Serogroup Prevalence, Virulence Profile and Antibiotic Resistance of Avian Pathogenic Escherichia coli Isolated from Broiler Chicken
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of Study and Bacterial Strains
2.2. Serogrouping
2.3. Virulence Genotyping
2.4. Antimicrobial Susceptibility Testing
2.5. Resistance Genotyping
2.6. Statistical Analysis
3. Results
3.1. Samples
3.2. Serogrouping
3.3. Virulence Genes
3.4. Antibiotic Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, T.A.; Elias, W.P.; Scaletsky, I.C.; Guth, B.E.; Rodrigues, J.F.; Piazza, R.M.; Ferreira, L.C.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47, 3–30. [Google Scholar]
- Wani, B.M.; Darzi, M.M.; Mir, M.S.; Adil, S.; Shakeel, I. Pathological and pharmacochemical evaluation of broiler chicken affected naturally with colibacillosis in Kashmir valley. Int. J. Pharmacol. 2017, 13, 388–395. [Google Scholar] [CrossRef]
- Amin, U.; Kamil, S.A.; Shah, S.A.; Dar, T.A.; Mir, M.S.; Ali, R.; Kashoo, Z.A.; Wani, B.M. Serotyping and prevalence of avian pathogenic Escherichia coli infection in broilers in Kashmir. Pharm. Innov. 2017, 6, 336–338. [Google Scholar]
- Bozcal, E. Distribution and virulence properties of extra-intestinal pathogenic Escherichia coli in Turkey. Microbiol. Med. 2016, 31, 99–102. [Google Scholar]
- Ewers, C.; Li, G.; Wilking, H.; Kiessling, S.; Alt, K.; Antáo, E.M.; Laturnus, C.; Diehl, I.; Glodde, S.; Homeier, T.; et al. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: How closely related are they? Int. J. Med. Microbiol. 2007, 297, 163–176. [Google Scholar] [CrossRef]
- Mehat, J.W.; Vanvliet, A.H.M.; La Ragione, R.M. The Avian Pathogenic Escherichia coli (APEC) pathotype is comprised of multiple distinct, independent genotypes. Avian. Pathol. 2021, 50, 402–416. [Google Scholar] [CrossRef]
- Amin, U.; Kamil, S.A.; Wani, B.M.; Qureshi, S.; Shah, S.A.; Dar, T.A.; Adil, S.; Mir, M.S. Haematological and biochemical alterations of broiler chicken affected naturally with colibacillosis. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1906–1913. [Google Scholar] [CrossRef]
- Shah, S.; Mir, M.; Kamil, S.; Shafi, M.; Adil, S.; Wani, P.; Rather, M. Prevalence and isolation of avian pathogenic Escherichia coli from colibacillosis affected broiler chicken in kashmir valley. Life Sci. Leaflets 2020, 125, 6–13. [Google Scholar]
- Xu, J.; Sangthong, R.; McNeil, E.; Tang, R.; Chongsuvivatwong, V. Antibiotic use in chicken farms in northwestern China. Antimicrob. Resist. Infect. Control 2020, 9, 10. [Google Scholar] [CrossRef]
- Ture, M.; Altinok, I. Detection of putative virulence genes of Lactococcus garvieae. Dis. Aquat. Organ. 2016, 119, 59–66. [Google Scholar] [CrossRef]
- Dang, H.T.; Park, H.K.; Myung, S.C.; Kim, W. Development of a novel PCR assay based on the 16S–23S rRNA internal transcribed spacer region for the detection of Lactococcus garvieae. J. Fish Dis. 2012, 35, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- CLSI M100; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021.
