Testicular Tumors and Environmental Pollution: A Comparative Oncoepidemiology Study in the Campania Region from 2020 to 2023
Simple Summary
Abstract
1. Introduction
1.1. Testicular Tumors
1.2. Environmental Disruptors and Testicular Cancer
1.3. The Aim of the Study
2. Materials and Methods
2.1. Oncological Data
2.2. Environmental Data
2.3. Geographic Information Systems
2.4. Sites of National Interest (SINs)
- Sites of National Interest transferred to regional jurisdiction (SIR): Area del Litorale Vesuviano, Litorale Domizio Flegreo, Bacino Idrografico del fiume Sarno, and Pianura.
- Sites of National Interest that remain under the jurisdiction of the Ministry of the Environment (SIN): Napoli Orientale (with quarters of Secondigliano, San Pietro a Patierno, Ponte della Maddalena, Sant’Erasmo, Poggioreale, San Giovanni a Teduccio, Barra, and Ponticelli) and Napoli Bagnoli–Coroglio.
- City of Giuliano: Undergoing perimeter definition, currently transitioning from a SIR to SIN.
2.5. Oncological and Environmental Approach
2.6. Statistical Analysis
2.7. Crude Incidence Rate (CIR) and Standardized Incidence Risk (SIR)
3. Results
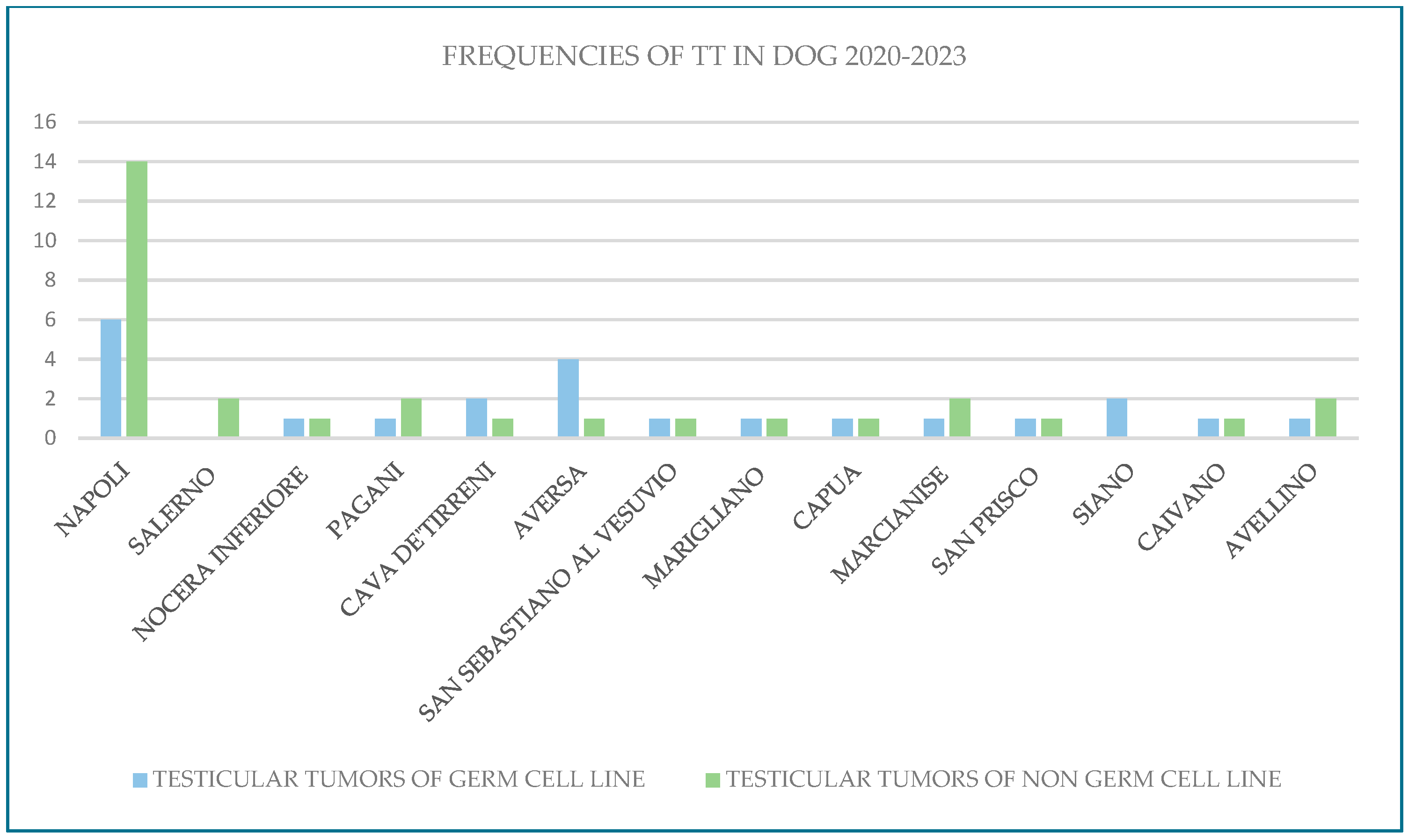
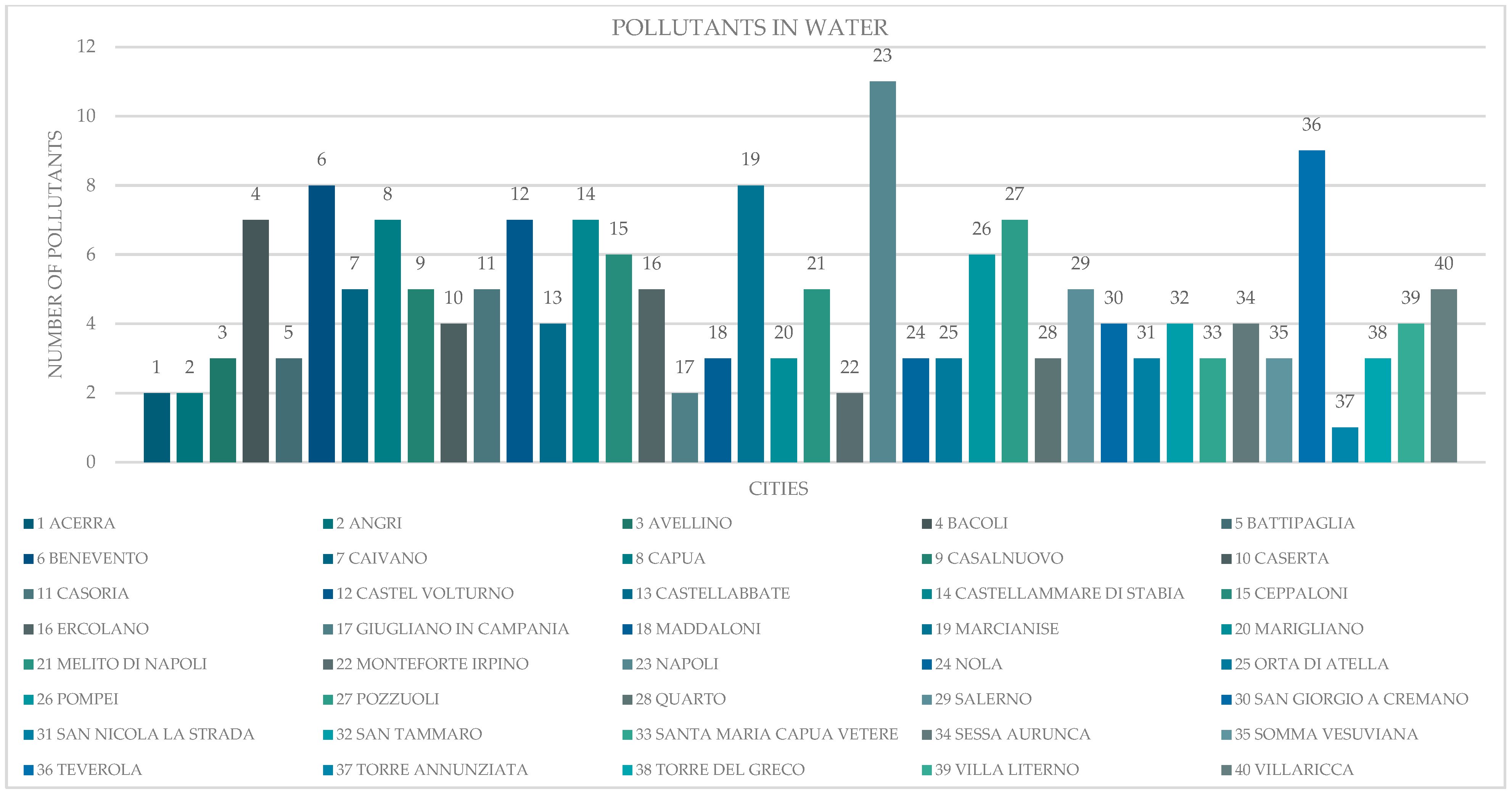
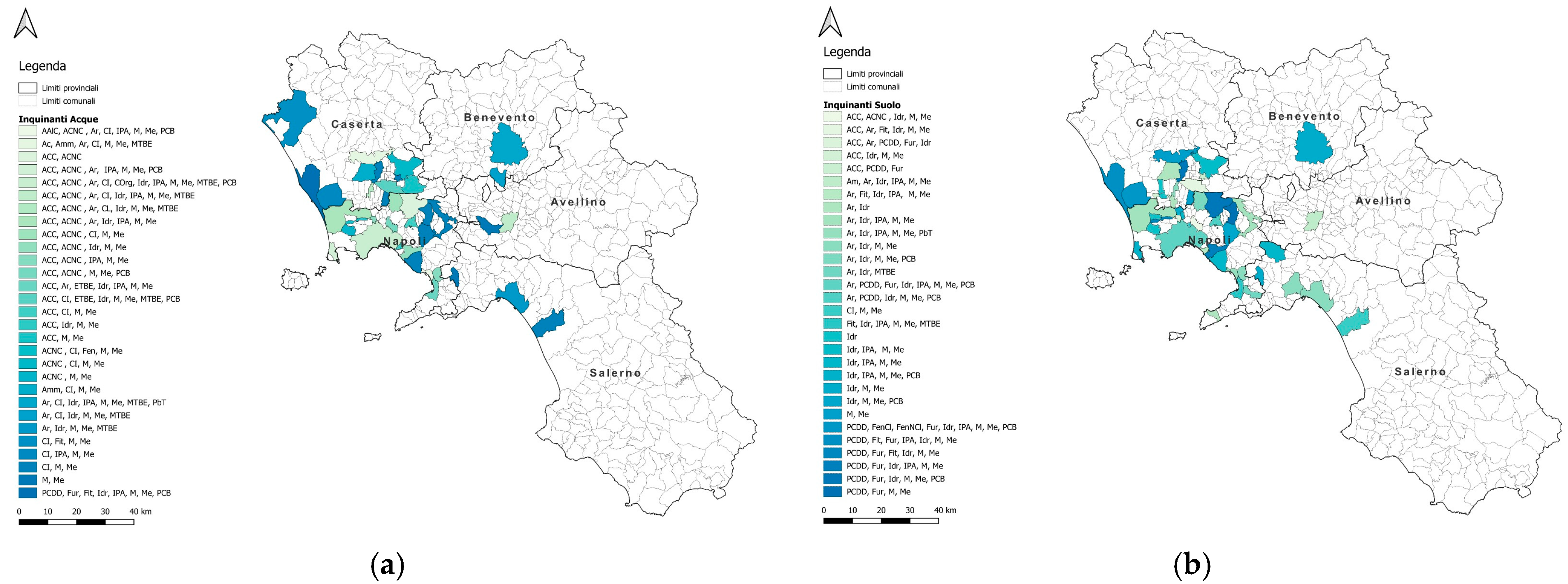
3.1. Environmental Approach
- In dogs:Naples (Secondigliano): Testicular tumors of the non-germ cell line (Leydig cell tumors).Naples (Poggioreale): Non-germ cell line testicular tumors (Leydig cell tumors).Naples (Ponticelli): Testicular tumors of the germ line (seminomas).
