Solitary Subcutaneous Nodular Lymphoid Lesions in Dogs: Histopathologic and Immunophenotypic Comparison of B-Cell Pseudolymphoma and Subcutaneous Panniculitis-like T-Cell Lymphoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Histopathology and Immunohistochemistry
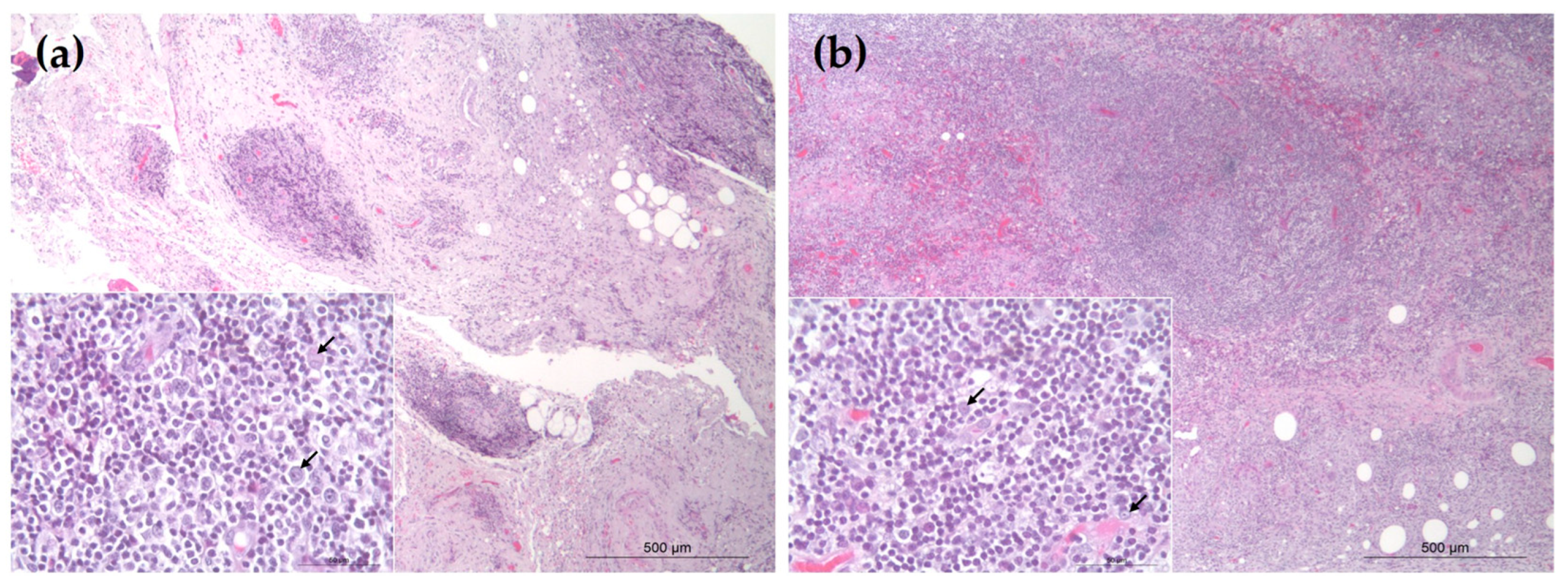
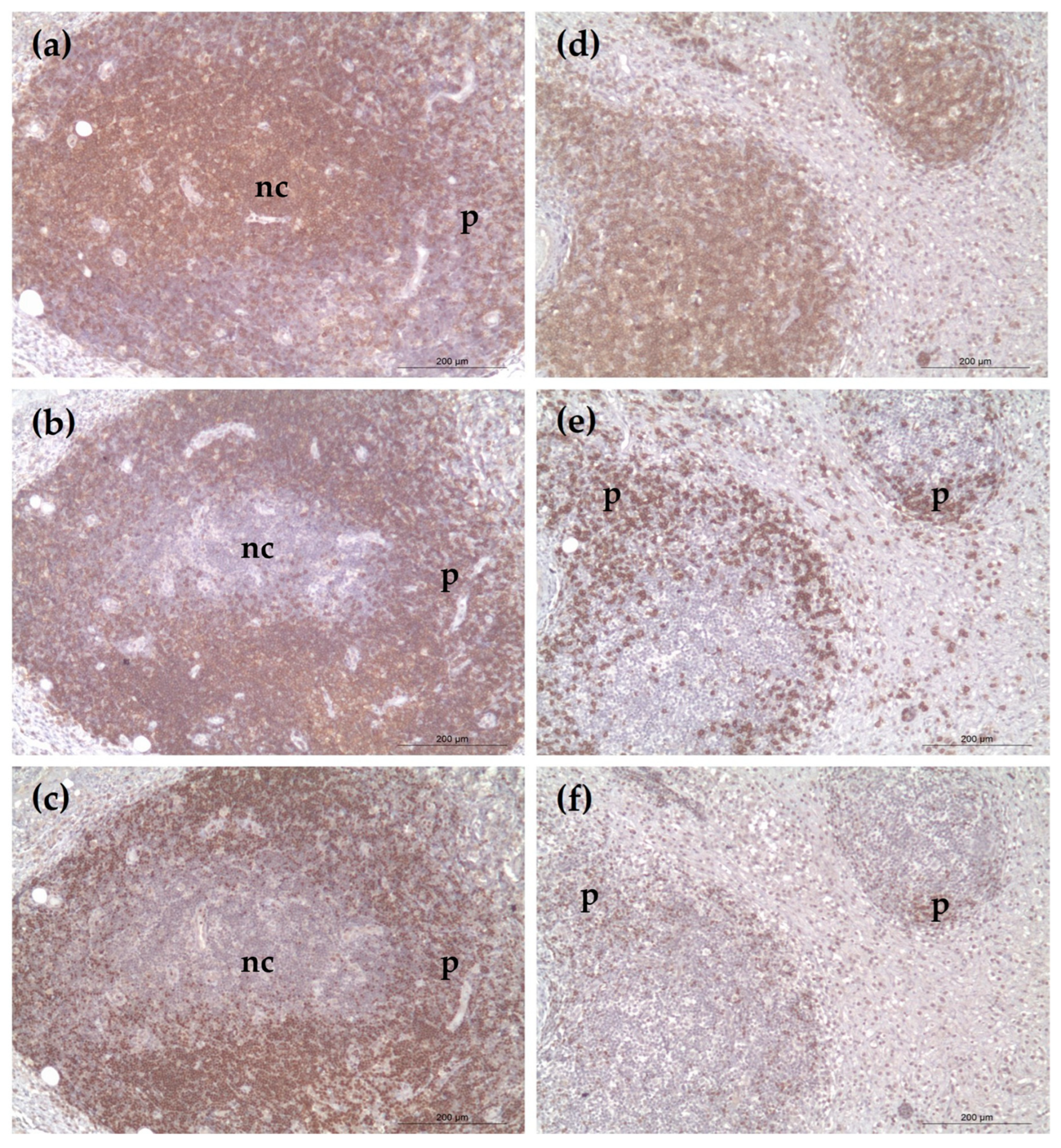
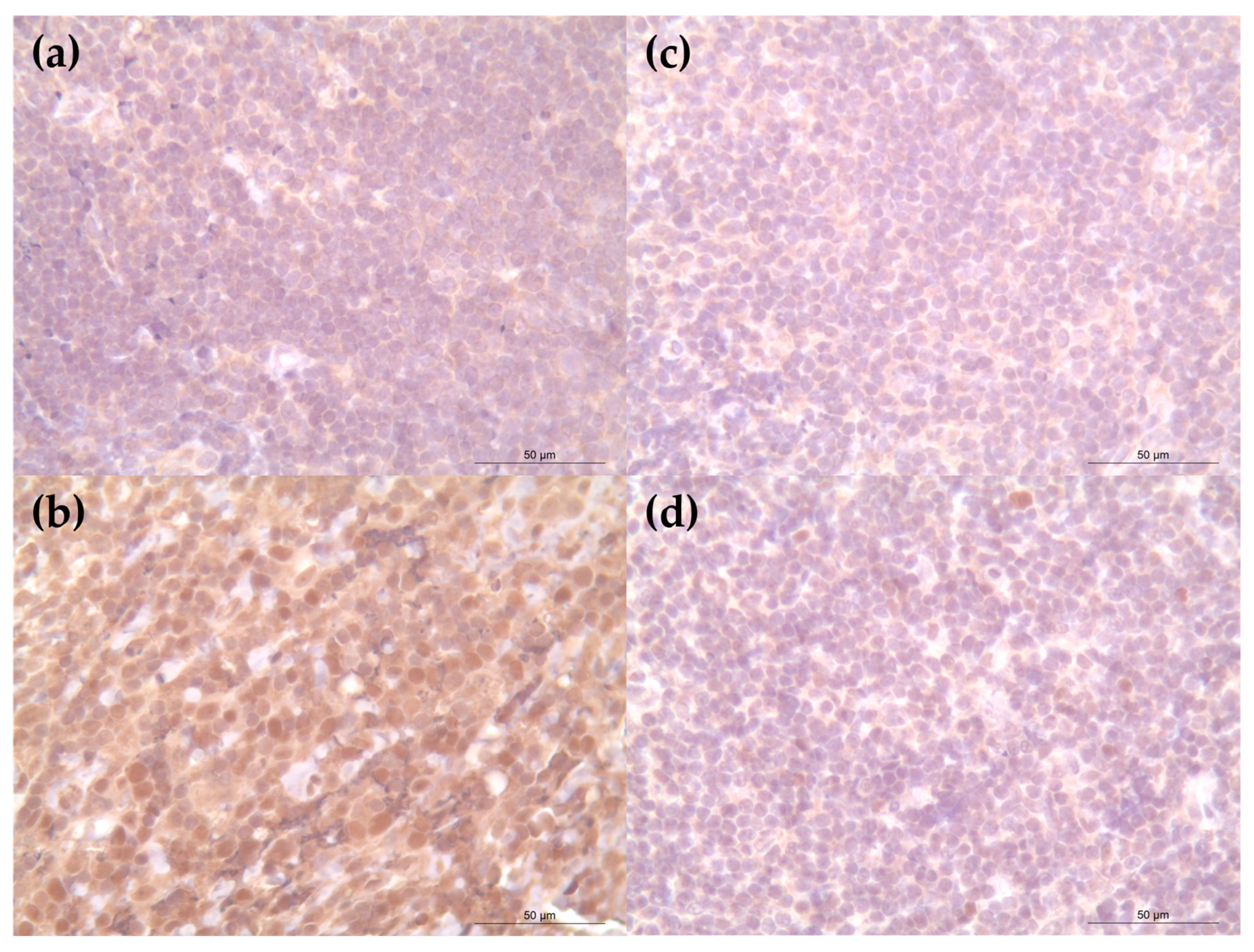
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Affolter, V.K.; Gross, T.L.; Moore, P.F. Indolent cutaneous T-cell lymphoma presenting as cutaneous lymphocytosis in dogs. Vet. Dermatol. 2009, 20, 577–585. [Google Scholar] [CrossRef]
- Bergman, R. Pseudolymphoma and cutaneous lymphoma: Facts and controversies. Clin. Dermatol. 2010, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.K.; Hegde, U.; Jagadish, L.; Shetty, C. Pseudolymphoma versus lymphoma: An important diagnostic decision. J. Oral Maxillofac. Pathol. 2016, 20, 328. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.A.; Sanders, D.S.A. Inflammatory dermatoses mimicking malignancy (pseudolymphoma). Curr. Diagn. Pathol. 2005, 11, 245–252. [Google Scholar] [CrossRef]
- Albanese, F.; Abramo, F.; Marino, M.; Massaro, M.; Marconato, L.; Minoli, L.; Martini, V.; Aresu, L. Feline and Canine Cutaneous Lymphocytosis: Reactive Process or Indolent Neoplastic Disease? Vet. Sci. 2022, 9, 26. [Google Scholar] [CrossRef]