- Chen, S. Characterization and Molecular Mechanisms of Antimicrobial Resistance in Foodborne Pathogens. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2004. [Google Scholar]
- Al Azad, M.A.R.; Rahman, M.M.; Amin, R.; Begum, M.I.A.; Fries, R.; Husna, A.; Khairalla, A.S.; Badruzzaman, A.T.M.; El Zowalaty, M.E.; Lampang, K.N. Susceptibility and multidrug resistance patterns of Escherichia coli isolated from cloacal swabs of live broiler chickens in Bangladesh. J. Pathog. 2019, 8, 118. [Google Scholar] [CrossRef]
- Schutzius, G.C. Antibiotic Resistance in Septic Sludge and Receiving Environments of Ho Chi Minh City, Vietnam. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2018. [Google Scholar]
- Armitage, P.; Berry, G.; Matthews, J.N.S. Statistical Methods in Medical Research; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Sharada, R.; Ruban, S.W.; Thiyageeswaran, M. Isolation, characterization and antibiotic resistance pattern of Escherichia coli isolated from poultry. Am. Eurasian. J. Sci. Res. 2010, 5, 18–22. [Google Scholar]
- Yaguchi, K.; Ogitani, T.; Osawa, R.; Kawano, M.; Kokumai, N.; Kaneshige, T.; Noro, T.; Masubuchi, K.; Shimizu, Y. Virulence factors of avian pathogenic Escherichia coli strains isolated from chickens with colisepticemia in Japan. Avian. Dis. 2007, 51, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Seifi, S.; Khoshbakht, R.; Atabak, A.R. Antibiotic susceptibility, serotyping and pathogenicity evaluation of avian Escherichia coli isolated from broilers in northern Iran. Bulgar. J. Vet. Med. 2015, 18, 173–179. [Google Scholar] [CrossRef]
- Magray, S.N.; Wani, S.A.; Kashoo, Z.A.; Bhat, M.A.; Adil, S.; Farooq, S.; Nishikawa, Y. Serological diversity, molecular characterisation and antimicrobial sensitivity of avian pathogenic Escherichia coli (APEC) isolates from broiler chickens in Kashmir, India. Anim. Prod. Sci. 2019, 59, 338–346. [Google Scholar] [CrossRef]
