Development of an ELISA for NNV-Specific Antibody Detection in Grouper Hatcheries in China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
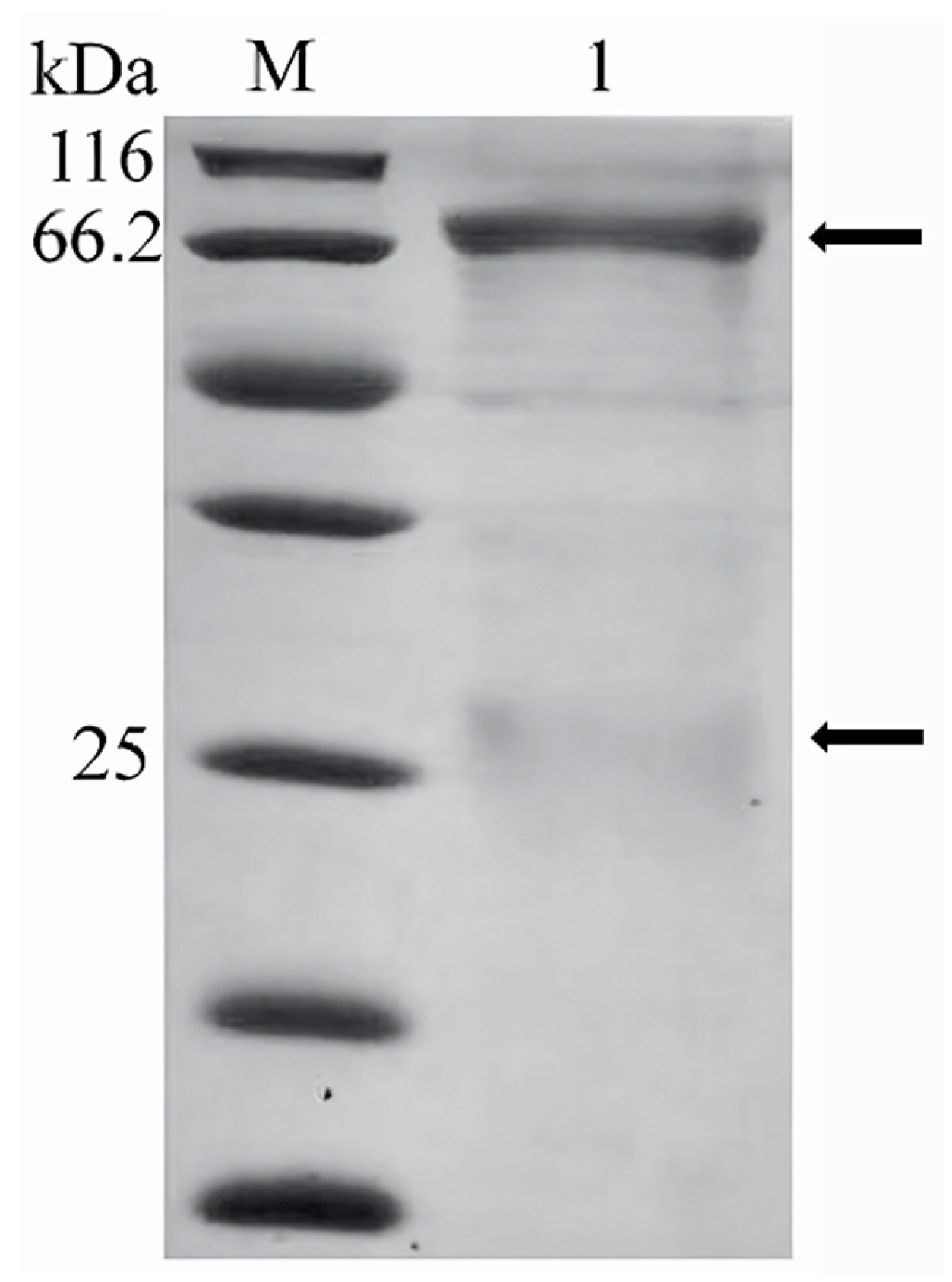
2.1. Antigen Preparation for ELISA
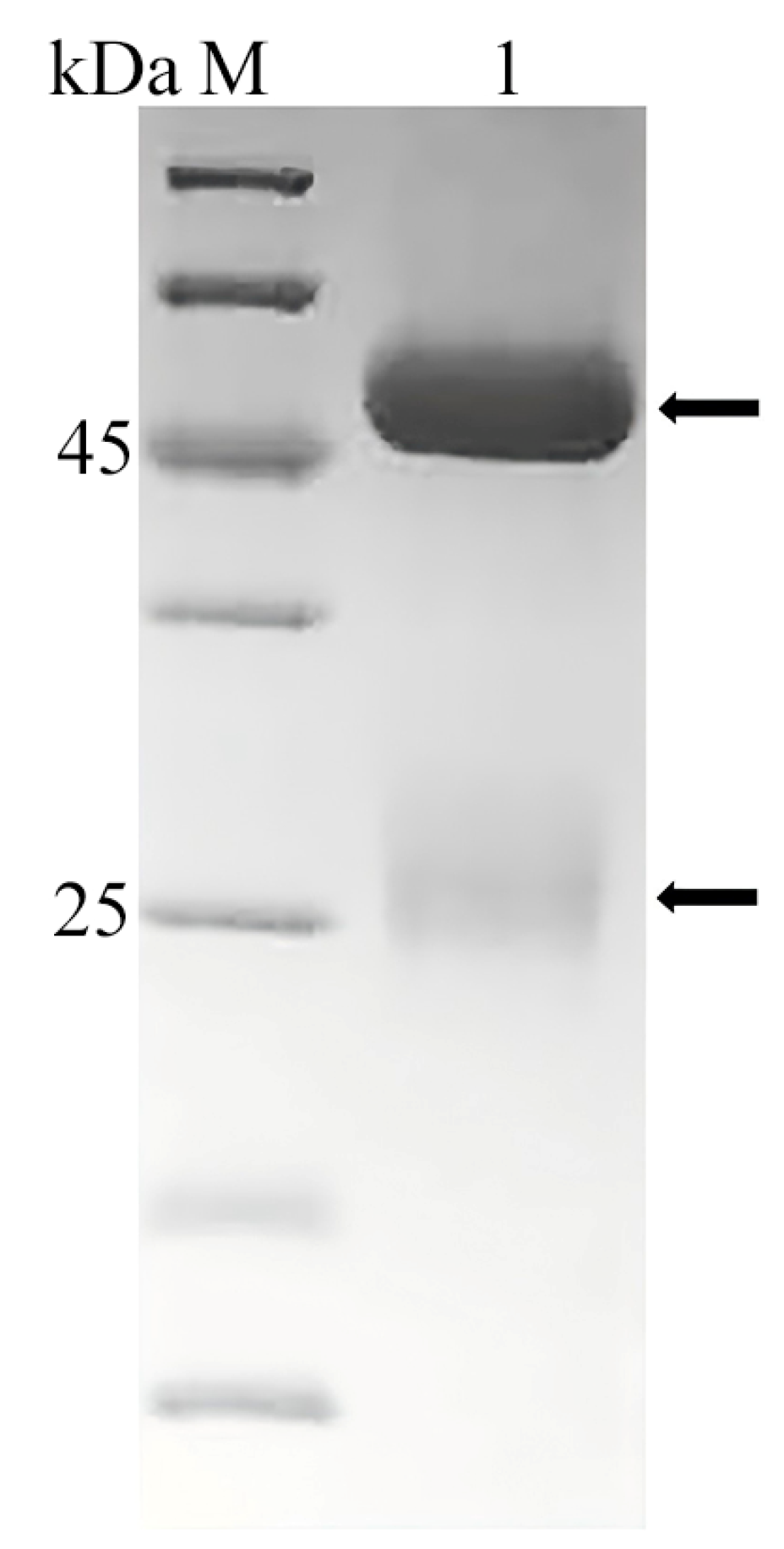
2.2. The Preparation of Grouper Antibody and HRP-Labeled Rabbit Anti-Grouper Antibody
2.3. ELISA System for Detection of Grouper Antibody
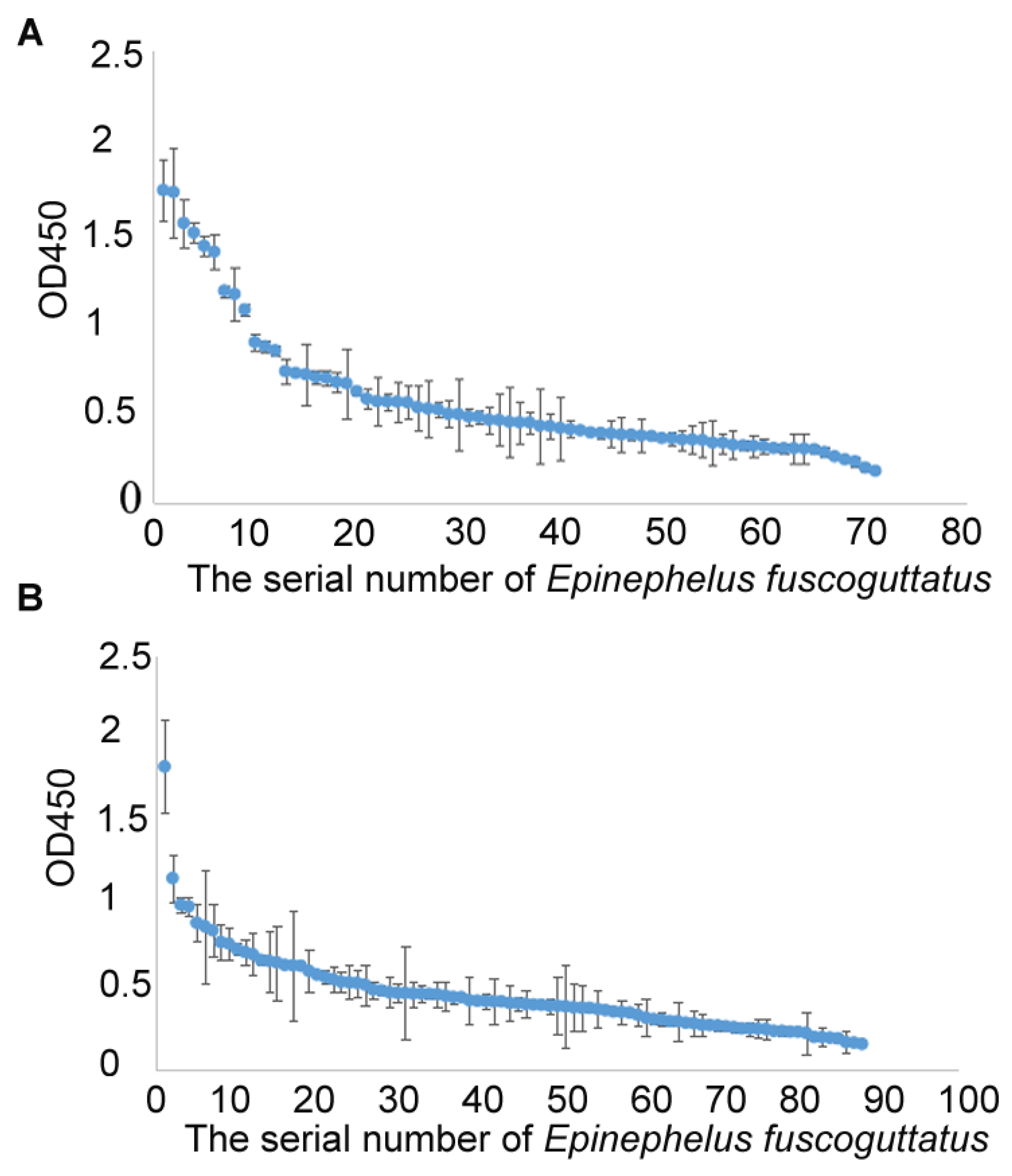
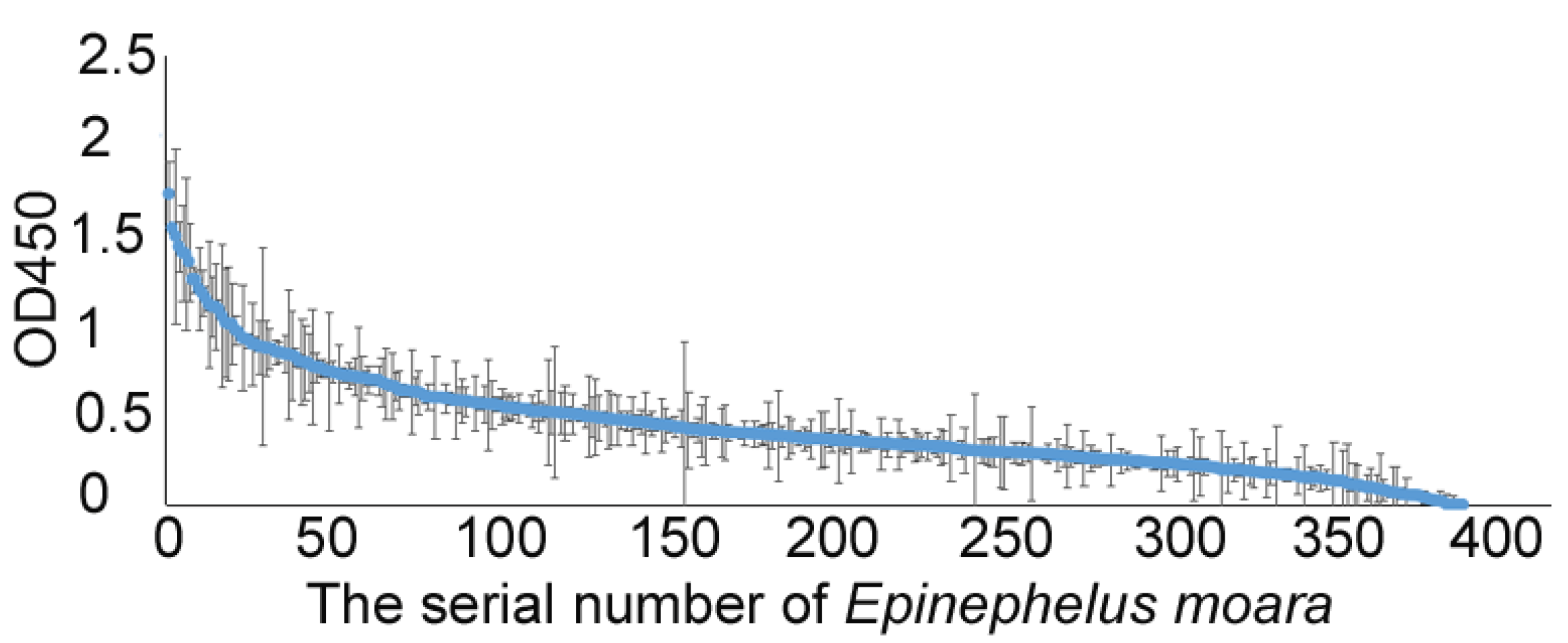
2.4. Surveillance of NNV-Specific Antibodies in Three Grouper Hatcheries of China
3. Results
3.1. Antigen for ELISA
3.2. The Preparation of Grouper Antibody and HRP-Labeled Rabbit Anti-Grouper Antibody
3.3. Surveillance of NNV-Specific Antibodies in Three Grouper Farms in China
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fishery Administration Bureau of the Ministry of Agriculture. China Fishery Statistical Year Book 2024; China Agriculture Press: Beijing, China, 2023. (In Chinese) [Google Scholar]
- Costa, J.; Thompson, K. Understanding the interaction between Betanodavirus and its host for the development of prophylactic measures for viral encephalopathy and retinopathy. Fish Shellfish Immunol. 2016, 53, 35–49. [Google Scholar] [CrossRef]
- Chen, S.; Tian, Y.; Liu, Y.; Li, Z.; Li, Z.; Duan, P.; Li, L.; Wang, X.; Wang, L.; He, X.; et al. Heterosis in growth and low temperature tolerance in Jinhu grouper (Epinephelus fuscoguttatus♀ × Epinephelus tukula♂). Aquaculture 2023, 562 (Suppl. C), 738751. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, Z.; Duan, H.; Ma, W.; Tang, J.; Li, W.; Liu, J.; Hou, Y.; Sun, Z.; Pang, Z.; et al. The family line establishment of the hybrid Epinephelus moara (♀)× E. lanceolatus (♂) by using cryopreserved sperm and the related genetic effect analysis. J. Fish. China 2017, 41, 1817–1828. (In Chinese) [Google Scholar]
