zoonotic Pathogens in Stray Cats in an Urban Area of Northeast Spain. Vet. Parasitol. Reg. Stud. Rep. 2024, 53, 101052. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; García, M.; Gálvez, R.; Checa, R.; Marino, V.; Sarquis, J.; Barrera, J.P.; Rupérez, C.; Caballero, L.; Chicharro, C.; et al. Implications of Zoonotic and Vector-Borne Parasites to Free-Roaming Cats in Central Spain. Vet. Parasitol. 2018, 251, 125–130. [Google Scholar] [CrossRef]
- Kochanowsky, J.A.; Koshy, A.A. Toxoplasma gondii . Curr. Biol. 2018, 28, R770–R771. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Longcore, T.; Barbieri, M.; Dabritz, H.; Hill, D.; Klein, P.N.; Lepczyk, C.; Lilly, E.L.; McLeod, R.; Milcarsky, J.; et al. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. Ecohealth 2019, 16, 378–390. [Google Scholar] [CrossRef]
- Huertas-López, A.; Álvarez-García, G.; Sánchez-Sánchez, R.; Cantos-Barreda, A.; Ibáñez-López, F.J.; Martínez-Subiela, S.; Cerón, J.J.; Martínez-Carrasco, C. A Systematic Review and Meta-Analysis of the Serological Diagnosis of Toxoplasma gondii Infection Highlight the Lack of a One Health Integrative Research. Res. Vet. Sci. 2023, 155, 137–149. [Google Scholar] [CrossRef]
- İnci, A.; Sohel, M.H.; Babür, C.; Uslu, S.; Karademir, G.K.; Yürük, M.; Düzlü, Ö.; Kızgın, A.D.; Yıldırım, A. An Overview of One Health Concept Focusing on Toxoplasmosis. Turk. Parazitol. Derg. 2023, 47, 256–274. [Google Scholar] [CrossRef]
- Gilot-Fromont, E.; Lélu, M.; Dardé, M.-L.; Richomme, C.; Aubert, D.; Afonso, E.; Mercier, A.; Gotteland, C.; Villena, I. The Life Cycle of Toxoplasma gondii in the Natural Environment. In Toxoplasmosis: Recent Advances; InTech: Houston TX, USA, 2012; pp. 3–36. ISBN 978-953-51-0746-0. [Google Scholar]
- Mercier, A.; Devillard, S.; Ngoubangoye, B.; Bonnabau, H.; Bañuls, A.-L.; Durand, P.; Salle, B.; Ajzenberg, D.; Dardé, M.-L. Additional Haplogroups of Toxoplasma gondii out of Africa: Population Structure and Mouse-Virulence of Strains from Gabon. PLoS Negl. Trop. Dis. 2010, 4, e876. [Google Scholar] [CrossRef]
- Dakroub, H.; Sgroi, G.; D’Alessio, N.; Russo, D.; Serra, F.; Veneziano, V.; Rea, S.; Pucciarelli, A.; Lucibelli, M.G.; De Carlo, E.; et al. Molecular Survey of Toxoplasma gondii in Wild Mammals of Southern Italy. Pathogens 2023, 12, 471. [Google Scholar] [CrossRef]