- Barnes, H.J.; Nolan, L.K.; Vaillancourt, J.F. Colibacilliosis. In Diseases of Poultry; Saif, Y.M., Fadly, A.M., Eds.; Blackwell: Ames, IA, USA, 2008; pp. 691–732. [Google Scholar]
- Johnson, T.J.; Wannemuehler, Y.; Johnson, S.J.; Stell, A.L.; Doetkott, C.; Johnson, J.R.; Kim, K.S.; Spanjaard, L.; Nolan, L.K. Comparison of Extra intestinal Pathogenic Escherichia coli Strains from Human and Avian Sources Reveals a Mixed Subset Representing Potential Zoonotic Pathogens. Appl. Environ. Microbiol. 2008, 74, 7043–7050. [Google Scholar] [CrossRef]
- Subedi, M.; Luitel, H.; Devkota, B.; Bhattarai, R.K.; Phuyal, S.; Panthi, P.; Shrestha, A.; Chaudhary, D.K. Antibiotic resistance pattern and virulence genes content in avian pathogenic Escherichia coli (APEC) from broiler chickens in Chitwan, Nepal. BMC Vet. Res. 2018, 14, 113. [Google Scholar] [CrossRef]
- Delicato, E.R.; de Brito, B.G.; Gaziri, L.C.J.; Vidotto, M.C. Virulence associated genes in Escherichia coli isolates from poultry with colibacillosis. Vet. Microbiol. 2003, 94, 97–103. [Google Scholar]
- McPeake, S.J.W.; Smyth, J.A.; Ball, H.J. Characterization of avian pathogenic Escherichia coli (APEC) associated with colisepticaemia compared to faecal isolates from healthy birds. Vet. Microbiol. 2005, 110, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Dziva, F.; Hauser, H.; Connor, T.R.; van Diemen, P.M.; Prescott, G.; Langridge, G.C.; Eckert, S.; Chaudhuri, R.R.; Ewers, C.; Mellata, M.; et al. Sequencing and functional annotation of avian pathogenic Escherichia coli serogroup O78 strains reveal the evolution of E. coli lineages pathogenic for poultry via distinct mechanisms. Infect. Immun. 2013, 81, 838–849. [Google Scholar] [CrossRef]
- Rodriguez-Siek, K.E.; Giddings, C.W.; Doetkott, C.; Johnson, T.J.; Fakhr, M.K.; Nolan, L.K. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology 2005, 151, 2097–2110. [Google Scholar] [CrossRef] [PubMed]