- Yang, Z.; Yue, G.; Wong, S. VNN disease and status of breeding for resistance to NNV in aquaculture. Aquac. Fish. 2022, 7, 147–157. [Google Scholar] [CrossRef]
- Shetty, M.; Maiti, B.; Shivakumar Santhosh, K.; Venugopal, M.N.; Karunasagar, I. Betanodavirus of marine and freshwater fish: Distribution, genomic organization, diagnosis and control measures. Indian J. Virol. 2012, 23, 114–123. [Google Scholar] [CrossRef]
- Clyde, C.; Tan, J.; Yeap, S.; Yong, C. Current updates on viral infections affecting tilapia. Aquac. Fish. 2025, 10, 355–371. [Google Scholar] [CrossRef]
- Munday, B.; Kwang, J.; Moody, N. Betanodavirus infections of teleost fish: A review. J. Fish Dis. 2002, 25, 127–142. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Y.; Cheng, M.; Li, Z.; Li, S.; Wu, Y.; Zhang, J.; Ma, W.; Li, W.; Pang, Z.; et al. Transcriptome comparative analysis of immune tissues from asymptomatic and diseased Epinephelus moara naturally infected with nervous necrosis virus. Fish Shellfish Immunol. 2019, 93, 99–107. [Google Scholar] [CrossRef]
- Peng, P.; Liu, C.; Li, Z.; Xue, Z.; Mao, P.; Hu, J.; Xu, F.; Yao, C.; You, M. Emerging ELISA derived technologies for in vitro diagnostics. TrAC Trends Anal. Chem. 2022, 152, 116605. [Google Scholar] [CrossRef]
- Das, S.; Sahoo, P.K. Markers for selection of disease resistance in fish: A review. Aquac. Int. 2014, 22, 1793–1812. [Google Scholar] [CrossRef]
