1. Introduction
Tall fescue (
Schedonorus arundinaceus (Schreb.)), more specifically Kentucky-31, is a cool-season perennial grass that is one of the most wide spread and heavily utilized forages in the United States [
1]. Tall fescue commonly has a symbiotic relationship with a fungal endophyte (
Epichloë coenophiala) [
2,
3] that confers productivity characteristics to the plant. These characteristics include improved insect resistance and drought tolerance, allowing for greater plant persistence and improved competitive ability [
4,
5,
6]. Due to these positive attributes, as well as improved plant nutritive qualities, tall fescue has been heavily utilized in livestock grazing systems [
1]. The positive characteristics conferred to tall fescue are due to the production of ergot alkaloids (EA) by the fungal endophyte. While EA production is beneficial to the plant, consumption by livestock can be detrimental to animal health by leading to bovine fat necrosis, fescue foot, and fescue toxicosis. Although fat necrosis and fescue foot are detrimental to health and productivity, fescue toxicosis is of greatest concern to beef producers due to large economic losses that are incurred [
7,
8].
Due to the consumption of EA, cattle grazing toxic, endophyte-infected (E+) tall fescue during the spring grazing season may experience compromised growth performance, poor health, compromised immune function [
9], and reduced dry matter intake (DMI). Ergot alkaloids function by binding to serotonergic and adrenergic receptors within the vascular system [
3]. By binding to vascular receptors, EA leads to vasoconstriction of blood vessels, reducing blood flow to both organs and peripheral tissues [
10]. Additionally, vasoconstriction of blood vessels disrupts thermoregulation by elevating body temperature, making cattle vulnerable to heat stress [
3,
10]. This is further exacerbated by persistently low serum prolactin concentrations which results in continued hair coat growth, leading to a delay in hair coat shedding well into the summer grazing season [
11].
Numerous management strategies, products, and compounds have been evaluated to reduce the negative impacts of tall fescue toxicosis but many, such as pasture renovation, have been impractical or unsuccessful in mitigation of the aforementioned impacts [
7,
11]. Several studies have evaluated inter-seeding of legumes with E+ tall fescue to dilute the effects of EA with variable responses. Inclusion of red clover (
Trifolium pratense) and white clover (
Trifolium repens) has been shown to be an effective strategy to increase animal performance when cattle are grazing E+ tall fescue [
11,
12,
13]. Improved performance when grazing inter-seeded pastures may be due to the presence of plant secondary metabolites (PSM) such as isoflavones and tannins. However, improvement in performance may not be a reflection of PSM but rather a higher plane of nutrition, particularly in relation to increased protein and energy, and improved intake [
13]. Additionally, while the inclusion of clovers with E+ tall fescue may be effective, it may not always be an economically feasible strategy. Therefore, there remains a need to identify a practical strategy for mitigating tall fescue toxicosis that is both practical and economically feasible.
Supplying supplemental plant extracts, such as tannins, as feed additives may improve the health status of cattle grazing E+ tall fescue. Modes of action of supplemental plant extract additives may vary but often include antioxidant properties, improved utilization of nutrient supply, and non-selective binding that reduces alkaloid absorption and metabolism [
11,
14]. As previously mentioned, tannins are polyphenolic compounds that are synthesized by legumes [
14,
15] and are frequently utilized in ruminant diets [
16]. They can be classified as hydrolysable tannins (HT), such as those found in chestnut leaves (
Castanea spp.) or condensed tannins, such as those found in quebracho (
Schinopsis spp.; CT) [
16]. Tannins may alleviate the effects of tall fescue toxicosis as they have a high binding affinity for nitrogenous compounds, such as those found in EA [
11]. This binding capability could reduce the toxic effects of EA by reducing the absorption of alkaloids through the gastrointestinal epithelia [
17,
18]. Villalba et al. [
17] indicated that performance and intake of lambs receiving E+ tall fescue seed improved when supplemented with the tannin-containing legume sainfoin (
Onobrychis viciifolia) when compared with a non-tannin-containing legume. However, sainfoin was not offered as a plant extract product nor was tall fescue grazed in this study.