- Sabri, M.; Leveillé, S.; Dozois, C.M. A SitABCD homologue from an avian pathogenic Escherichia coli strain mediates transport of iron and manganese and resistance to hydrogen peroxide. Microbiology 2006, 152, 745–758. [Google Scholar] [CrossRef]
- Eltai, N.O.; Yassine, H.M.; El-Obeid, T.; Al-Hadidi, S.H.; Al Thani, A.A.; Alali, W.Q. Prevalence of Antibiotic-Resistant Escherichia coli Isolates from Local and Imported Retail Chicken Carcasses. J. Food Prot. 2020, 83, 2200–2208. [Google Scholar] [CrossRef]
- Van, T.T.; Chin, J.; Chapman, T.; Tran, L.T.; Coloe, P.J. Safety of raw meat and shellfish in Vietnam: An analysis of Escherichia coli isolations for antibiotic resistance and virulence genes. Int. J. Food. Microbiol. 2008, 124, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Fabrega, A.; Madurga, S.; Giralt, E.; Villa, J. Mechanism of action of and resistance to quinolones. Microb. Biotechnol. 2008, 2, 40–61. [Google Scholar] [CrossRef]
- Sarker, M.S.; Mannan, M.S.; Ali, M.Y.; Bayzid, M.; Ahad, A.; Bupasha, Z.B. Antibiotic resistance of Escherichia coli isolated from broilers sold at live bird markets in Chattogram, Bangladesh. J. Adv. Vet. Anim. Res. 2019, 6, 272. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; WHO Press: Geneva, Switzerland, 2015; Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 15 May 2025).
- World Organisation for Animal Health. OIE Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials. 2021. Available online: https://www.woah.org/en/what-we-do/global-initiatives/antimicrobial-resistance/ (accessed on 15 May 2025).
| S.No. | Target Gene | Sequence 5′–3′ | Primer Conc. (µM) | Product Size | Reference |
|---|---|---|---|---|---|
| 1 | iss | (F)GTGGCGAAAACT AGTAAAACAGC | 1.0 | 762 | [10] |
| (R)CGCCTCGGGGTGGATAA | |||||
| 2 | iucC | (F) CGCCGTGGCTGGGGTAAG | 1.0 | 541 | [11] |
| (R) CAGCCGGTTCAC CAAGTATCACTG | |||||
| 3 | sitA | (F)AGGGGGCACAACTGATTCTCG | 0.5 | 608 | [12] |
| (R)TACCGGGCCGTTTTCTGTGC | |||||
| 4 | tsh | (F) GGTGGTGCACTGGAGTGG | 1.0 | 642 | [10] |
| (R) AGTCCAGCGTGATAGTGG | |||||
