Prevalence and Risk Factors of Bovine Viral Diarrhea Virus Antibodies in Dairy Herds of Bangladesh
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Study Population, and Target Population
2.2. Sampling Size Calculation and Sampling Protocol
2.3. Collection of Milk Samples
2.4. Herd- and Cow-Level Data Collection
2.5. Processing of Milk Samples
2.6. Competitive Enzyme-Linked Immunosorbent Assay (cELISA)
2.7. Test Validation and Interpretation
2.8. Data Analysis
2.8.1. Descriptive Statistics
2.8.2. Identification of Risk Factors
Herd-Level Risk Factors
Cow-Level Risk Factors
3. Results
3.1. Summary Statistics
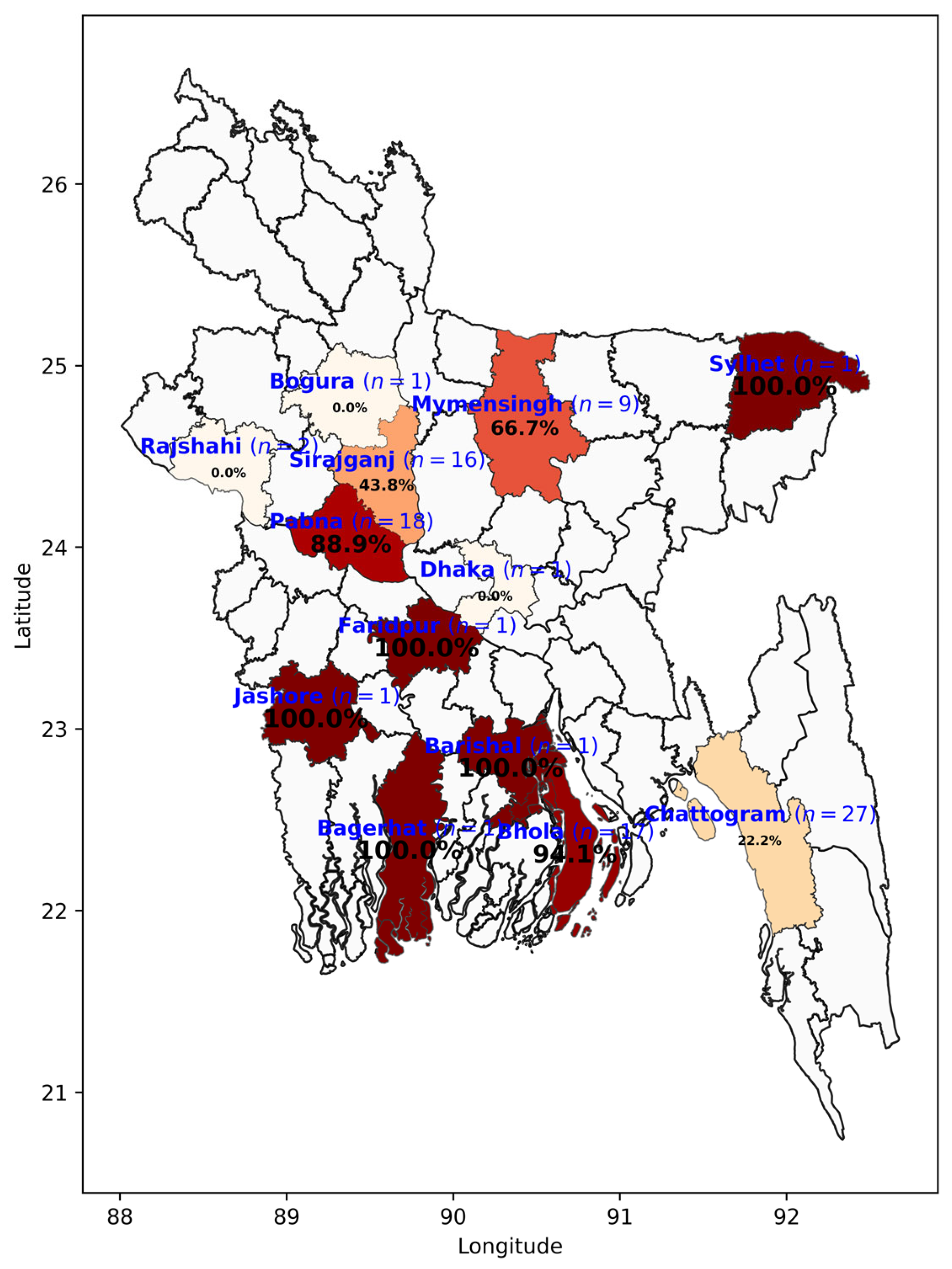
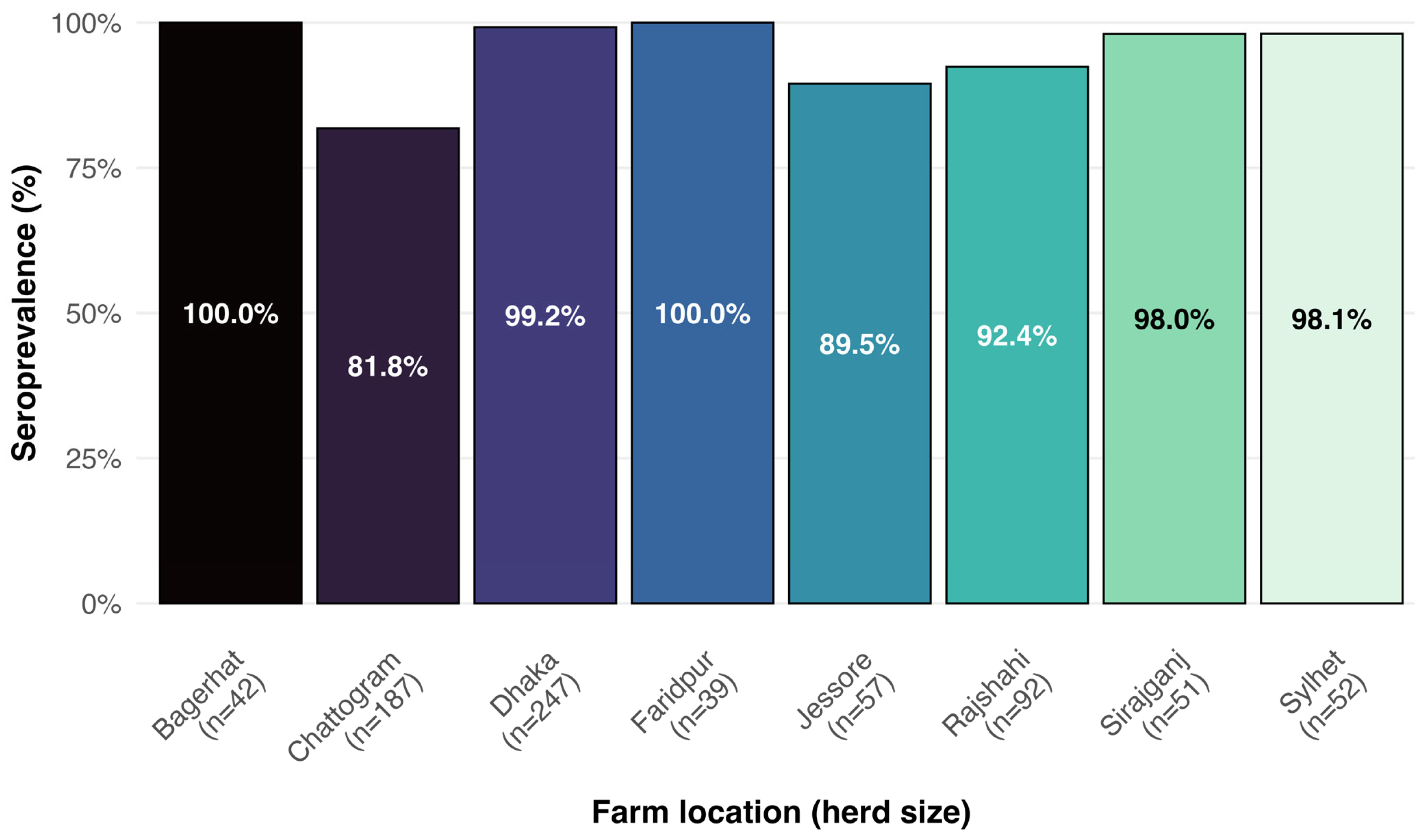
3.2. Herd- and Cow-Level BVD Seroprevalence
3.3. Risk Factors
3.3.1. Herd
3.3.2. Cow
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LDDP | Livestock and Dairy Development Project |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| BVD | Bovine Viral Diarrhea |
| CI | Confidence Interval |
| OD | Optical Density |
| S/N | Sample to Negative Ratio |
| OR | Odds ratio |
| VIF | Variance Inflation Factor |
| cELISA | Competitive Enzyme-Linked Immunosorbent Assay |
References
- Wang, Y.; Pang, F. Diagnosis of Bovine Viral Diarrhea Virus: An Overview of Currently Available Methods. Front. Microbiol. 2024, 15, 1370050. [Google Scholar] [CrossRef]