- Wei, R.; Liu, H.; Zhang, Z.; Chen, F.; Chen, J.; Xu, Q.; Yu, H.; Liang, J.; Yao, Z. Coexistence of Subcutaneous Panniculitis-Like T-Cell Lymphoma and Dermatomyositis in a 12-Year-Old Boy. Ann. Dermatol. 2023, 35, S79–S83. [Google Scholar] [CrossRef]
- Valli, V.E.; Bienzle, D.; Meuten, D.J. Tumors of the Hemolymphatic System. In Tumors in Domestic Animals; Wiley-Blackwell: Ames, IA, USA, 2016; pp. 235–236. [Google Scholar]
- Zandvliet, M. Canine lymphoma: A review. Vet. Q. 2016, 36, 76–104. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.M.; Anderson, S.; Harrington, B. Characterization of CD3+/CD20+ canine large-cell lymphoma. J. Vet. Diagn. Investig. 2024, 36, 86–94. [Google Scholar] [CrossRef]
- Henrich, M.; Bauknecht, A.; Hecht, W.; Reinacher, M. Lack of Bcl-2 expression in feline follicular lymphomas. J. Vet. Diagn. Investig. 2019, 31, 809–817. [Google Scholar] [CrossRef]
- Rook, K.A. Canine and Feline Cutaneous Epitheliotropic Lymphoma and Cutaneous Lymphocytosis. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Noland, E.L.; Keller, S.M.; Kiupel, M. Subcutaneous Panniculitis-Like T-cell Lymphoma in Dogs: Morphologic and Immunohistochemical Classification. Vet. Pathol. 2018, 55, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Nguyen, K.T.; Le, L.T.; Nguyen, K.T.; Trinh, C.T.; Hoang, V.T. Subcutaneous Panniculitis-Like T-Cell Lymphoma With Hemophagocytic Lymphohistiocytosis. J. Investig. Med. High Impact Case Rep. 2024, 12, 23247096241253337. [Google Scholar] [CrossRef]