| 5 | papC | (F) GTGGCAGTATG AGTAATGACCGTTA | 0.5 | 205 | [10] |
| (R) ATATCCTTTCTGC AGGGATGCAATA |
| Antimicrobial Agent | Resistance Gene | Sequence | Product Size | Primer Conc. | Reference |
|---|---|---|---|---|---|
| Quinolones | qnrA | (F) GGT ATG GAT ATT ATT GAT AAA G (R) CTA ATC CGG CAG CAC TAT TTA | 670 | 0.2 | [14] |
| Sulfonamide | Sul1 | (F) TTC GGC ATT CTG AAT CTC AC (R) ATG ATC TAA CCC TCG GTC TC | 822 | 0.2 | [15] |
| Streptomycin | aadA1 | (F) TAT CCA GCT AAG CGC GAA CT (R) ATT TGC CGA CTA CCT TGG TC | 447 | 0.2 | [15] |
| Tetracycline | tet(A) | (F) GGT TCA CTC GAA CGA CGT CA (R) CTG TCC GAC AAG TTG CAT GA | 577 | 0.2 | [16] |
| tet(B) | (F) CCT CAG CTT CTC AAC GCG TG (R) GCA CCT TGC TGA TGA CTC TT | 634 | 0.2 | [16] |
| S. No. | Serogroup | Number of Isolates | Percentage A |
|---|---|---|---|
| 1. | O2 | 40 | 16.0 g |
| 2. | O1 | 31 | 12.4 fg |
| 3. | O8 | 29 | 11.6 efg |
| 4. | O76 | 23 | 9.2 ef |
| 5. | O114 | 11 | 6.66 de |
| 6. | O45 | 10 | 4.0 cd |
| 7. | O26 | 09 | 3.6 bcd |
| 8. | O20 | 08 | 3.2 abcd |
| 9. | O89 | 08 | 3.2 abcd |
| 10. | R | 07 | 2.8 abc |
| 11. | O59 | 04 | 1.6 abc |
| 12. | O88 | 04 | 1.6 abc |
| 13. | O101 | 03 | 1.2 ab |
| 14. | O11 | 03 | 1.2 ab |
| 15. | O126 | 02 | 0.8 a |
| 16. | UT | 58 | 23.2 h |
| Total | 250 | ||
| Serogroup | Virulence-Associated Gene | No. of Isolates | ||||
|---|---|---|---|---|---|---|
| iss | tsh | iucC | papC | sitA | ||
| O2 | + | + | + | - | - | 16 |
| + | + | - | - | + | 12 | |
| + | - | + | - | + | 6 | |
| - | - | + | + | + | 2 | |
| + | - | - | - | - | 2 | |
| - | + | - | - | + | 2 | |
| O1 | + | + | + | + | + | 4 |
| + | + | - | - | + | 5 | |
| + | + | - | + | + | 8 | |
| - | + | + | + | - | 3 | |
| - | - | - | + | + | 2 | |
| + | - | + | - | - | 9 | |
| O8 | + | + | + | + | + | 5 |
| + | + | + | - | - | 6 | |
| + | - | - | - | + | 3 | |
| - | - | - | + | - | 4 | |
| - | + | + | + | - | 3 | |
| + | + | - | - | - | 8 | |
| O76 | + | + | - | - | - | 6 |
| + | + | + | - | + | 4 | |
| + | - | + | + | - | 3 | |