- In humans:Naples: Germline and non-germ cell line testicular tumors (seminomas, mixed, embryonal carcinoma, liposarcoma, yolk sac teratoma, neuroendocrine tumor, teratoma).Naples (Ponticelli): Testicular tumors of the germ cell line (seminomas).Naples (Secondigliano): Testicular tumors of the germ cell line (seminomas).
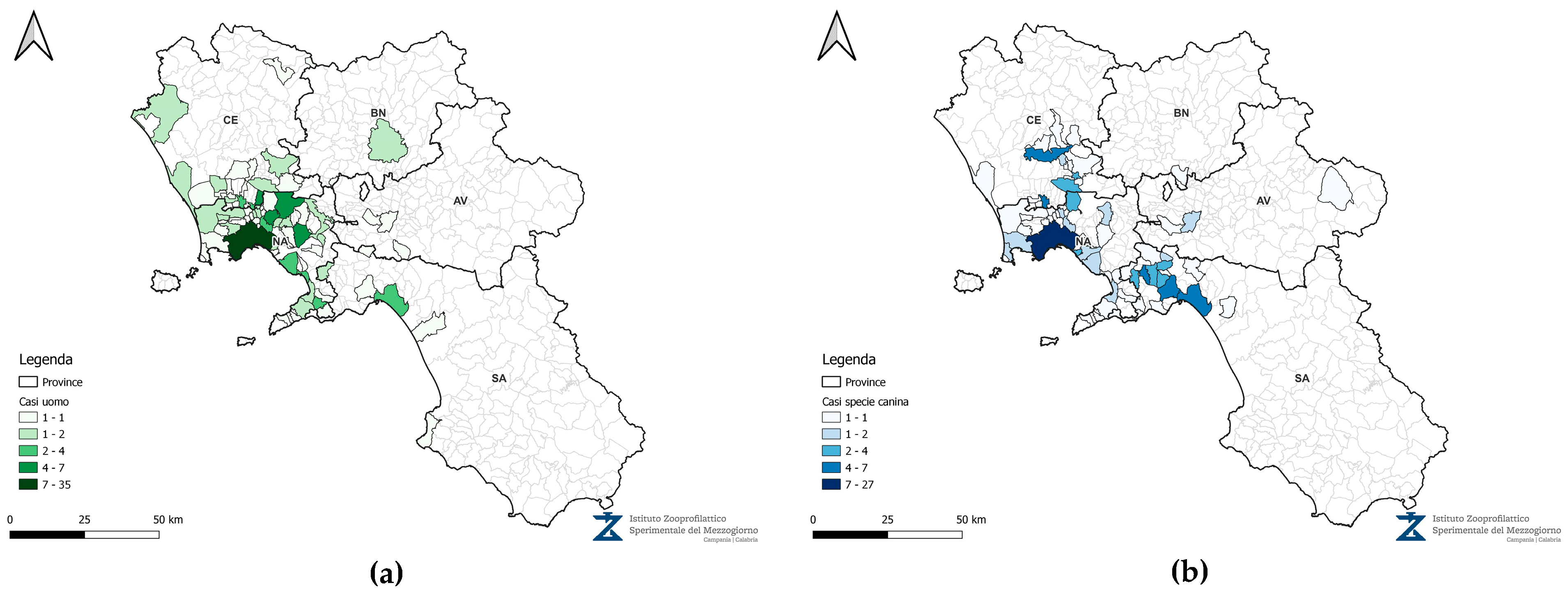
3.2. Oncological Approach
3.3. Correlation Between Cancer and Pollutants
3.4. Statistical Analysis
3.5. Crude Incidence Rate (CIR) and Standardized Incidence Risk (SIR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ranieri, G.; Gadaleta, C.D.; Patruno, R.; Zizzo, N.; Daidone, M.G.; Hansson, M.G.; Paradiso, A.; Ribatti, D. A model of study for human cancer: Spontaneous occurring tumors in dogs. Biological features and translation for new anticancer therapies. Crit. Rev. Oncol. Hematol. 2013, 88, 187–197. [Google Scholar] [CrossRef]
- Sarver, A.L.; Makielski, K.M.; DePauw, T.A.; Schulte, A.J.; Modiano, J.F. Increased risk of cancer in dogs and humans: A consequence of recent extension of lifespan beyond evolutionarily determined limitations? Aging Cancer 2022, 3, 3–19. [Google Scholar] [CrossRef]
- Vaccaro, E.; Navas, L.; Ercolano, M.; Piegari, G.; Di Napoli, E.; Papparella, S.; Inverso, D.; Brunetti, B.; Paciello, O.; Russo, V. Immunohistochemical Investigation of Cyclooxygenase-2 Expression in Rabbit Uterine Adenocarcinoma and the Potential Use of COX-2 Inhibitors in Cancer Therapy. Animals 2024, 14, 3169. [Google Scholar] [CrossRef] [PubMed]
- De Biase, D.; De Leo, M.; Piegari, G.; d’Aquino, I.; Di Napoli, E.; Mercogliano, C.; Calabria, A.; Pula, A.; Navas, L.; Russo, V.; et al. Investigation of the Theragnostic Role of KIT Expression for the Treatment of Canine Mast Cell Tumors with Tyrosine Kinase Inhibitors. Vet. Sci. 2024, 11, 492. [Google Scholar] [CrossRef]
- Available online: https://isde-treviso.it/wp-content/uploads/2021/06/Inquinamento-chimico-interferenti-endocrini-e-rischio-feto materno.pdf (accessed on 20 January 2020).