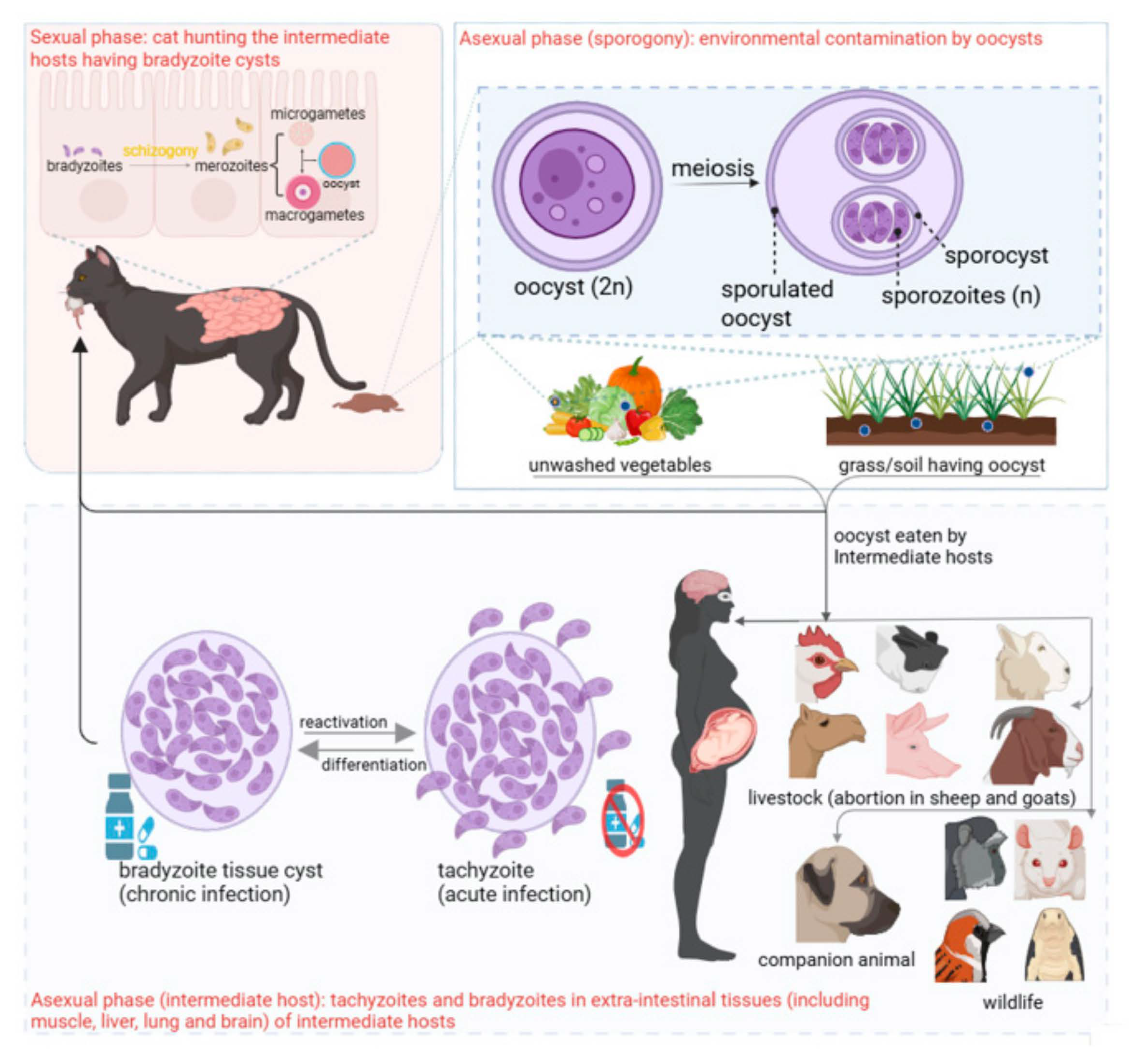

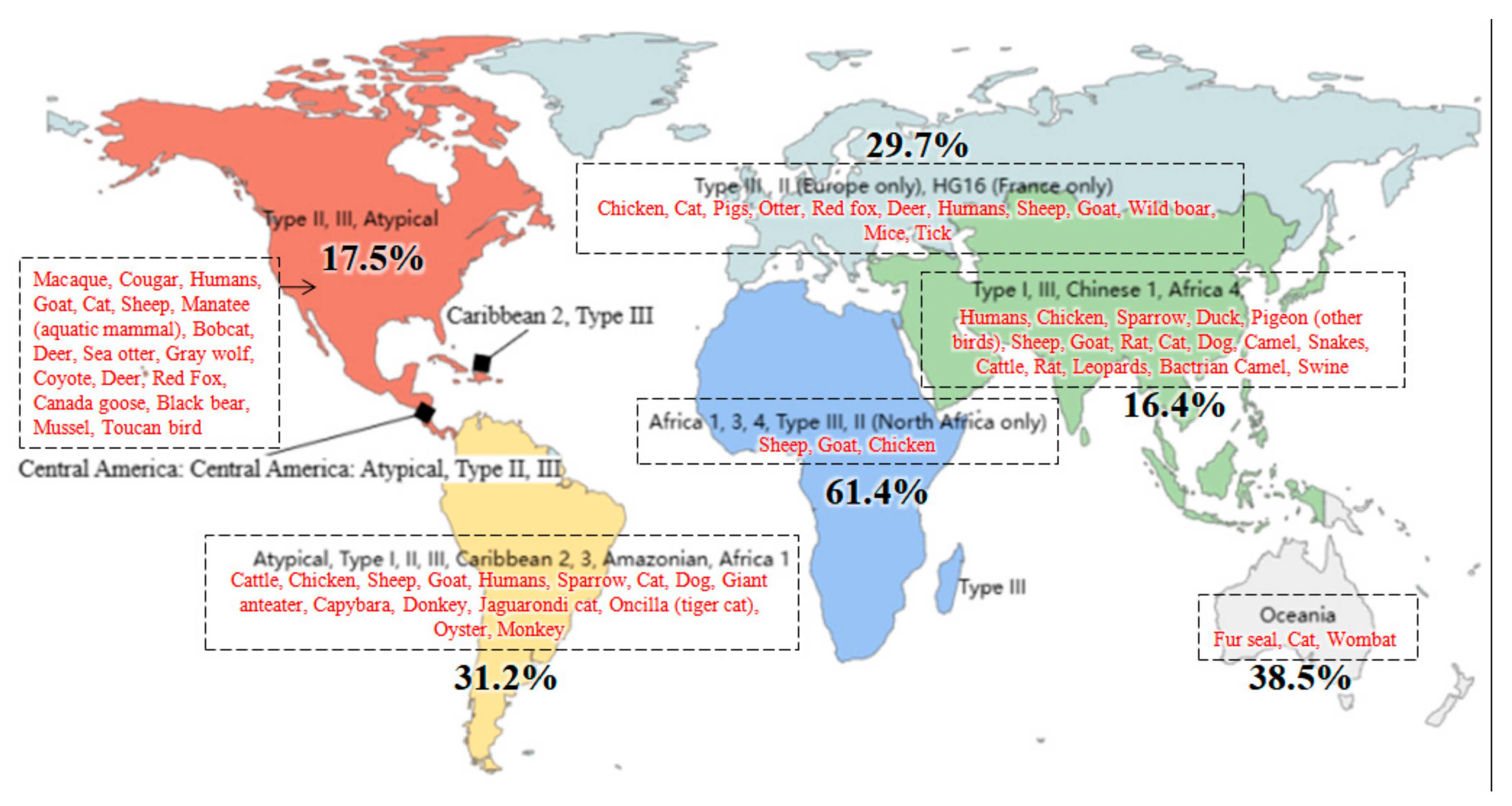
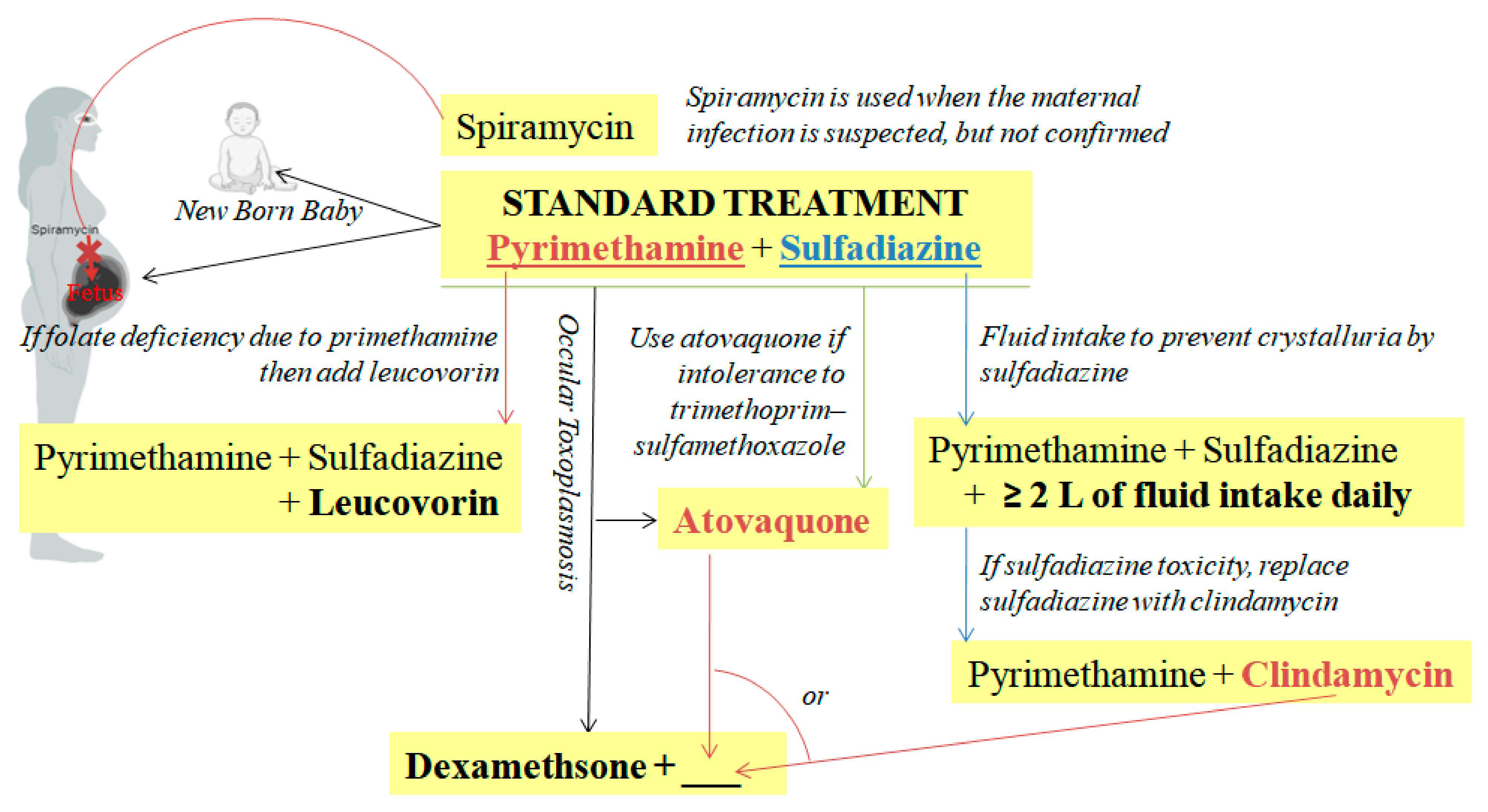
| Year | Finding | Ref. |
|---|---|---|
| History | ||
| 1909 | Name “Toxoplasma gondii” proposed | [20] |
| 1937 | Successful isolation of viable T. gondii | [10] |
| 1992 | T. gondii was classified as Type I, II, III, and atypical | [12] |
| 2005 | Gene mapping achieved | [12,21] |
| Parasite Morphology and Life Cycle | ||
| 1928 | Cyst recognized | [4] |
| 1958 | Tachyzoites division by endodyogeny described | [22] |
| 1960 | Bradyzoites resistance to digestive enzymes recognized | [19] |
| 1970 | Oocyst described | [23] |
| 1970 | Asexual and sexual stages were reported in the intestine of cats | [24] |
| 1972 | Asexual enteroepithelial stages were designated as types A–E | [24] |
| 1973 | The term “bradyzoite”, also called cystozoites, was proposed for the cystic organisms | [25] |
| 1973 | Term tachyzoite proposed (tachy = fast, zoite = life) | [25] |
| 1988 | Term tissue cyst proposed | [12] |
| 2019 | T. gondii sexual reproduction is associated with delta-6-desaturase gene | [26] |
| 2023 | ROCY1 gene activates T. gondii bradyzoite formation | [13] |
| Transmission | ||
| 1959 | Congenital transmission was observed in mice for up to 10 generations | [27] |
| 1954 | Transmission by carnivorism explained | [28] |
| 1965 | T. gondii infectivity is associated with cat feces | [29] |
| 1970– 1972 | Sexual life cycle to defined only in felines, including excretion of oocysts only by felids | [12] |
| 2008 | Congenital transmission found in a large wild animal species, the white-tailed deer | [12] |
| Diagnosis | ||
| 1958 | Sabin–Feldman dye test | [30] |
| 1968 | IgM antibody detection from cord blood or infant serum for detecting congenital toxoplasmosis proposed | [12] |
| 1965 | Direct agglutination test (DAT) in humans and other animals | [12] |
| 1969 | Detection of IgM in cord blood through the indirect fluorescent antibody test and ELISA | [12] |
| 1989 | Detection of T. gondii DNA was achieved, using the B1 gene from tachyzoite, through PCR | [12] |
