Bluetongue’s New Frontier—Are Dogs at Risk?
Simple Summary
Abstract
1. Introduction
2. Overview of the Epidemiological Context of Bluetongue Virus
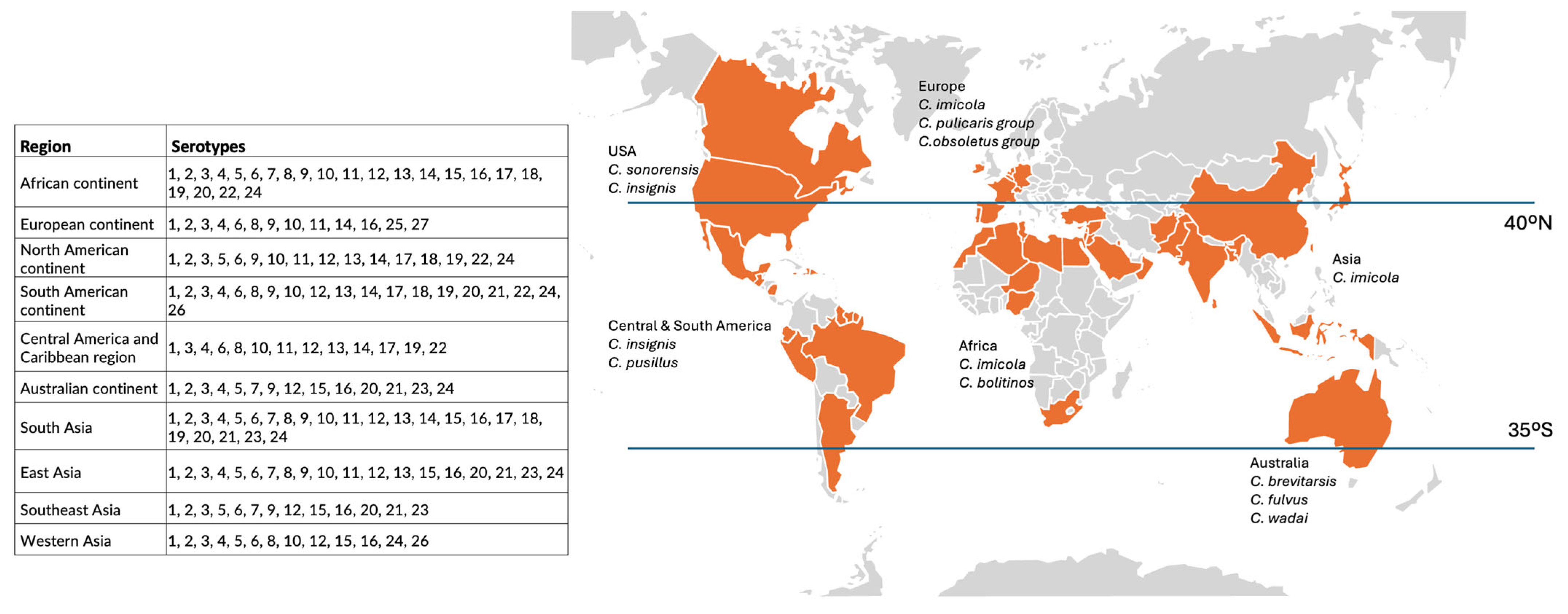
2.1. Bluetongue Virus in Brief
2.2. Host Range and Species Susceptibility
2.3. Transmission Pathways and Epidemiological Patterns
2.4. Bluetongue in Ruminants and Non-Ruminant Species: Epidemiological Considerations
2.4.1. Disease Expression in Ruminants
2.4.2. Disease Expression in Dogs and Other Non-Ruminant Hosts
2.5. Knowledge Gaps in Carnivores Role in Bluetongue Epidemiology
3. Pathogenesis of Bluetongue Infection
3.1. Vector-Borne Infection
3.2. Non-Vectorial Route of Infection
3.2.1. Transplacental Viral Transmission
3.2.2. The Mucosal Route of Infection
4. Challenges in Diagnosing BTV in Dogs
5. Are Biosecurity Risk Assessment Frameworks in Need for Expansion?
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthijnssens, J.; Attoui, H.; Bányai, K.; Brussaard, C.P.D.; Danthi, P.; del Vas, M.; Dermody, T.S.; Duncan, R.; Fāng, Q.; Johne, R.; et al. ICTV virus taxonomy profile: Sedoreoviridae. J. Gen. Virol. 2022, 103, 001782. [Google Scholar]
- Wilson, A.J.; Mellor, P.S. Bluetongue in Europe: Past, present and future. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2669–2681. [Google Scholar] [CrossRef]
- Maclachlan, N.J.; Drew, C.P.; Darpel, K.E.; Worwa, G. The pathology and pathogenesis of Bluetongue. J. Comp. Pathol. 2009, 141, 1–16. [Google Scholar] [CrossRef]
- Rushton, J.; Lyons, N. Economic impact of Bluetongue: A review of the effects on production. Vet. Ital. 2015, 51, 401–406. [Google Scholar] [CrossRef]
- Baldini, M.H.M.; de Moraes, A.N. Bluetongue and epizootic haemorrhagic disease in wildlife with emphasis on the South American scenario. Vet. Ital. 2021, 57, 97–103. [Google Scholar] [CrossRef]
- Kim, H.-J.; Choi, J.-G.; Seong, D.-S.; Jeong, J.-U.; Kim, H.-J.; Park, S.-W.; Yun, S.-P.; Roh, I.-S. The first report on the complete sequence characterization of bluetongue virus serotype 3 in the Republic of Korea. Vet. Sci. 2024, 11, 29. [Google Scholar] [CrossRef]
- Maclachlan, N.J.; Mayo, C.E.; Daniels, P.W.; Savini, G.; Zientara, S.; Gibbs, E.P. Bluetongue. Rev. Sci. Tech. 2015, 34, 329–340. [Google Scholar] [CrossRef]
- Saminathan, M.; Singh, K.P.; Khorajiya, J.H.; Dinesh, M.; Vineetha, S.; Maity, M.; Rahman, A.F.; Misri, J.; Malik, Y.S.; Gupta, V.K.; et al. An updated review on Bluetongue virus: Epidemiology, pathobiology, and advances in diagnosis and control with special reference to India. Vet. Q. 2020, 40, 258–321. [Google Scholar] [CrossRef]
- Maclachlan, N.J. Bluetongue: History, global epidemiology, and pathogenesis. Prev. Vet. Med. 2011, 102, 107–111. [Google Scholar] [CrossRef]
- Maclachlan, N.J.; Mayo, C.E. Potential strategies for control of Bluetongue, a globally emerging, culicoides-transmitted viral disease of ruminant livestock and wildlife. Antivir. Res. 2013, 99, 79–90. [Google Scholar] [CrossRef]