- Available online: https://www.micuro.it/enciclopedia/benessere/interferenti-endocrini (accessed on 23 February 2020).
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; García-Montalvo, E.A.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef]
- Harbo Poulsen, A.; Arthur Hvidtfeldt, U.; Sørensen, M.; Puett, R.; Ketzel, M.; Brandt, J.; Christensen, J.H.; Geels, C.; Raaschou-Nielsen, O. Components of particulate matter air-pollution and brain tumors. Environ. Int. 2020, 144, 106046. [Google Scholar] [CrossRef]
- Pacifico, L.R.; Pizzolante, A.; Guarino, A.; Iannone, A.; Esposito, M.; Albanese, S. Wildfires as a Source of Potentially Toxic Elements (PTEs) in Soil: A Case Study from Campania Region (Italy). Int. J. Environ. Res. Public. Health 2023, 20, 4513. [Google Scholar] [CrossRef]
- Temkin, A.M.; Hocevar, B.A.; Andrews, D.Q.; Naidenko, O.V.; Kamendulis, L.M. Application of the Key Characteristics of Carcinogens to Per and Polyfluoroalkyl Substances. Int. J. Environ. Res. Public. Health 2020, 17, 1668. [Google Scholar] [CrossRef]
- Frehse, M.S.; Martins, M.I.; Ono, E.Y.; Bracarense, A.P.; Bissoqui, L.Y.; Teixeira, E.M.; Santos, N.J.; Freire, R.L. Aflatoxins ingestion and canine mammary tumors: There is an association? Food Chem. Toxicol. 2015, 84, 74–78. [Google Scholar] [CrossRef]
- Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/asbestos (accessed on 20 January 2020).
- Available online: https://www.airc.it/cancro/informazioni-tumori/corretta-informazione/tutti-i-tipi-di-amianto-sono-cancerogeni (accessed on 20 January 2020).
- Glickman, L.T.; Domanski, L.M.; Maguire, T.G.; Dubielzig, R.R.; Churg, A. Mesothelioma in pet dogs associated with exposure of their owners to asbestos. Environ. Res. 1983, 32, 305–313. [Google Scholar] [CrossRef]
- Available online: https://www.osservatorioamianto.com/dipartimenti/ricerca-e-curadelmesotelioma/mesotelioma-nuove-terapie (accessed on 1 March 2021).