| Treatment | ||
| 1942 | Sulfonamides were reported to be effective against murine toxoplasmosis | [31,32] |
| 1953 | Combined therapy with sulfonamides and pyrimethamine was discovered to have satisfactory results in treating toxoplasmosis in humans | [31] |
| 1958 | Spiramycin found to have anti-T. gondii activity in mice | [33] |
| 1973 | Clindamycin was documented to have anti-T. gondii effects | [34,35] |
| Prevention and Control | ||
| 1972 | Susceptible populations should avoid contact with oocysts | [12] |
| 1990s | Keeping cats out of pig farms can reduce T. gondii infection in pigs | [12] |
| 1995 | Vaccination of sheep to reduce neonatal mortality in lambs is made available commercially | [36] |
| First Confirmed Cases of Toxoplasmosis in Animals | ||
| 1908 | First report of T. gondii (bradyzoites) in tissue (cyst) of Ctenodactylus gundi, in Tunisia | [1] |
| 1914 | Eurasian red squirrels (Sciurus vulgaris) | [37] |
| 1916 | Howler monkey (Alouatta seinculus) | [38] |
| 1951 | Squirrel monkeys (Saimiri sciureus) in the Philadelphia Zoo | [38] |
| 1974 | Pallas’s cats (Otocolobus manul) | [39] |
| 1985 | Wild turkeys from Blairsville, Georgia, USA | [40] |
| 1987 | Bobcat (Lynx rufus) from Ronan, Montana, USA | [41] |
| 1990 | Koalas (Phascolarctos cinereus); an arboreal marsupial, Sydney, Australia | [42] |
| 1990 | Dolphins (Stenella longirostris) | [43] |
| 1995 | Common mole (Talpa europaea) from Germany | [44] |
| 1997 | Golden lion tamarins (Leontopithecus rosalia) | [45] |
| 1997 | Barred owl (Strix varia) from Quebec, Canada | [46] |
| 2000 | Sea otters (Enhydra lutris nereis) | [47] |
| 2000 | Hawaiian crow (Corvus hawaiiensis) | [48] |
| 2004 | Striped skunk (Mephitis mephitis); T. gondii genotype III from Mississippi, USA | [49] |
| 2004 | Beaver (Castor canadensis) | [50] |
| 2004 | Captive nilgais (Boselaphus tragocamelus) | [51] |
| 2004 | Saiga antelope (Saiga tatarica) | [51] |
| 2004 | Bald eagle (Haliaeetus leucocephalus) from New Hampshire | [52] |
| 2004 | Canada goose (Branta canadensis); T. gondii genotype III from Mississippi, USA | [49] |
| 2004 | Black-winged lory (Eos cyanogenia); T. gondii genotype III from South Carolina, USA | [49] |