Studies have shown that tannins may improve final body weight (BW), dry matter intake (DMI), and average daily gain (ADG) [
16,
19]. Additionally, tannins have been shown to possess properties demonstrating antimicrobial and antiparasitic effects [
20,
21] although impacts on health are highly variable based on the origin and type of tannin utilized [
21]. While tannins have the potential to mitigate the post-ingestive impacts of EA, the extent of these benefits may vary depending on alkaloid structure, rumen and abomasum pH, and tannin concentration [
11,
22]. For example, structural differences between HT and CT may influence their ability to bind and neutralize EA, with research suggesting that CT form more stable complexes with alkaloids due to their higher molecular weight and greater affinity for protein-like compounds. Excessive inclusion of dietary tannins may be detrimental by decreasing palatability and DMI, which can decrease animal productivity and feed efficiency [
22]. This presents challenges in determining the correct concentration and type of tannin to include to mitigate the effects of tall fescue toxicosis while simultaneously avoiding potential negative impacts from excessive dietary tannin inclusion. The objective of this study was to assess the dietary inclusion of two proprietary tannin-based feed additives compared to soybean hulls only on animal performance and physiological measures of yearling beef heifers grazing E+ tall fescue pastures.
2. Materials and Methods
2.1. Animal Care and Use
This project was approved by the University of Arkansas Division of Agriculture’s institutional animal care and use committee (#24025) and was conducted at the Livestock and Forestry Research Station (LFST; 35.826683N, 91.774796W) near Batesville, Arkansas from early April to late June 2024.
2.2. Weather Conditions
As previously described by Gadberry et al. [
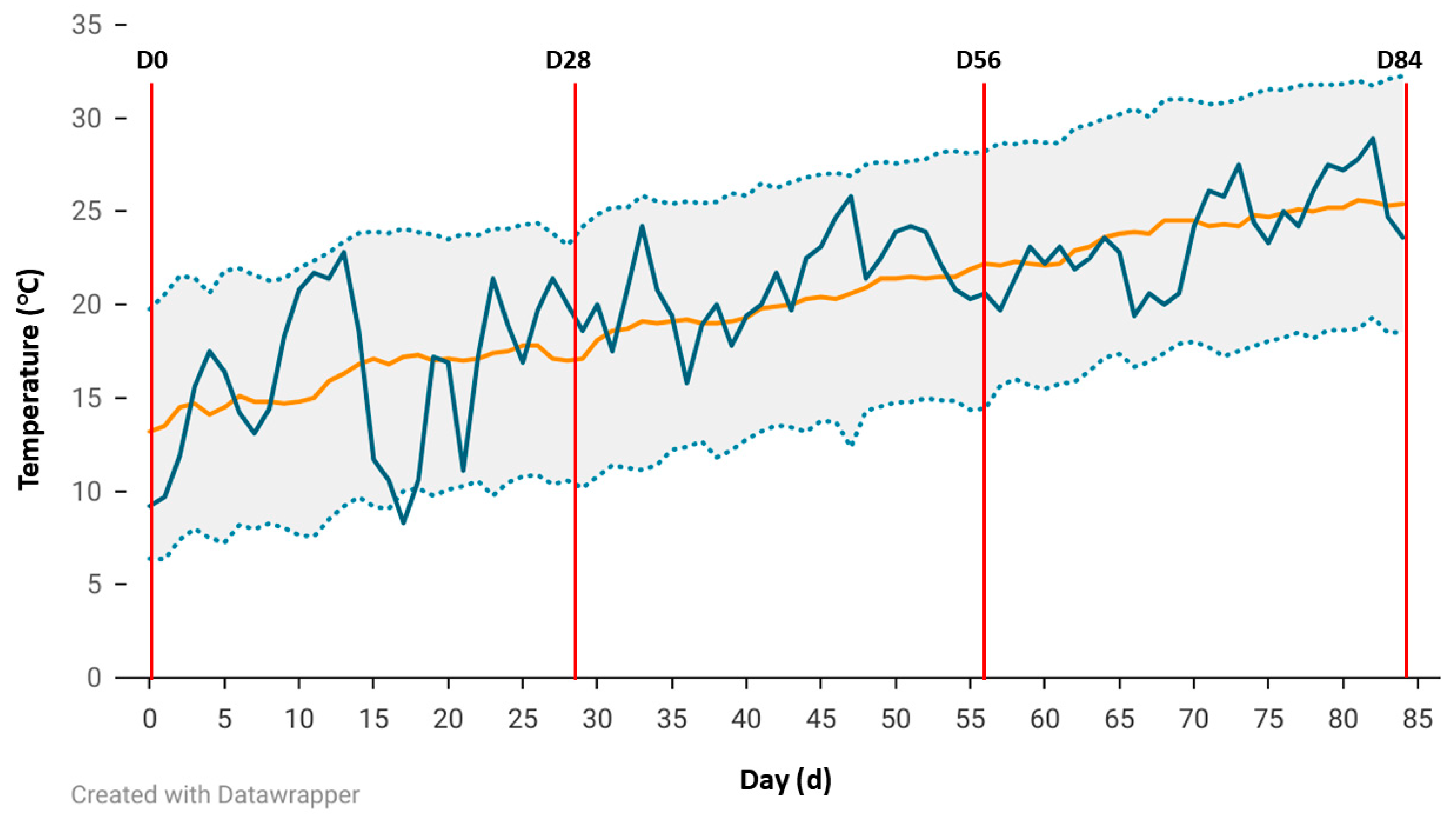
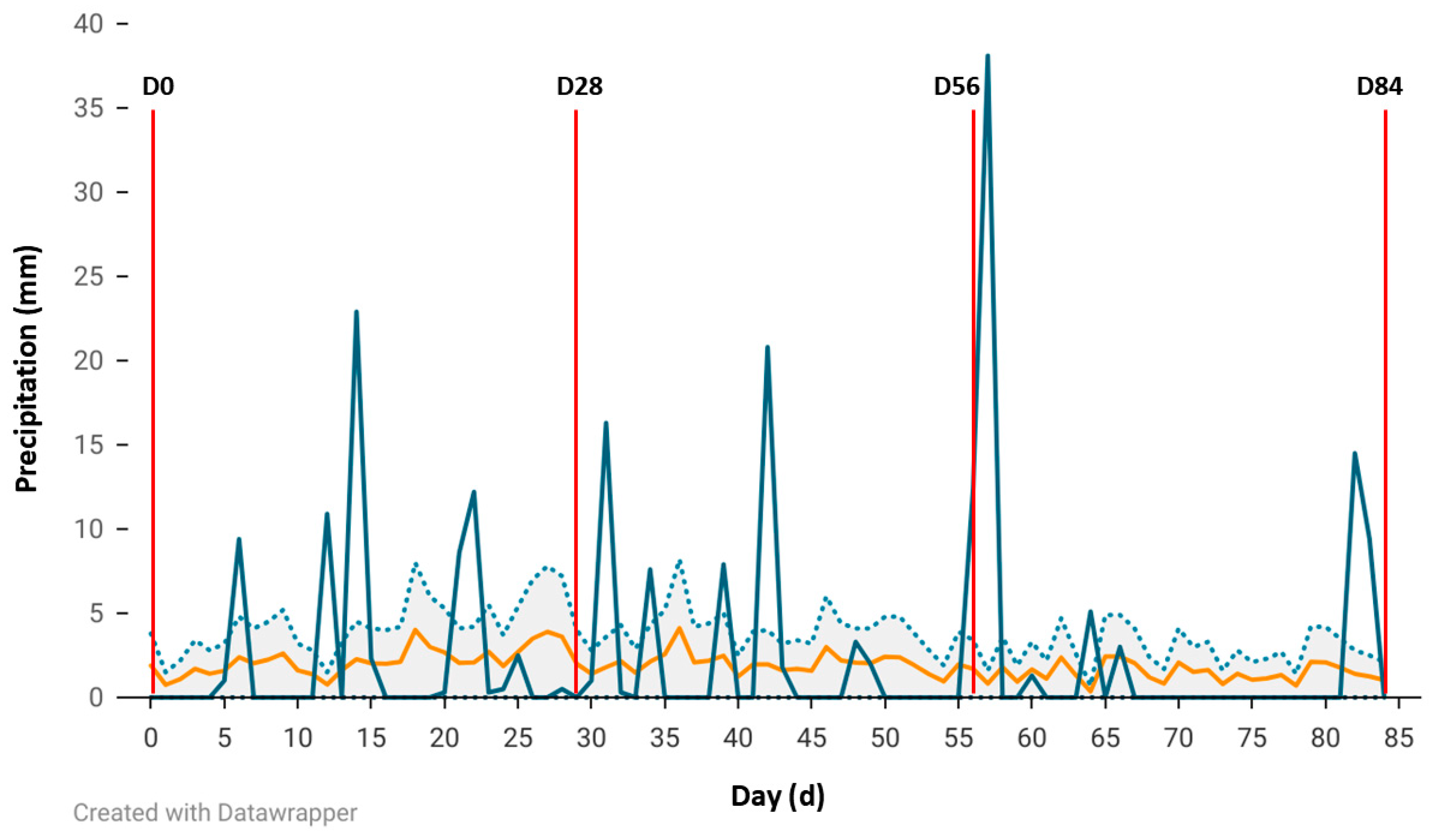
23], the LFST is an official reporting station for the National Weather Service (Station ID USC00030458). Additionally, the LFST has a weather station that is part of the Climate Reference Network. National Weather Service temperature (°C) and rainfall (mm) data for the year (yr) 2024 and historical data (1942–2023) were available through the National Oceanic and Atmospheric Administration climate data online portal (
https://www.ncei.noaa.gov/access/past-weather/72501 (accessed on 30 May 2025)).
2.3. Animal Management