| - | + | - | + | - | 2 | |
| - | - | + | - | + | 3 | |
| + | + | - | - | + | 5 | |
| O114 | + | + | + | - | - | 2 |
| + | + | - | - | - | 2 | |
| + | - | - | - | + | 2 | |
| + | + | - | + | - | 3 | |
| + | + | + | + | - | 2 | |
| O45 | + | + | + | + | + | 2 |
| + | - | - | + | + | 2 | |
| + | - | + | + | - | 2 | |
| - | + | + | - | - | 2 | |
| + | + | - | - | + | 1 | |
| + | - | - | - | - | 1 | |
| O26 | + | + | + | - | - | 2 |
| + | + | - | - | - | 1 | |
| + | + | - | - | + | 2 | |
| - | - | + | + | + | 1 | |
| - | + | + | - | - | 1 | |
| + | - | - | + | + | 2 | |
| O20 | + | + | - | - | + | 2 |
| + | + | + | - | - | 1 | |
| + | - | - | - | + | 1 | |
| - | - | + | - | + | 1 | |
| + | + | - | - | - | 2 | |
| + | - | + | - | - | 1 | |
| O89 | + | + | + | - | - | 1 |
| + | + | - | - | - | 1 | |
| + | + | - | - | + | 2 | |
| - | + | - | - | - | 2 | |
| + | - | - | + | + | 2 | |
| O59 | + | + | + | - | + | 1 |
| + | + | - | - | - | 1 | |
| + | + | - | - | + | 1 | |
| + | + | + | - | - | 1 | |
| O88 | + | + | + | + | + | 1 |
| + | + | - | - | - | 1 | |
| + | + | - | + | - | 1 | |
| + | - | - | - | - | 1 | |
| O101 | + | + | + | - | - | 1 |
| + | + | + | - | + | 1 | |
| + | + | - | - | + | 1 | |
| O11 | + | + | - | - | + | 1 |
| + | - | + | - | + | 1 | |
| - | + | + | - | + | 1 | |
| O126 | + | + | + | - | + | 1 |
| + | + | + | - | - | 1 | |
| Rough | + | + | + | - | - | 2 |
| + | + | - | - | - | 1 | |
| + | - | - | + | + | 1 | |
| + | + | - | - | + | 1 | |
| - | - | + | - | + | 1 | |
| + | + | + | - | + | 1 | |
| UT | + | + | + | + | + | 6 |
| + | + | + | - | + | 4 | |
| + | + | - | - | - | 8 | |
| + | - | - | + | - | 3 | |
| - | - | - | - | - | 3 | |
| - | + | - | - | - | 3 | |
| + | - | + | - | + | 4 | |
| + | + | - | - | + | 4 | |
| - | + | + | - | - | 5 | |
| - | - | - | - | + | 3 | |
| - | - | + | - | - | 3 | |
| + | - | - | - | - | 3 | |
| - | + | - | + | - | 4 | |
| + | + | - | + | - | 5 | |
| TOTAL NO. | 199 | 178 | 117 | 73 | 115 | 250 |