- Wang, J.P.; Qi, L.; Moore, M.R.; Ng, J.C. A review of animal models for the study of arsenic carcinogenesis. Toxicol. Lett. 2002, 133, 17–31. [Google Scholar] [CrossRef]
- Craun, K.; Luethcke, K.R.; Shafer, M.; Stanton, N.; Zhang, C.; Schauer, J.; Faulkes, J.; Sundling, K.E.; Kurtycz, D.; Malecki, K.; et al. Environmental chemical exposures in the urine of dogs and people sharing the same households. J. Clin. Transl. Sci. 2020, 5, e54. [Google Scholar] [CrossRef] [PubMed]
- Spatari, G.; Allegra, A.; Carrieri, M.; Pioggia, G.; Gangemi, S. Epigenetic Effects of Benzene in Hematologic Neoplasms: The Altered Gene Expression. Cancers 2021, 13, 2392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sui, P.; Wu, B.; Chen, A.; Lu, Y.; Hou, F.; Cheng, X.; Cui, S.; Song, J.; Huang, G.; et al. Benzene induces rapid leukemic transformation after prolonged hematotoxicity in a murine model. Leukemia. 2021, 35, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Koestel, Z.L.; Backus, R.C.; Tsuruta, K.; Spollen, W.G.; Johnson, S.A.; Javurek, A.B.; Ellersieck, M.R.; Wiedmeyer, C.E.; Kannan, K.; Xue, J.; et al. Bisphenol A (BPA) in the serum of pet dogs following short-term consumption of canned dog food and potential health consequences of exposure to BPA. Sci. Total Environ. 2017, 579, 1804–1814. [Google Scholar] [CrossRef]
- Wang, K.; Huang, D.; Zhou, P.; Su, X.; Yang, R.; Shao, C.; Wu, J. Bisphenol A exposure triggers the malignant transformation of prostatic hyperplasia in beagle dogs via cfa-miR-204/KRAS axis. Ecotoxicol. Environ. Saf. 2022, 235, 113430. [Google Scholar] [CrossRef]
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced cancers in animals and in humans. Int. J. Occup. Environ. Health 2007, 13, 202–212. [Google Scholar] [CrossRef]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef]
- Smith, N.; Luethcke, K.R.; Craun, K.; Trepanier, L. Risk of bladder cancer and lymphoma in dogs is associated with pollution indices by county of residence. Vet. Comp. Oncol. 2022, 20, 246–255. [Google Scholar] [CrossRef]
- Wong, I.C.; Ng, Y.K.; Lui, V.W. Cancers of the lung, head and neck on the rise: Perspectives on the genotoxicity of air pollution. Chin. J. Cancer 2014, 33, 476–480. [Google Scholar] [CrossRef]
- Ledford, H. How air pollution causes lung cancer—without harming DNA. Nature 2023, 616, 419–420. [Google Scholar] [CrossRef]
- Rébé, C.; Ghiringhelli, F. Interleukin-1β and Cancer. Cancers 2020, 12, 1791. [Google Scholar] [CrossRef]
- Jiang, C.L.; He, S.W.; Zhang, Y.D.; Duan, H.X.; Huang, T.; Huang, Y.C.; Li, G.F.; Wang, P.; Ma, L.J.; Zhou, G.B.; et al. Air pollution and DNA methylation alterations in lung cancer: A systematic and comparative study. Oncotarget 2017, 8, 1369–1391. [Google Scholar] [CrossRef] [PubMed]
- Bettini, G.; Morini, M.; Marconato, L.; Marcato, P.S.; Zini, E. Association between environmental dust exposure and lung cancer in dogs. Vet. J. 2010, 186, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Di Lella, B.N. Analisi e Valutazione Dell’esposizione al Fumo Passivo di Tabacco di Sigaretta Come Fattore di Rischio Per Le patologie Orali Nei Gatti. Bachelor’s Thesis, University of Naples Federico II, Naples, Italy, 2018. [Google Scholar]
- Available online: https://www.efsa.europa.eu/en/topics/topic/dioxins-and-pcbs (accessed on 15 April 2022).