| 2005 | Hawaiian monk seal | [53] |
| 2005 | Fisher (Martes pennanti) from Garrett County, Maryland, USA | [54] |
| 2007 | Cheetah (Acinonyx jubatus) from Dubai, UAE | [55] |
| 2007 | American Red squirrels (Tamiasciurus hudsonicus) from New York, USA | [56] |
| 2007 | Woodchuck (Marmota monax) from New York, USA | [56] |
| 2008 | Sand cats (F. margarita) from Sharjah, UAE | [57] |
| 2008 | Gordon’s wildcat (Felis silvestris gordoni) from Sharjah, UAE | [58] |
| 2008 | Nicobar pigeons from South Africa | [59] |
| 2011 | Red-handed howler monkey (Alouatta belzebul) | [60] |
| 2011 | Black-footed penguin (Spheniscus demersus) | [61] |
| 2012 | Flying-foxes (megachiropteran bats) | [62] |
| 2013 | Northern shoveller (Anas clypeata) from Tuscany, Italy | [63] |
| 2013 | Common teal (Anas crecca) from Tuscany, Italy | [63] |
| 2015 | Giant panda (Ailuropoda melanoleuca) from Zhengzhou, China | [64] |
| 2015 | Wombats (Vombatus ursinus) | [65] |
| 2016 | Swinhoe’s striped squirrel (Tamiops swinhoei) from Germany | [66] |
| 2016 | Opossums (Didelphis virginiana) from Yucatan, Mexico | [67] |
| 2019 | Eastern fox squirrels (Sciurus niger) | [68] |
| Domestic Animals | ||
| Pet animals | ||
| 1908 | Domestic rabbits (Oryctolagus cuniculus) from Brazil | [1,20] |
| 1932 | Ferrets (Mustela putorius furo) from New Zealand | [69] |
| 1942 | Cats from the Middletown, New York, USA | [12] |
| 1950 | Dogs | [70] |
| 1956 | Mink | [71] |
| 1985 | Red foxes (Vulpes vulpes) | [72] |
| Production Animals | ||
| 1952 | Swine | [73] |
| 1957 | Sheep | [74] |
| 1961 | Chickens | [75] |
| 1979 | Goats | [76] |
| 1990 | Camels | [77] |
| 2003 | Ducks | [78] |
| 2014 | Alpaca | [79] |
| 2014 | Turkeys | [80] |
| 2014 | Geese from Hainan, China | [81] |
| 2021 | Horses | [17,18] |
| 2024 | Buffalos | [82] |
| 2025 | Cattle | [16] |
| Year | Animal Specie | Affected/Total | Region | Ref | |
|---|---|---|---|---|---|
| 1952 | Chickens | High mortality (flock eradicated) | South-Eastern Norway | [103] | |
| 1992 | Canaries | 7/30 with ophthalmic lesions | New Zealand | [101] | |
| 2004 | Lories | 5/5 died | Columbia, South Carolina, USA | [49] | |
| 2008 | Nicobar pigeons | 3/10 died | South Africa | [59] | |