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.Q.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Mushegian, A.R.; et al. Changes to Taxonomy and the International Code of Virus Classification and Nomenclature Ratified by the International Committee on Taxonomy of Viruses (2017). Arch. Virol. 2017, 162, 2505–2538. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Meyers, G.; Bukh, J.; Gould, E.A.; Monath, T.; Scott Muerhoff, A.; Pletnev, A.; Rico-Hesse, R.; Stapleton, J.T.; Simmonds, P.; et al. Proposed Revision to the Taxonomy of the Genus Pestivirus, Family Flaviviridae. J. Gen. Virol. 2017, 98, 2106–2112. [Google Scholar] [CrossRef]
- Haider, N.; Rahman, M.S.; Khan, S.U.; Mikolon, A.; Gurley, E.S.; Osmani, M.G.; Shanta, I.S.; Paul, S.K.; Macfarlane-Berry, L.; Islam, A.; et al. Identification and Epidemiology of a Rare HoBi-Like Pestivirus Strain in Bangladesh. Transbound. Emerg. Dis. 2014, 61, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Lanyon, S.R.; Hill, F.I.; Reichel, M.P.; Brownlie, J. Bovine Viral Diarrhoea: Pathogenesis and Diagnosis. Vet. J. 2014, 199, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, L.N.; Surendra, K.S.N.L.; Rana, S.K.; Thodangala, N.; Prasad, A.; Muthappa, P.N.; Sharma, G.K. Seroprevalence of Bovine Viral Diarrhoea in Organized Herds in India. Vet. Arh. 2023, 93, 389–398. [Google Scholar] [CrossRef]
- Devi, B.V.; Sireesha, G.; Neeraja, D.; Kumari, L.R.; Amarendrakumar, R. Prevalence of Bovine Viral Diarrhoea Infection in Cattle and Buffaloes from Andhra Pradesh, India. J. Vet. Anim. Sci. 2023, 54, 729–735. [Google Scholar] [CrossRef]
- Thapa, A.; Acharya, M.P.; Raut, R.; Rimal, S. Seroprevalence and Risk Factors of Bovine Viral Diarrhea in Improved Cattle of Chitwan, Nawalpur and Rupandehi Districts of Nepal. Nepal. Vet. J. 2019, 36, 93–97. [Google Scholar] [CrossRef]