- Gye, H.; Lee, H.; Nishizawa, T. Spreading of farmed sevenband grouper Hyporthodus septemfasciatus naturally convalesced from prior mild nervous necrosis virus (NNV) infection. Aquaculture 2021, 543, 736927. [Google Scholar] [CrossRef]
- Jaramillo, D.; Hick, P.; Dyrting, K.; Anderson, I.; Whittington, R.J. Surveillance for nervous necrosis virus-specific antibodies in barramundi Lates calcarifer in Australian hatcheries. Dis. Aquat. Org. 2017, 124, 543. [Google Scholar] [CrossRef]
- Breuil, G.; Romestand, B. A rapid ELISA method for detecting specific antibody level against nodavirus in the serum of the sea bass, Dicentrarchus labrax (L.): Application to the screening of spawners in a sea bass hatchery. J. Fish Dis. 1999, 22, 45–52. [Google Scholar] [CrossRef]
- Watanabe, K.; Nishizawa, T.; Yoshimizu, M. Selection of brood stock candidates of barfin flounder using an ELISA system with recombinant protein of barfin flounder nervous necrosis virus. Dis. Aquat. Org. 2000, 41, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, N.; Makesh, M.; Sain, A.; Jayaprakash, N.S.; Kailasam, M.; Vijayan, K.K. Non-lethal screening of Asian seabass (Lates calcarifer) by monoclonal antibody based indirect enzyme linked immunosorbent assay for viral nervous necrosis. Fish Shellfish Immunol. Rep. 2021, 2, 100011. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, J.; Zhang, Z.; Liu, J.; Zhang, C.; Zhu, B.; Wang, G. An immersion subunit vaccine loaded by single-walled carbon nanotube protects pearl gentian grouper from viral nervous necrosis virus. Aquaculture 2021, 541, 736813. [Google Scholar] [CrossRef]