- Gonzalez, C.L.; Medeiros, L.J.; Braziel, R.M.; Jaffe, E.S. T-Cell Lymphoma Involving Subcutaneous Tissue: A Clinicopathologic Entity Commonly Associated with Hemophagocytic Syndrome. Am. J. Surg. Pathol. 1991, 15, 17–27. [Google Scholar] [CrossRef]
- Sugeeth, M.T.; Narayanan, G.; Jayasudha, A.V.; Nair, R.A. Subcutaneous panniculitis-like T-cell lymphoma. Bayl. Univ. Med. Cent. Proc. 2017, 30, 76–77. [Google Scholar] [CrossRef]
- Roccabianca, P.; Dell’Aere, S.; Avallone, G.; Zamboni, C.; Bertazzolo, W.; Crippa, L.; Giudice, C.; Caniatti, M.; Affolter, V.K. Subcutaneous panniculitis-like T-cell lymphoma: Morphological, immunophenotypical and clonality assessment in six cats. Vet. Dermatol. 2024, 35, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.M.; Vernau, W.; Moore, P.F. Clonality Testing in Veterinary Medicine: A Review With Diagnostic Guidelines. Vet. Pathol. 2016, 53, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.C.; Vernau, W.; Modiano, J.F.; Olver, C.S.; Moore, P.F.; Avery, A.C. Diagnosis of Canine Lymphoid Neoplasia Using Clonal Rearrangements of Antigen Receptor Genes. Vet. Pathol. 2003, 40, 32–41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, Y.-H.; Kim, H.