- Ferrante, M.C.; Di Vaio, P.; Magli, E.; Frecentese, F.; Melì, R.; Caliendo, G.; Corvino, A.; Fiorino, F.; Giordano, F.; Monnolo, A.; et al. PCB levels in adipose tissue of dogs from illegal dumping sites in Campania region (Italy). Chemosphere 2020, 244, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Donato, F.; Moneda, M.; Portolani, N.; Rossini, A.; Molfino, S.; Ministrini, S.; Contessi, G.B.; Pesenti, S.; De Palma, G.; Gaia, A.; et al. Polychlorinated biphenyls and risk of hepatocellular carcinoma in the population living in a highly polluted area in Italy. Sci. Rep. 2021, 11, 3064. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 2013, 14, 287–288. [Google Scholar] [CrossRef]
- Dimatteo, M.; Di Napoli, E.; Paciello, O.; d’Aquino, I.; Iaccarino, D.; D’amore, M.; Guida, M.; Cozzolino, L.; Serpe, F.P.; Fusco, G.; et al. Pathological Changes and CYP1A1 Expression as Biomarkers of Pollution in Sarpa Salpa and Diplodus Sargus. Animals 2024, 14, 3160. [Google Scholar] [CrossRef]
- Faja, F.; Esteves, S.; Pallotti, F.; Cicolani, G.; Di Chiano, S.; Delli Paoli, E.; Lenzi, A.; Lombardo, F.; Paoli, D. Environmental disruptors and testicular cancer. Endocrine 2022, 78, 429–435. [Google Scholar] [CrossRef]
- Soto-Heras, S.; Reinacher, L.; Wang, B.; Oh, J.E.; Bunnell, M.; Park, C.J.; Hess, R.A.; Ko, C.J. Cryptorchidism and testicular cancer in the dog: Unresolved questions and challenges in translating insights from human studies. Biol. Reprod. 2024, 111, 269–291. [Google Scholar] [CrossRef]
- Wanjari, U.R.; Gopalakrishnan, A.V. Cadmium as a male reproductive toxicant and natural and non-natural ways to tackle it: A review. Environ. Sci. Pollut. Res. Int. 2024, 31, 18340–18361. [Google Scholar] [CrossRef]
- Pocar, P.; Grieco, V.; Aidos, L.; Borromeo, V. Endocrine-Disrupting Chemicals and Their Effects in Pet Dogs and Cats: An Overview. Animals 2023, 13, 378. [Google Scholar] [CrossRef] [PubMed]
- Nava-Castro, K.E.; Ramírez-Nieto, R.; Méndez-García, L.A.; Girón-Pérez, M.I.; Segovia-Mendoza, M.; Navidad-Murrieta, M.S.; Morales Montor, J. Environmental Pollution as a Risk Factor in Testicular Tumour Development: Focus on the Interaction between Bisphenol A and the Associated Immune Response. Int. J. Environ. Res. Public. Health. 2019, 16, 4113. [Google Scholar] [CrossRef] [PubMed]
- Sumner, R.N.; Byers, A.; Zhang, Z.; Agerholm, J.S.; Lindh, L.; England, G.C.W.; Lea, R.G. Environmental chemicals in dog testes reflect their geographical source and may be associated with altered pathology. Sci. Rep. 2021, 11, 7361. [Google Scholar] [CrossRef]
- Thorvaldsen, T.E.; Nødtvedt, A.; Grotmol, T.; Gunnes, G. Morphological and immunohistochemical characterisation of seminomas in Norwegian dogs. Acta Vet. Scand. 2012, 54, 52. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours: Urinary and Male Genital Tumours, 5th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2022. [Google Scholar]
- Kennedy, P.C.; Cullen, J.M.; Edwards, J.F.; Goldschmidt, M.H.; Larsen, S.; Munson, L.; Nielsen, S. Histological Classification of Tumors of the Genital System of Domestic Animals; Armed Forces Institute of Pathology and American Registry of Pathology: Washington, DC, USA, 2007; Volume IV. [Google Scholar]
- Pinello, K.; Baldassarre, V.; Steiger, K.; Paciello, O.; Pires, I.; Laufer-Amorim, R.; Oevermann, A.; Niza-Ribeiro, J.; Aresu, L.; Rous, B.; et al. Vet-ICD-O-Canine-1, a System for Coding Canine Neoplasms Based on the Human ICD-O-3.2. Cancers 2022, 14, 1529. [Google Scholar] [CrossRef]
- Available online: https://www.arpacampania.it/web/guest/siti-contaminati (accessed on 10 July 2023).