- Cho, S.; Kim, H.; Lan, N.; Han, H.; Lee, K.; Hwang, J.; Kwon, M.; Kang, B.; Han, S.; Moon, H.; et al. Oral vaccination through voluntary consumption of the convict grouper Epinephelus septemfasciatus with yeast producing the capsid protein of red-spotted grouper nervous necrosis virus. Vet. Microbiol. 2017, 204, 159–164. [Google Scholar] [CrossRef]
- Lu, T.; Chen, C.; Yang, Y.; Liao, C. Modelling the effect of vaccination on transmission dynamics of nervous necrosis virus in grouper larvae Epinephelus coioides. J. Fish Dis. 2020, 43, 1155–1165. [Google Scholar] [CrossRef]
- Liu, J.; Qin, Y.; Yan, L.; Liu, W.; Shi, H.; Lu, Y.; Liu, X. Protective effects of egg yolk immunoglobulins (IgY) on juvenile groupers (Epinephelus fuscoguttatus × Epinephelus lanceolatus) with red-spotted grouper nervous necrosis virus infection. Aquaculture 2021, 11, 737218. [Google Scholar] [CrossRef]
- Chen, H.; Xu, B.; Lin, N.; Liu, X.; Chen, Q.; Lin, T. Production and Characterization of Monoclonal Antibodies Against Epinephelus akaara Ig. Fujian J. Agric. Sci. 2011, 26, 687–690. (In Chinese) [Google Scholar]
- Feng, J.; Hu, C. Purification and characterization of serum immunoglobulins of four major marine fishes in China. J. Trop. Oceanogr. 2002, 21, 8–13. (In Chinese) [Google Scholar]
- Dauben, C.; Pröll-Cornelissen, M.; Heuß, E.; Appel, A.K.; Henne, H.; Roth, K.; Schellander, K.; Tholen, E.; Große-Brinkhaus, C. Genome-wide associations for immune traits in two maternal pig lines. BMC Genom. 2021, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Srisapoome, P.; Chatchaiphan, S.; Bunnoy, A.; Koonawootrittriron, S.; Na-Nakorn, U. Heritability of immunity traits and disease resistance of bighead catfish, Clarias macrocephalus Günther, 1864. Fish Shellfish Immunol. 2019, 92, 209–215. [Google Scholar] [CrossRef]