| TOTAL % | 79.6 | 71.2 | 46.8 | 29.2 | 46.0 | |
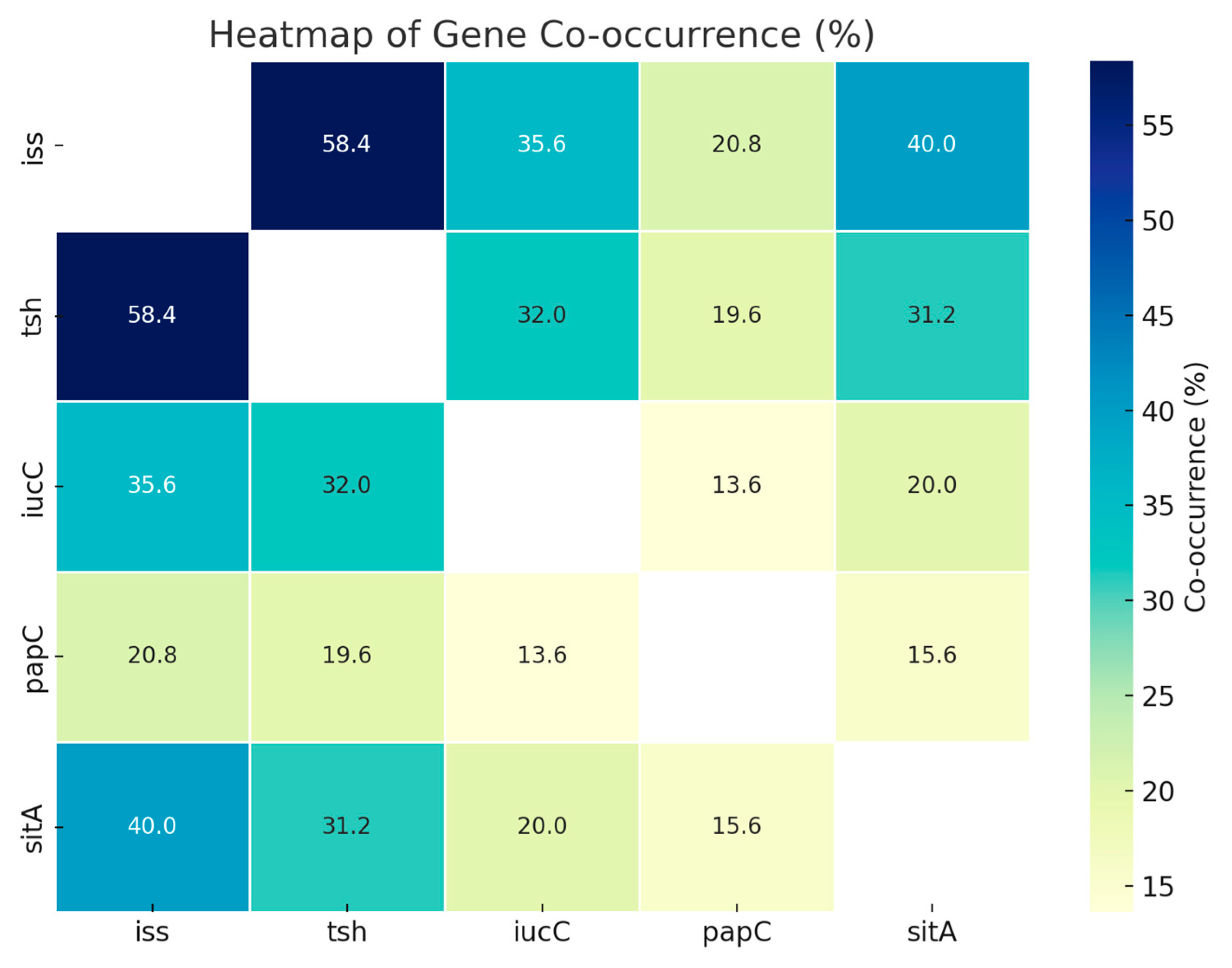
| iss | tsh | iucC | papC | sitA | |
|---|---|---|---|---|---|
| iss | - | ||||
| tsh | 58.4 * | - | |||
| iucC | 35.6 | 32.0 * | - | ||
| papC | 20.8 | 19.6 | 13.6 | - | |
| sitA | 40.0 * | 31.2 | 20.0 | 15.6 | - |
| Antibiotic | Disc Content (µg) | Susceptible No. (%) | Intermediate No. (%) | Resistant No. (%) |
|---|---|---|---|---|
| Penicillins | ||||
| Ampicillin (Amp) | 10 | 75 (30) | 59 (23.6) | 156 (62.4) |
| Aminoglycosides | ||||
| Amikacin (Amk) | 30 | 217 (86.8) | 23 (9.2) | 10 (4) |
| Gentamicin (Gen) | 10 | 225 (90) | 15 (6) | 10 (4) |
| Kanamycin (Kan) | 145 (58) | 28 (11.2) | 77 (30.8) | |
| Streptomycin (Str) | 10 | 34 (13.6) | 0 | 216 (86.4) |
| Tetracyclines | ||||
| Tetracycline (Tet) | 30 | 5 (2) | 15 (6) | 230 (92) |
| Doxycycline (Dox) | 30 | 20 (8) | 10 (4) | 220 (88) |
| Macrolides | ||||
| Erythromycin (Ery) | 15 | 13 (5.2) | 0 | 237 (94.8) |
| Azithromycin (Azi) | 15 | 20 (8) | 10 (4) | 220 (88) |
| Fluoroquinolones | ||||
| Ciprofloxacin (Cip) | 5 | 168 (67.2) | 43 (17.2) | 39 (15.6) |
| Norfloxacin (Nor) | 10 | 60 (24) | 21 (8.4) | 169 (67.6) |
| Ofloxacin (Ofl) | 5 | 75 (30) | 58 (23.2) | 117 (46.8) |
| Enrofloxacin (Enr) | 5 | 30 (12) | 26 (10.4) | 194 (77.6) |
| Chloramphenicol (chl) | 30 | 139 (55.6) | 75 (30) | 36 (14.4) |
| Sulfonamides | ||||
| Sulfadiazine (Sul) | 300 | 10 (4) | 10 (4) | 230 (92) |
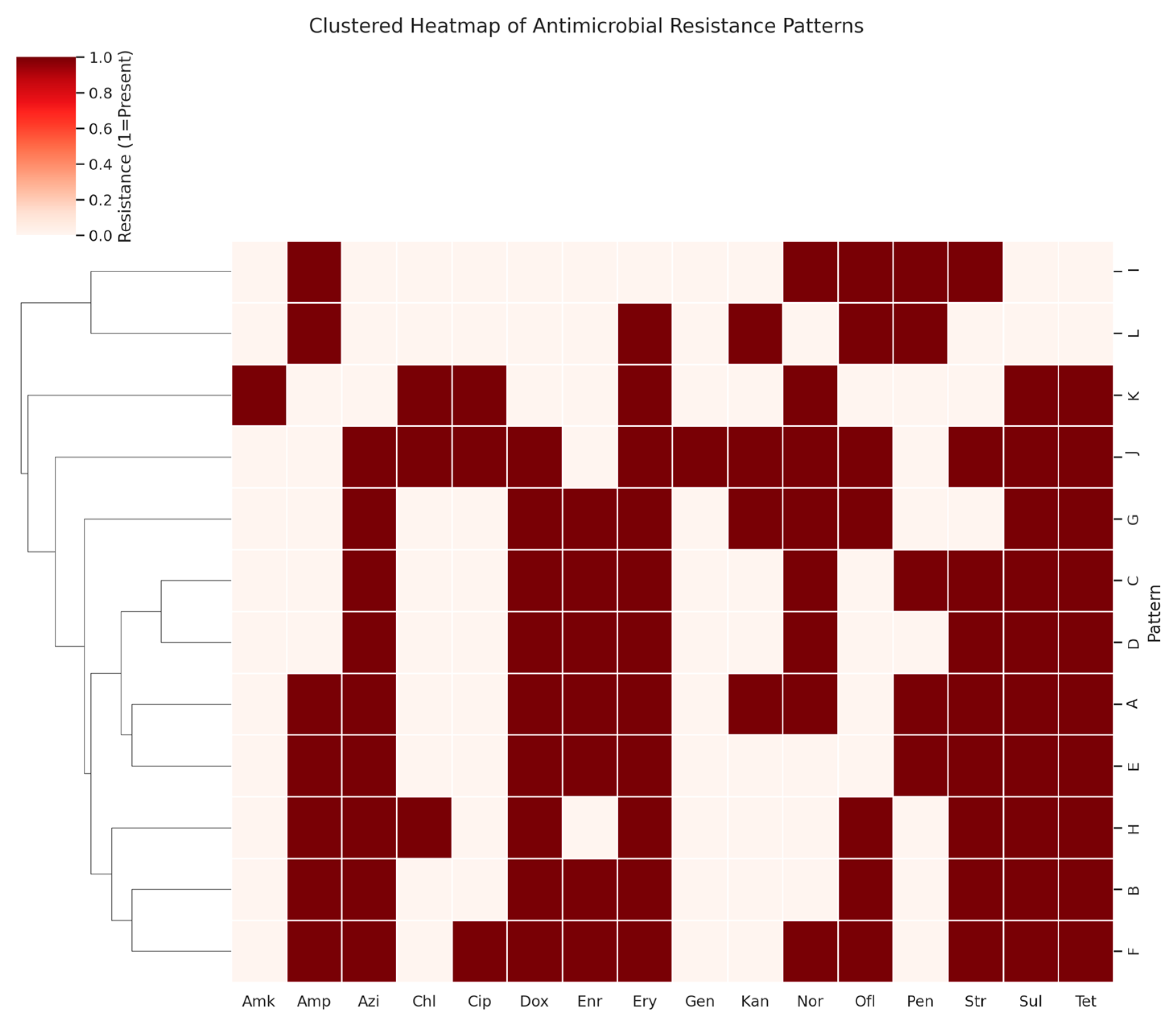
| Pattern | No. of Isolates | Resistance Pattern |
|---|---|---|
| A | 43 | Tet, Amp, Str, Kan, Dox, Ery, Azi, Nor, Enr, Sul |
| B | 35 | Tet, Amp, Str, Dox, Ery, Azi, Ofl, Enr, Sul, |
| C | 30 | Tet, Str, Dox, Ery, Azi, Nor, Enr, Sul |
| D | 27 | Tet, Str, Dox, Ery, Azi, Nor, Enr, Sul |
| E | 23 | Tet, Amp, Str, Dox, Ery, Azi, Enr, Sul |
| F | 19 | Tet, Amp, Str, Dox, Ery, Azi, Nor, Ofl, Enr, Sul, Cip |
| G | 17 | Tet, Kan, Dox, Ery, Azi, Nor, Enr, Sul, Ofl |
| H | 16 | Tet, Amp, Str, Dox, Ery, Azi, Chl, Sul, Ofl |