A total of 45 yearling beef heifers of primarily Angus background grazed E+ tall fescue as a single group prior to the start of the treatment period in spring 2024. Before assignment to treatment pastures, heifers were evaluated and confirmed negative for persistent infection with bovine viral diarrhea virus (PI-BVD) based on an ELISA antigen capture test (Cattle Stats, Oklahoma City, OK, USA). Cattle were placed on assigned study pastures 3 d prior to the beginning of the study and were stratified by hair coat color and body weight (BW). Pastures were composed of a gravelly silt loam soil type as described by Diaz et al. [
24]. All replicate pastures were provided a trace mineralized salt block (United Salt Corporation, Houston, TX, USA) as well as artificial or natural shade and water pumped from a well into water tanks (MiraFount, Miraco Livestock Water Systems, Grinnell, IA, USA). Upon assignment to pastures, fecal samples were collected and submitted to the University of Arkansas System Division of Agriculture Parasitology lab for strongyle egg counts (FEC). After samples were collected for FEC, heifers were given an oral anthelmintic (Safeguard, Merck Animal Health Intervet, Inc., Rahway, NJ, USA) at the beginning of the study (D0) as well as a trace mineral injection dose (Multimin 90, Multimin USA, Inc., Fort Collins, CO, USA). Fecal egg counts were assessed again at the conclusion of the study (D84). Fecal samples utilized for FEC were processed as described by Gadberry et al. [
23].