- Coetzee, P.; Van Vuuren, M.; Stokstad, M.; Myrmel, M.; Venter, E.H. Bluetongue virus genetic and phenotypic diversity: Towards identifying the molecular determinants that influence virulence and transmission potential. Vet. Microbiol. 2012, 161, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.L.B.; Rosa, J.C.C.; Matos, A.C.D.; Cruz, R.A.S.; Guedes, M.I.M.C.; Dorella, F.A.; Figueiredo, H.C.P.; Pavarini, S.P.; Sonne, L.; Lobato, Z.I.P.; et al. Identification of Bluetongue virus serotypes 1, 4, and 17 co-infections in sheep flocks during outbreaks in Brazil. Res. Vet. Sci. 2017, 113, 87–93. [Google Scholar] [CrossRef]
- Maclachlan, N.J.; Crafford, J.E.; Vernau, W.; Gardner, I.A.; Goddard, A.; Guthrie, A.J.; Venter, E.H. Experimental reproduction of severe Bluetongue in sheep. Vet. Pathol. 2008, 45, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Akita, G.; Ianconescu, M.; MacLachlan, N.; Osburn, B. Bluetongue disease in dogs associated with contaminated vaccine. Vet. Rec. 1994, 134, 283–284. [Google Scholar] [CrossRef]
- Wilbur, L.A.; Evermann, J.F.; Levings, R.L.; Stoll, I.R.; Starling, D.E.; Spillers, C.A.; Gustafson, G.A.; McKeirnan, A.J. Abortion and death in pregnant bitches associated with a canine vaccine contaminated with Bluetongue virus. J. Am. Vet. Med. Assoc. 1994, 204, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Evermann, J.F.; McKeiman, A.J.; Wilbur, L.A.; Levings, R.L.; Trueblood, E.S.; Baldwin, T.J.; Hughbanks, F.G. Canine fatalities associated with the use of a modified live vaccine administered during late stages of pregnancy. J. Vet. Diagn. Investig. 1994, 6, 353–357. [Google Scholar] [CrossRef]
- Levings, R.L.; Wilbur, L.A.; Evermann, J.F.; Stoll, I.R.; Starling, D.E.; Spillers, C.A.; Gustafson, G.A.; McKeiman, A.J.; Rhyan, J.C.; Halverson, D.H.; et al. Abortion and death in pregnant bitches associated with a canine vaccine contaminated with Bluetongue virus. Dev. Biol. Stand. 1996, 88, 219–220. [Google Scholar]
- Alexander, K.A.; MacLachlan, N.J.; Kat, P.W.; House, C.; O’Brien, S.J.; Lerche, N.W.; Sawyer, M.; Frank, L.G.; Holekamp, K.; Smale, L.; et al. Evidence of natural Bluetongue virus infection among african carnivores. Am. J. Trop. Med. Hyg. 1994, 51, 568–576. [Google Scholar] [CrossRef]
- Dubovi, E.J.; Hawkins, M.; Griffin, R.A.; Johnson, D.J.; Ostlund, E.N. Isolation of Bluetongue virus from canine abortions. J. Vet. Diagn. Investig. 2013, 25, 490–492. [Google Scholar] [CrossRef]
- Hanekom, J.; Hoepner, S.; du Preez, K.; Leisewitz, A. The clinical presentation and management of a naturally occurring Bluetongue virus infection in a pregnant rottweiler dog. J. S. Afr. Vet. Assoc. 2022, 93, 151–155. [Google Scholar] [CrossRef]
- Oura, C.A.L.; EL Harrak, M. Midge-transmitted Bluetongue in domestic dogs. Epidemiol. Infect. 2011, 139, 1396–1400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caporale, M.; Di Gialleonorado, L.; Janowicz, A.; Wilkie, G.; Shaw, A.; Savini, G.; Van Rijn, P.A.; Mertens, P.; Di Ventura, M.; Palmarini, M. Virus and host factors affecting the clinical outcome of Bluetongue virus infection. J. Virol. 2014, 88, 10399–10411. [Google Scholar] [CrossRef]