- Aasish, G.; Sulav, D.; Umesh, S.; Dojraj, K.; Krishna, K. Seroprevalence and Its Associated Risk Factors of Bovine Neosporosis and Bovine Viral Diarrhea in Cattle of Tilottama Municipality, Rupandehi, Nepal. Int. J. Vet. Sci. Res. 2022, 8, 127–132. [Google Scholar] [CrossRef]
- Aye, Y.M.; Aung, M.; Kyaw, W.O.; Naing, T.; Po, S.P. Prevalence and Associated Factors with Bovine Viral Diarrhoea Virus Antibodies in the Bulk Tank Milk of Small Scale Dairy Herds in Central Myanmar. Adv. Anim. Vet. Sci. 2017, 5, 316–323. [Google Scholar]
- Uddin, M.A.; Ahasan, A.S.M.L.; Islam, K.; Islam, M.Z.; Mahmood, A.; Islam, A.; Islam, K.M.F.; Ahad, A. Seroprevalence of Bovine Viral Diarrhea Virus in Crossbred Dairy Cattle in Bangladesh. Vet. World 2017, 10, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.M.R.; Afrin, F.; Saha, S.S.; Jhontu, S.; Asgar, M.A. Prevalence and Haematological Parameters for Bovine Viral Diarrhoea (BVD) in South Bengal Areas in Bangladesh. Bangladesh Vet. 2015, 32, 48–54. [Google Scholar] [CrossRef][Green Version]
- Moennig, V.; Becher, P. Control of Bovine Viral Diarrhea. Pathogens 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Ahmmed, T.; Prapti, B.B.R.; Rahman, A.; Islam, T.; Chouhan, C.S.; Rahman, A.K.M.A.; Siddique, M.P. First Report of MDR Virulent Pseudomonas Aeruginosa in Apparently Healthy Japanese Quail (Coturnix Japonica) in Bangladesh. PLoS ONE 2025, 20, e0316667. [Google Scholar] [CrossRef]