| 2011 | Black-footed penguins | 3 chicks died | Netherlands | [61] | |
| 2019 | Chickens and guinea fowl | 15/76 died | Viamão, Rio Grande do Sul, Southern Brazil | [102] | |
| 1973 | Goats | 3 does congenital toxoplasmosis (Flock 1) | Tasmania, Australia | [76] | |
| 1977 | 5 stillbirths (Flock 2) | ||||
| 1978 | An abortion having twin kids (Flock 3) | ||||
| 1954 | Sheep | 15–20% abortion rate | Wellington, New Zealand | [14,15] | |
| 2005 | 38 abortions + 15 resorbed | Texas, USA | [83] | ||
| 2006 | 2 of 26 infected lambs | Texas, USA | [83] | ||
| 2010 | 30/239 abortions in a dairy flock | Palencia, Spain | [86] | ||
| 70/210 abortions in a meat flock | Segovia, Spain | ||||
| 2010 | 40/100 pregnant ewes aborted | Serro, Minas Gerais State, Brazil | [85] | ||
| 2015– 2018 | 146/242 from 11 abortion episodes | Spain | [84] | ||
| 213/342 from two slaugheterhouses | |||||
| 1999 | monkeys | Wooly monkeys | 3 died | Brazil | [98] |
| 2001 and 2006 | Squirrel monkeys (Saimiri sciureus) | 50 monkeys died and none recovered spontaneously | French Guiana | [99] | |
| 2018 | 7 of 13 dead | Seoul, South Korea | [105] | ||
| 2019 | 4 died | Hokkaido, Japan | [106] | ||
| 2020 | Howler monkeys (Alouatta sp.) | 7 died | Brazil | [100] | |
| 1994 | Pigs | 50–60% morbidity, 10–42% mortality 200/800 pigs died | Mantova, Lombardia, Italy | [88] | |
| 810/2080 pigs died | Modena, Emilia-Romagna, Italy | ||||
| ~31/345 pigs died | |||||
| 34/80 pigs died | Mantova, Lombardia, Italy | ||||
| 2001 | 42% affected, 8% mortality | China | [88] | ||
| 2001 | 33% affected, 2% mortality | China | [5] | ||
| 2004 | 19/260 sows died | Jinchang, Gansu Province, China | [87] | ||
| Marine | |||||
| 2001– 2015 | Hawaiian Monk Seals (Neomonachus Schauinslandi) | 8/183 confirmed T. gondii | Hawaii, USA | [91] | |
| 2009 | Bottlenose dolphins | 2 died (all seropositive) | Canada | [90] | |
| 2013 | Hector’s dolphins | 7/28 died (25%) | New Zealand | [89] | |
| 2015–2019 | Northern sea otters (Enhydra lutris kenyoni) | 22/44 infected | Washington, USA | [96] | |
| Miscellaneous | |||||
| 1957 | Hares (Lepus timidus ainu) | 8/13 died | Sapporo, Japan | [104] | |
| 1964 | Chinchillas | 44/56 died + 4 abortions | Not specified | [92] | |