- Available online: https://bonifichesiticontaminati.mite.gov.it/sin/anagrafica-denominazione-caratteristiche/ (accessed on 10 July 2023).
- Available online: https://www.isprambiente.gov.it/it/pubblicazioni/manuali-e-linee-guida/manuale-per-le-indagini-ambientali-nei-siti (accessed on 10 July 2023).
- Available online: https://www.isprambiente.gov.it/files/temi/tec-confronto-tabelle-limiti.pdf (accessed on 10 July 2023).
- Available online: https://www.esri.com/it-it/what-is-gis/overview (accessed on 10 July 2023).
- Available online: https://www.geospatialworld.net/blogs/managing-the-environment-using-gis/ (accessed on 18 July 2023).
- Available online: http://www.campaniatrasparente.it/gis.php (accessed on 22 July 2023).
- Available online: https://it.wikipedia.org/wiki/Siti_di_interesse_nazionale#Contaminanti_principali (accessed on 25 July 2023).
- Available online: https://www.regione.campania.it/regione/it/la-tua-campania/portale-gisa-sicurezza-alimentare-e-veterinaria/portale-gisa-sicurezza-alimentare-e-veterinaria?page=1 (accessed on 15 July 2025).
- Available online: https://demo.istat.it/app/?i=POS&l=it (accessed on 15 July 2025).
- Zhu, L.; Hajeb, P.; Fauser, P.; Vorkamp, K. Endocrine—Disrupting Chemicals in Indoor Dust: A Review of Temporal and Spatial Trends and Human Exposure. Sci. Total Environ. 2023, 874, 162374. [Google Scholar] [CrossRef]
- Wooten, K.J.; Smith, P.N. Canine Toys and Training Devices as Sources of Exposure to Phthalates and Bisphenol A: Quantitation of Chemicals in Leachate and In Vitro Screening for Endocrine Activity. Chemosphere 2013, 93, 2245–2253. [Google Scholar] [CrossRef]




| Male Population | Male Observed Cases (O) | Male Populations at Risk (N) |
|---|---|---|
| Dog | 221 | 512.791 |
| Human | 174 | 28.876.799 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Napoli, E.; De Biase, D.; degli Uberti, B.; Dimatteo, M.; Baldi, L.; Cavallo, S.; Rosato, G.; Izzillo, D.; Piegari, G.; Russo, V.; et al. Testicular Tumors and Environmental Pollution: A Comparative Oncoepidemiology Study in the Campania Region from 2020 to 2023. Vet. Sci. 2025, 12, 695. https://doi.org/10.3390/vetsci12080695
Di Napoli E, De Biase D, degli Uberti B, Dimatteo M, Baldi L, Cavallo S, Rosato G, Izzillo D, Piegari G, Russo V, et al. Testicular Tumors and Environmental Pollution: A Comparative Oncoepidemiology Study in the Campania Region from 2020 to 2023. Veterinary Sciences. 2025; 12(8):695. https://doi.org/10.3390/vetsci12080695
Chicago/Turabian StyleDi Napoli, Evaristo, Davide De Biase, Barbara degli Uberti, Maria Dimatteo, Loredana Baldi, Stefania Cavallo, Guido Rosato, Daniela Izzillo, Giuseppe Piegari, Valeria Russo, and et al. 2025. "Testicular Tumors and Environmental Pollution: A Comparative Oncoepidemiology Study in the Campania Region from 2020 to 2023" Veterinary Sciences 12, no. 8: 695. https://doi.org/10.3390/vetsci12080695
APA StyleDi Napoli, E., De Biase, D., degli Uberti, B., Dimatteo, M., Baldi, L., Cavallo, S., Rosato, G., Izzillo, D., Piegari, G., Russo, V., Rossetti, S., Bruzzese, F., Palmieri, C., Budillon, A., & Paciello, O. (2025). Testicular Tumors and Environmental Pollution: A Comparative Oncoepidemiology Study in the Campania Region from 2020 to 2023. Veterinary Sciences, 12(8), 695. https://doi.org/10.3390/vetsci12080695