- Islam, S.K.S.; Rumi, T.B.; Kabir, S.M.L.; van der Zanden, A.G.M.; Kapur, V.; Rahman, A.K.M.A.; Ward, M.P.; Bakker, D.; Ross, A.G.; Rahim, Z. Bovine Tuberculosis Prevalence and Risk Factors in Selected Districts of Bangladesh. PLoS ONE 2020, 15, e0241717. [Google Scholar] [CrossRef]
- Kumar, S.K.; Palanivel, K.M.; Sukumar, K.; Ronald, B.S.M.; Selvaraju, G.; Ponnudurai, G. Herd-Level Risk Factors for Bovine Viral Diarrhea Infection in Cattle of Tamil Nadu. Trop. Anim. Health Prod. 2018, 50, 793–799. [Google Scholar] [CrossRef]
- Bushra, A.; Zaman, R.U.; Rahman, A.S.; Runa, M.A.; Tasnuva, S.; Peya, S.S.; Parvin, M.S.; Islam, T. Biosecurity, Health and Disease Management Practices among the Dairy Farms in Five Districts of Bangladesh. Prev. Vet. Med. 2024, 225, 106142. [Google Scholar] [CrossRef]
- Demil, E.; Fentie, T.; Vidal, G.; Jackson, W.; Lane, J.; Mekonnen, S.A.; Smith, W. Prevalence of Bovine Viral Diarrhea Virus Antibodies and Risk Factors in Dairy Cattle in Gondar City, Northwest Ethiopia. Prev. Vet. Med. 2021, 191, 105363. [Google Scholar] [CrossRef]
- Terrestrial Animal Health Code, 2024. Chapter 4.7, Article 4.7.2. Conditions Applicable to Testing of Bulls and Teaser Animals. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/ (accessed on 20 June 2025).
- Government of the People’s Republic of Bangladesh Animal Disease Rules. 2008. Available online: https://mofl.portal.gov.bd/sites/default/files/files/mofl.portal.gov.bd/policies/09437850_67a7_4c51_9991_e76a68552e2a/Animal%20Disease%20rule-2008.pdf (accessed on 20 June 2025).