- Berghof, T.; Matthijs, M.; Arts, J.; Bovenhuis, H.; Dwars, R.M.; van der Poel, J.J.; Visker, M.H.P.W.; Parmentier, H.K. Selective breeding for high natural antibody level increases resistance to avian pathogenic Escherichia coli (APEC) in chickens. Dev. Comp. Immunol. 2019, 93, 45–57. [Google Scholar] [CrossRef]

| Source of Fish | Species | Sample Number | Average Weight | Purpose |
| Yantai hatchery | E. Fuscoguttatus | 70 | - | Preparation for secondary antibody |
| Hainan hatchery1 | E. Fuscoguttatus | 71 | 2510.3 ± 541.6 g | Surveillance of NNV-specific antibodies |
| Hainan hatchery2 | E. Fuscoguttatus | 88 | 3674.7 ± 369.5 g | Surveillance of NNV-specific antibodies |
| Yantai hatchery | E. Moara | 390 | 5846.9 ± 1675.6 g | Surveillance of NNV-specific antibodies |
| Reports | Species | ELISA System | Recombinant Expression System | The Chromogenic Agent | Detection | Characteristics |
|---|---|---|---|---|---|---|
| [12] | Hyporthodus septemfasciatus | (Experimental fish sera, NNV suspension, anti-NNV rabbit serum, HRP-conjugated swine antiserum against rabbit Ig) | - | o-phenylenediamine | OD492 | Cumbersome and toxic |
| [14] | Dicentrarchus labrax | (Rabbit IgG raised against nodavirus, experimental fish sera, biotinylated monoclonal antibody against sea bass IgM, avidin horseradish peroxidase conjugate) | - | o-phenylenediamine | 0D492 | Cumbersome and toxic |