-S.; Kim, W.-J.; Oh, H.-J.; Lee, B.-J.; Im, C.-K.; Do, S.-H. Solitary Subcutaneous Nodular Lymphoid Lesions in Dogs: Histopathologic and Immunophenotypic Comparison of B-Cell Pseudolymphoma and Subcutaneous Panniculitis-like T-Cell Lymphoma. Vet. Sci. 2025, 12, 532. https://doi.org/10.3390/vetsci12060532
Koo Y-H, Kim H-S, Kim W-J, Oh H-J, Lee B-J, Im C-K, Do S-H. Solitary Subcutaneous Nodular Lymphoid Lesions in Dogs: Histopathologic and Immunophenotypic Comparison of B-Cell Pseudolymphoma and Subcutaneous Panniculitis-like T-Cell Lymphoma. Veterinary Sciences. 2025; 12(6):532. https://doi.org/10.3390/vetsci12060532
Chicago/Turabian StyleKoo, Young-Hyun, Hyo-Sung Kim, Woo-Jin Kim, Hye-Ji Oh, Byoung-Je Lee, Chang-Kyun Im, and Sun-Hee Do. 2025. "Solitary Subcutaneous Nodular Lymphoid Lesions in Dogs: Histopathologic and Immunophenotypic Comparison of B-Cell Pseudolymphoma and Subcutaneous Panniculitis-like T-Cell Lymphoma" Veterinary Sciences 12, no. 6: 532. https://doi.org/10.3390/vetsci12060532
APA StyleKoo, Y.-H., Kim, H.-S., Kim, W.-J., Oh, H.-J., Lee, B.-J., Im, C.-K., & Do, S.-H. (2025). Solitary Subcutaneous Nodular Lymphoid Lesions in Dogs: Histopathologic and Immunophenotypic Comparison of B-Cell Pseudolymphoma and Subcutaneous Panniculitis-like T-Cell Lymphoma. Veterinary Sciences, 12(6), 532. https://doi.org/10.3390/vetsci12060532