| 1992 | Black-footed ferrets (Mustela nigripes) | 8 acute deaths + 13 chronic | Kentucky, USA | [93] | |
| 1999 | Mink | 10,408 kits died | Wisconsin, USA | [97] | |
| 2006–2010 | Tammar wallabies | Deaths; 6 confirmed + 11 suspected | Budapest, Hungary | [94] | |
| 2017 | Red-necked wallabies | 9 died | Virginia, USA | [95] | |
| Year | Finding | Ref. |
|---|---|---|
| 1939 | First isolate of T. gondii from human. | [11,12] |
| 1939 | First report of congenital transmission demonstrated in human. | [11] |
| 1940 | T. gondii isolated in heart, spleen, and other tissues of humans. | [12] |
| 1941 | Report of acquired toxoplasmosis, whose isolate became the famous RH strain. | [12] |
| 1941 | T. gondii isolated from blood of humans. | [12] |
| 1953 | Combined therapy with sulfonamides and pyrimethamine was discovered to have satisfactory results in treating toxoplasmosis in humans. | [31] |
| 1958 | Spiramycin has been used prophylactically in pregnant women. | [33] |
| 1960 | Transmission by carnivorism reported. | [11] |
| 1965 | Feco-oral transmission reported. | [11] |
| 1972 | Susceptible populations should avoid contact with oocysts. | [12] |
| 1974 | (1) An infection acquired during early pregnancy is more damaging to the fetus. | [12] |
| (2) Not all women who acquired the infection transmitted it to the fetus. | ||
| (3) Women seropositive before pregnancy did not transmit infection to the fetus. | ||
| (4) Treatment with spiramycin reduced congenital transmission, but not clinical disease in infants. | ||
| 1976 | The RH strain has lost the capacity to produce oocysts in cats. | [12] |
| 1979 | The first human toxoplasmosis outbreak described, was through oocyst inhalation/ingestion. | [12] |
| 1983 | Reported fatal acute toxoplasmosis-induced encephalitis, almost all of whom were HIV infected individuals. | [12] |
| 1995 | Canadian waterborne outbreak of toxoplasmosis. | [12] |
| 2006 | T. gondii outbreak in Brazil. | [12] |
| Molecular Methods | Purpose | DNA Target Regions | Ref. |
|---|---|---|---|
| Conventional PCR | Species confirmation | B1 gene, 529-bp repetitive element, 18S rDNA gene, SAG1, SAG2, and GRA1 | [268,269] |