| I | 13 | Amp, Str, Nor, Ofl |
| J | 10 | Tet, Str, Kan, Dox, Ery, Azi, Nor, Ofl, Chl, Sul, Cip, Gen |
| K | 10 | Tet, Ery, Nor, Chl, Sul, Cip, Amk |
| L | 7 | Amp, Kan, Ery, Ofl |
| Antimicrobial Agent | Phenotypic Resistance | Resistance Gene | No. of Positive Isolates (%) |
|---|---|---|---|
| Tetracycline | 230 | tetA | 126 (54.8) |
| tetB | 119 (51.7) | ||
| Sulfadiazine | 230 | sul1 | 115 (50.0) |
| Streptomycin | 216 | aadA1 | 63 (29.2) |
| Ciprofloxacin | 39 | qnrA | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.A.; Mir, M.S.; Kamil, S.A.; Shafi, M.; Rather, M.A.; Khan, A.A.; Wani, Z.A.; Adil, S.; Alqahtani, F.M.; Alhomrani, M.; et al. Serogroup Prevalence, Virulence Profile and Antibiotic Resistance of Avian Pathogenic Escherichia coli Isolated from Broiler Chicken. Vet. Sci. 2025, 12, 592. https://doi.org/10.3390/vetsci12060592
Shah SA, Mir MS, Kamil SA, Shafi M, Rather MA, Khan AA, Wani ZA, Adil S, Alqahtani FM, Alhomrani M, et al. Serogroup Prevalence, Virulence Profile and Antibiotic Resistance of Avian Pathogenic Escherichia coli Isolated from Broiler Chicken. Veterinary Sciences. 2025; 12(6):592. https://doi.org/10.3390/vetsci12060592
Chicago/Turabian StyleShah, Showkat A., Masood S. Mir, Shayaib A. Kamil, Majid Shafi, Mudasir A. Rather, Azmat A. Khan, Zahoor A. Wani, Sheikh Adil, Fatmah M. Alqahtani, Majid Alhomrani, and et al. 2025. "Serogroup Prevalence, Virulence Profile and Antibiotic Resistance of Avian Pathogenic Escherichia coli Isolated from Broiler Chicken" Veterinary Sciences 12, no. 6: 592. https://doi.org/10.3390/vetsci12060592
APA StyleShah, S. A., Mir, M. S., Kamil, S. A., Shafi, M., Rather, M. A., Khan, A. A., Wani, Z. A., Adil, S., Alqahtani, F. M., Alhomrani, M., & Wani, M. (2025). Serogroup Prevalence, Virulence Profile and Antibiotic Resistance of Avian Pathogenic Escherichia coli Isolated from Broiler Chicken. Veterinary Sciences, 12(6), 592. https://doi.org/10.3390/vetsci12060592