2.4. Experimental Treatments, Pastures, and Forage Management
Nine experimental pastures were located within 3 blocks with 3 pasture replicates per treatment. As described by Diaz et al. [
24], pastures were previously fertilized with 60 kg/ha N prior to grazing. Each 1.62-ha pasture was stocked with 5 heifers. Pastures were sampled at the beginning of the study (D0) and every 28 days (d) until the conclusion of the study for forage mass and nutritive value (University of Arkansas Agricultural Diagnostics Laboratory, Fayetteville, AR, USA). At a study mid-point (D42), pastures were assessed for species composition using a step-point method to ensure pastures were primarily composed of E+ tall fescue. Briefly, the percentage of fescue in pastures was approximated by traversing pastures in a zig-zag pattern. Plant species were recorded at the point of the shoe at every fifth step. Additionally, pastures were evaluated for endophyte infection by collecting 20 fescue tillers/pasture and analyzing via an
Epichloë-specific immunoblot assay (Agronostics Ltd., Watkinsville, GA, USA) with Kentucky bluegrass (
Poa pratensis), annual ryegrass (
Lolium multiflorum), cheatgrass (
Bromus tectorum), and orchardgrass (
Dactylis glomerata) as negative controls.
Treatments were offered over an 84 d period between early April and late June 2024. All heifers grazed primarily E+ tall fescue pastures for the duration of the study. Experimental treatments included: (1) soybean hulls (SH; control; n = 3 pastures), (2) SH and BX tannin-based feed additive (10.0 g heifer−1 d−1; BX; n = 3 pastures), and (3) SH and ATX tannin-based feed additive (5.0 g heifer−1 d−1; ATX; n = 3 pastures). Additive inclusion levels were determined based on product and company recommendations. Treatments were offered on an as-fed basis (1.8 kg/heifer) between 0800 and 0900 daily and fed via trough within each pasture. Each week (wk), BX and ATX additives were prepared in 3 batches with the BX and ATX additives blended with SH prior to feeding. The BX additive (Silvateam S.p.A, San Michele Mondovì, Italy) was composed of a blend of quebracho (Schinopsis lorentzii) and chestnut (Castanea sativa) tannins as well as mixed screenings selected for their specific saponin contents. The ATX additive (Silvateam S.p.A, San Michele Mondovì, Italy) was composed of a blend of quebracho and chestnut tannins as well as mixed screenings selected for their polyphenolic-rich profiles.
2.5. Animal Performance and Health Response Measures
Heifers were individually weighed every 28 d prior to morning feeding. Additionally, hair coat scores, fecal staining scores, fecal samples (25 g) for moisture content, and rectal temperatures were collected every 28 d. Fecal samples for moisture content were dried at 50 °C for 96 h in a forced air oven. Rectal temperature was recorded (GLA M900, Tech Instrumentation Inc., Elizabeth, CO) following temperature reading stabilization for at least 10 s. Hair coat scores (
Table 1) and fecal staining scores (
Table 2) were recorded by 2 trained observers with the average score between observers used for treatment comparisons. Average daily gain and weight gained/period were calculated at the end of the study.