| [15] | Verasper rnoseri | (recombinant coat protein of BFNN, fish serum, rabbit serum against barfin flounder IgM, HRP-conjugated antibody against rabbit Ig) | PET-25b (+); insoluble NNV capsid protein | o-phenylenediarnin | OD492 | cumbersome and toxic |
| [13] | Lates calcarifer | (sheep anti-NNV primary antibodies, semi-purified NNV, experimental fish sera, rabbit anti-Australian bass antibodies, donkey anti-rabbit horseradish peroxidase (HRP) conjugate) | - | o-phenylenediarnin | OD492 | Cumbersome and toxic |
| This study | E. Fuscoguttatus | (recombinant coat protein of RGNNV, experimental fish sera, HRP-conjugated rabbit antibody against grouper IgM) | pGEX-6P-1; soluble NNV capsid protein | TMB | OD450 | Simpler, less costly and safe |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Tian, Y.; Li, Z.; Qiu, Y.; Ding, X.; Li, L. Development of an ELISA for NNV-Specific Antibody Detection in Grouper Hatcheries in China. Vet. Sci. 2025, 12, 754. https://doi.org/10.3390/vetsci12080754
Wang L, Tian Y, Li Z, Qiu Y, Ding X, Li L. Development of an ELISA for NNV-Specific Antibody Detection in Grouper Hatcheries in China. Veterinary Sciences. 2025; 12(8):754. https://doi.org/10.3390/vetsci12080754
Chicago/Turabian StyleWang, Linna, Yongsheng Tian, Zhentong Li, Yishu Qiu, Xiaoyu Ding, and Linlin Li. 2025. "Development of an ELISA for NNV-Specific Antibody Detection in Grouper Hatcheries in China" Veterinary Sciences 12, no. 8: 754. https://doi.org/10.3390/vetsci12080754
APA StyleWang, L., Tian, Y., Li, Z., Qiu, Y., Ding, X., & Li, L. (2025). Development of an ELISA for NNV-Specific Antibody Detection in Grouper Hatcheries in China. Veterinary Sciences, 12(8), 754. https://doi.org/10.3390/vetsci12080754