| Real-time PCR | B1 gene, 529-bp repetitive element, 18S rDNA gene, SAG1 | ||
| LAMP | 529-bp repetitive element, B1, SAG1, SAG2, GRA1, oocyst wall protein genes | [270] | |
| Microsatellite analysis | Genotyping | TUB2, W35, TgM-A, B18, B17; M33, IV.1, XI.1, M48, M102, N60, N82, AA, N61, and N83 | [271] |
| Multilocus sequence typing | BTUB, SAG2, GRA6, and SAG3 | [272] | |
| PCR-RFLP | SAG1, SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1 and Apico | [272,273] | |
| RAPD-PCR | Genomic DNA | ||
| High-resolution melting (HRM) analysis | B1 gene | [274] |
| Sr. | Drugs | Dose * | Specie | Ref. |
|---|---|---|---|---|
| 1 | Sulfadiazine | 15–25 mg/kg | all animals | [278,279] |
| Pyrimethamine | 0.44 mg/kg | [280] | ||
| 2 | Trimethoprim-sulfamethoxazole | 15 mg/kg | dogs and cats | [279] |
| 3 | Clindamycin | 10–12.5 mg/kg | dogs | [281] |
| 25–50 mg/kg | cats | [127] |
| Sr. | Vaccine Type | Description | Advantages | Limitation | Ref. |
|---|---|---|---|---|---|
| 1 | ESA vaccines | Excretory–secretory antigens (ESAs) are used as antigenic agents | Reduces parasitemia of highly virulent strains. Propranolol and alum-adjuvanted ESE vaccine improve the protective effect of ESA and extend the survival time of mice. | Does not protect against all strains | [293] |
| 2 | Live attenuated vaccines e.g., modified T. gondii strain (S48) vaccine ΔHAP2 parasites by CRISPR/Cas9 ΔCDPK2 and ΔADSL knock-out vaccine | T. gondii is attenuated through gamma radiation, chemical treatment and passages. | Currently, live attenuated vaccine is the most effective anti-T. gondii vaccine. If oral live attenuated vaccines are prepared, they can mimic natural T. gondii infection and induce host cellular and humoral immunity against T. gondii without causing disease. | Short shelf life, may revert to virulence | [294] |
| 3 | Subunit vaccines e.g., rTgHSP70 subunit vaccine rROP18 and rCDPK6 combined with PLG subunit vaccine | Immunogenic parts of T. Gondii. | Multi-epitope subunit vaccines with different T-cell and B-cell epitopes are more promising for research. | Poor immunogenicity, requires carrier | [298] |