Jugular blood samples were collected on D56, D70, and D84 of the study with an 18G needle into a 10 mL red-top vacutainer (BD, Franklin Lakes, NJ, USA). Samples were placed on ice until processing. Following blood collection on D56, heifers received a subcutaneous injection of a modified live multivalent bovine respiratory disease (BRD) vaccine (One Shot, Zoetis, Kalamazoo, MI, USA) including strains to protect cattle against respiratory disease caused by infectious bovine rhinotracheitis (IBR) virus, parainfluenza3 (PI3) virus, and bovine respiratory syncytial virus (BRSV); respiratory disease and viremia caused by bovine viral diarrhea (BVD) Type 1, including 1b, and Type 2 virus; and bovine pneumonia caused by Mannheimia haemolytica type A1. At processing, blood samples were allowed to clot at room temperature and centrifuged for 20 min at 1700× g. Serum was collected and stored at −20 °C until analysis. Samples corresponding to D0, D14, and D28 post-vaccination were processed and submitted to the Iowa State Veterinary Diagnostics Lab for evaluation for infectious bovine rhinotracheitis (bovine herpesvirus type 1; BHV-1) virus neutralizing (VN) antibody titers. Serum titers were reported as the log2 of the greatest serum dilution of serum that provided complete protection of cells with the lowest dilution tested being 1:4 and the greatest 1:256. Serum samples with a neutralization value of <2 were considered negative and samples ≥2 were considered positive. Additional serum collected on D56 was analyzed for blood urea nitrogen (BUN) using a colorimetric kit (TECO Diagnostics, Anaheim, CA, USA). Assays were performed in a flat-bottom 96-well plate. Samples were analyzed in duplicate on a microplate reader (Biotek EPOCH, Biotek Instruments Inc., Winooksi, VT, USA) at a λ of 340 nm. Additional serum samples collected on D14 post-vaccination were analyzed for haptoglobin concentrations using a commercial ELISA kit (Immunology Consultants Lab, Tigard, OR, USA) with samples analyzed in duplicate. Blood drawn on the final d of the study (D84) was analyzed for serum prolactin using a radioimmunoassay (RIA; University of Tennessee, Knoxville, TN, USA). The intra- and inter-assay coefficients of variation were 4.51% and 9.18%, respectively.
During the D28 collection timepoint, a BX treatment heifer was observed to be unthrifty with loose stool and poor weight gain. This heifer was noted as having a high FEC and treated with a coccidiostat (Corid 9.6% Oral Solution, Huvepharma, Peachtree City, GA, USA) and anthelmintic (Dectomax, Zoetis Inc., NJ, USA) and removed from the study based on study protocols. To assess the impact of removal, analyses were conducted with and without the affected animal. Results remained consistent, indicating minimal effect on overall treatment outcomes. An ATX treatment heifer was never observed visiting the bunk following supplemental feed delivery. This was not accompanied by any signs of illness or stress and was considered to be an outlier. With 14 out of 15 ATX treatment heifers readily appearing at the trough each feeding and heifers consuming the full portion of provided supplement, we do not believe there is any evidence to suggest that this response is attributed to the ATX feed additive. Further, statistical analysis with and without this heifer showed no impact on results.
2.6. Statistical Analysis
Linear mixed models were constructed and analyzed using the LME4 package for R. Measured responses were plotted to determine if any outlier data points were evident. Following raw data assessment, response values were aggregated to the pasture level prior to analysis as pasture was the experimental unit. When appropriate, the initial observation on D0 was included as a covariate and pasture location block was included as a random effect. The Kenward–Rogers method was used for estimating denominator degrees of freedom for ANOVA using the lmerTest package for R and pairwise comparisons using the emmeans package for R. Assumptions for normality, homogeneity, and independence were assessed where appropriate for all models with transformations applied when assumptions were not met. A log transformation was applied to BHV-1 titer data prior to analysis and titers were aggregated to the pasture level using the geometric mean instead of the arithmetic mean prior to further statistical analyses. The FEC was tested for normality using the Shapiro–Wilk test. Kendall’s tau was calculated between observers across all sample dates for both hair coat scores and fecal staining scores. Confidence intervals for historical temperature and precipitation data between 1942 and 2023 were evaluated using the MEANS procedure of SAS 9.4 (SAS Inc., Cary, NC, USA). Weight gain, serum prolactin, BUN, and haptoglobin measures were evaluated using the MIXED procedure of SAS 9.4 with treatment as a fixed effect and block as a random effect. Haptoglobin data was log-transformed after analysis for normalcy. Data were considered significant at p ≤ 0.10 with tendencies recognized at 0.10 < p ≤ 0.15. Due to limited sample size, these thresholds were used to identify potential trends that might warrant further investigation.