- Antos, A.; Rola, J.; Bednarski, M.; Krzysiak, M.K.; Kęsik-Maliszewska, J.; Larska, M. Is Contamination of Bovine-Sourced Material with Bovine Viral Diarrhea Virus Still a Problem in Countries with Ongoing Eradication Campaigns? Ann. Anim. Sci. 2021, 21, 173–192. [Google Scholar] [CrossRef]
- Aragaw, K.; Sibhat, B.; Ayelet, G.; Skjerve, E.; Gebremedhin, E.Z.; Asmare, K. Seroprevalence and Factors Associated with Bovine Viral Diarrhea Virus (BVDV) Infection in Dairy Cattle in Three Milksheds in Ethiopia. Trop. Anim. Health Prod. 2018, 50, 1821–1827. [Google Scholar] [CrossRef]
- Stephenson, M.K.; Palomares, R.A.; White, B.J.; Engelken, T.J.; Brock, K.V. Prevalence of Bovine Viral Diarrhea Virus (BVDV) Persistently Infected Calves in Auction Markets from the Southeastern United States; Association between Body Weight and BVDV-Positive Diagnosis. Prof. Anim. Sci. 2017, 33, 426–431. [Google Scholar] [CrossRef]
- Yue, X.; Steeneveld, W.; van der Voort, M.; van Schaik, G.; Vernooij, J.C.M.; van Duijn, L.; Veldhuis, A.M.B.; Hogeveen, H. The Effect of Bovine Viral Diarrhea Virus Introduction on Milk Production of Dutch Dairy Herds. J. Dairy Sci. 2021, 104, 2074–2086. [Google Scholar] [CrossRef]
| Variable | Categories | Estimate (SE) | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Herd category | ||||
| ≤13 | 0.56 (0.70) | 1.27 (0.33–4.93) | 0.42 | |
| >13 to 23 | - | - | - | |
| >23 to 70 | 0.36 (0.67) | 1.94 (0.49–7.65) | 0.59 | |
| >70 | 2.61 (0.76) | 31.95 (4.9–208.43) | <0.001 | |
| Semen source | ||||
| Source 1 | Not analyzed | Not analyzed | Not analyzed | |
| Source 2 | 1.01 (0.93) | 2.75 (0.44−17.11) | 0.28 | |
| Source 3 | 3.19 (0.91) | 24.47 (4.09−146.53) | <0.001 | |
| Source 4 | Not analyzed | Not analyzed | Not analyzed | |
| Source 5 | 2.19 (0.71) | 8.99 (2.23−36.32) | 0.002 | |
| Source 6 | Reference | Reference | Reference | |
| Source 7 | Not analyzed | Not analyzed | Not analyzed | |
| Source 8 | 3.16 (0.93) | 23.55 (3.8−146.04) | <0.001 |
| Variables | Category | Estimate | SE | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|
| Age (years) | |||||
| Up to 4 | −0.13 | 0.56 | 0.87 (0.28–2.67) | 0.81 | |
| 4 to 5.6 | Reference | – | – | – | |
| 5.6 to 8 | 0.64 | 0.46 | 1.89 (0.75–4.76) | 0.17 | |
| >8 | 1.51 | 0.44 | 4.53 (1.90–10.77) | <0.001 | |
| Physical condition | |||||
| Thin | 2.56 | 1.04 | 13.02 (1.67–101.82) | 0.01 | |
| Normal | Reference | – | – | – | |
| Milk yield (Kg) | |||||
| ≤8.8 | Reference | – | – | – | |
| >8.8 | −0.88 | 0.44 | 0.41 (0.17–0.98) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajeeb, M.S.M.; Alam, M.S.; Islam, M.N.; Islam, M.M.; Adhikari, B.J.; Islam, S.; Rahman, M.S.; Rahman, A.K.M.A. Prevalence and Risk Factors of Bovine Viral Diarrhea Virus Antibodies in Dairy Herds of Bangladesh. Vet. Sci. 2025, 12, 739. https://doi.org/10.3390/vetsci12080739
Sajeeb MSM, Alam MS, Islam MN, Islam MM, Adhikari BJ, Islam S, Rahman MS, Rahman AKMA. Prevalence and Risk Factors of Bovine Viral Diarrhea Virus Antibodies in Dairy Herds of Bangladesh. Veterinary Sciences. 2025; 12(8):739. https://doi.org/10.3390/vetsci12080739
Chicago/Turabian StyleSajeeb, Md. Saifullah Mahmud, Md. Shaffiul Alam, Md. Nazmul Islam, Md. Monirul Islam, Bishwo Jyoti Adhikari, Shanta Islam, Md. Siddiqur Rahman, and A. K. M. Anisur Rahman. 2025. "Prevalence and Risk Factors of Bovine Viral Diarrhea Virus Antibodies in Dairy Herds of Bangladesh" Veterinary Sciences 12, no. 8: 739. https://doi.org/10.3390/vetsci12080739
APA StyleSajeeb, M. S. M., Alam, M. S., Islam, M. N., Islam, M. M., Adhikari, B. J., Islam, S., Rahman, M. S., & Rahman, A. K. M. A. (2025). Prevalence and Risk Factors of Bovine Viral Diarrhea Virus Antibodies in Dairy Herds of Bangladesh. Veterinary Sciences, 12(8), 739. https://doi.org/10.3390/vetsci12080739