| 4 | DNA vaccines e.g., ROP, GRA, MIC, and SAG antigen vaccines | Made by antigenic proteins such as rhoptry proteins (ROPs), microsomal proteins (MICs), and surface antigens (SAGs). | Inexpensive, easy to administer, and induces a strong immune response. | Poor immunogenicity in large animals, may trigger antibodies against the DNA vector | [137,298] |
| 5 | Multiple epitope vaccines SAPNs vaccine and SAPNs scaffolded peptide epitopes vaccines | Antigenic peptide epitopes from SAG1, SAG2C, GRA6, GRA5 are used as antigenic agents. | Generates stronger Th1 responses and allows epitope optimization. | Poor immunogenicity, short half-life, may cause immune tolerance | [300] |
| 6 | mRNA vaccines e.g., TgNTPase-II-LNP vaccine | mRNA-stimulating antigenic protein. | Safe, no risk of gene recombination. | Short intracellular half-life, not very stable in vivo | [301] |
| 7 | Carbohydrate-based vaccines | T. gondii proteins tagged with surface carbohydrates to stabilize and transport them, eliciting carbohydrate-specific antibodies. | TLR-2 and TLR-4 can recognize T. gondii GPI. | Poor immunogenicity, autoimmunity | [302] |
| 8 | Exosome vaccines e.g., DC2.4 exosome vaccine | Exosomes from T. gondii, and DC2.4 cell-derived exosomes as antigenic agents. | Induce humoral and cell-mediated immunity. | Poor biocompatibility | [303] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhab, M.; Aziz, M.W.; Shaukat, A.; Cao, M.-X.; Hou, Z.; Huang, S.-Y.; Li, L.; Yuan, Y.-G. Review of Toxoplasmosis: What We Still Need to Do. Vet. Sci. 2025, 12, 772. https://doi.org/10.3390/vetsci12080772
Farhab M, Aziz MW, Shaukat A, Cao M-X, Hou Z, Huang S-Y, Li L, Yuan Y-G. Review of Toxoplasmosis: What We Still Need to Do. Veterinary Sciences. 2025; 12(8):772. https://doi.org/10.3390/vetsci12080772
Chicago/Turabian StyleFarhab, Muhammad, Muhammad Waqar Aziz, Aftab Shaukat, Ming-Xing Cao, Zhaofeng Hou, Si-Yang Huang, Ling Li, and Yu-Guo Yuan. 2025. "Review of Toxoplasmosis: What We Still Need to Do" Veterinary Sciences 12, no. 8: 772. https://doi.org/10.3390/vetsci12080772
APA StyleFarhab, M., Aziz, M. W., Shaukat, A., Cao, M.-X., Hou, Z., Huang, S.-Y., Li, L., & Yuan, Y.-G. (2025). Review of Toxoplasmosis: What We Still Need to Do. Veterinary Sciences, 12(8), 772. https://doi.org/10.3390/vetsci12080772