- Samy, A.M.; Peterson, A.T. Climate change influences on the global potential distribution of Bluetongue virus. PLoS ONE 2016, 11, e0150489. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.C.; Maroco, D.; Henriques, A.M.; Costa, M.L.; Alves, A.; Ramos, F.; Duarte, A.; Fagulha, T.; Varanda, I.C.; dos Santos, F.A.; et al. Fatal Bluetongue virus serotype 3 infection in female dogs: A case report from Alentejo, Portugal, 2024. Viruses 2025, 17, 159. [Google Scholar] [CrossRef] [PubMed]
- Hanekom, J.; Ebersohn, K.; Penzhorn, L.; Quan, M.; Leisewitz, A.; Guthrie, A.; Fosgate, G.T. Bluetongue Virus infection in farm dogs exposed to an infected sheep flock in South Africa. Transbound. Emerg. Dis. 2024, 2024, 2446398. [Google Scholar] [CrossRef]
- Wageninger University & Research. Bluetongue Found in Dutch Dog. Wageninger University, 2023. Available online: https://www.wur.nl/en/research-results/research-institutes/bioveterinary-research/show-bvr/bluetongue-found-in-dutch-dog.htm (accessed on 5 March 2025).
- Osburn, B.I. The impact of Bluetongue virus on reproduction. Comp. Immunol. Microbiol. Infect. Dis. 1994, 17, 189–196. [Google Scholar] [CrossRef]
- Roy, P. Bluetongue virus structure and assembly. Curr. Opin. Virol. 2017, 24, 115–123. [Google Scholar] [CrossRef]
- Niedbalski, W. The evolution of Bluetongue virus: Genetic and phenotypic diversity of field strains. Pol. J. Vet. Sci. 2013, 16, 611–616. [Google Scholar] [CrossRef]
- Ftaich, N.; Ciancia, C.; Viarouge, C.; Barry, G.; Ratinier, M.; van Rijn, P.A.; Breard, E.; Vitour, D.; Zientara, S.; Palmarini, M.; et al. Turnover rate of NS3 proteins modulates Bluetongue virus replication kinetics in a host-specific manner. J. Virol. 2015, 89, 10467–10481. [Google Scholar] [CrossRef]
- Bissett, S.L.; Roy, P. Impact of VP2 structure on antigenicity: Comparison of BTV1 and the highly virulent BTV8 serotype. J. Virol. 2024, 98, e00953-24. [Google Scholar] [CrossRef]
- Fay, P.C.; Jaafar, F.M.; Batten, C.; Attoui, H.; Saunders, K.; Lomonossoff, G.P.; Reid, E.; Horton, D.; Maan, S.; Haig, D.; et al. Serological cross-reactions between expressed vp2 proteins from different Bluetongue virus serotypes. Viruses 2021, 13, 1455. [Google Scholar] [CrossRef] [PubMed]
- Carpi, G.; Holmes, E.C.; Kitchen, A. The evolutionary dynamics of Bluetongue virus. J. Mol. Evol. 2010, 70, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Sanders, C.; Veronesi, E.; Rajko-Nenow, P.; Mertens, P.P.C.; Batten, C.; Gubbins, S.; Carpenter, S.; Darpel, K. Field-reassortment of Bluetongue virus illustrates plasticity of virus associated phenotypic traits in the arthropod vector and mammalian host. J. Virol. 2022, 96, e0053122. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, P.; Van Vuuren, M.; Stokstad, M.; Myrmel, M.; van Gennip, R.G.; van Rijn, P.A.; Venter, E.H. Viral replication kinetics and in vitro cytopathogenicity of parental and reassortant strains of Bluetongue virus serotype 1, 6 and 8. Vet. Microbiol. 2014, 171, 53–65. [Google Scholar] [CrossRef]
- Nomikou, K.; Hughes, J.; Wash, R.; Kellam, P.; Breard, E.; Zientara, S.; Palmarini, M.; Biek, R.; Mertens, P. Widespread reassortment shapes the evolution and epidemiology of Bluetongue virus following European invasion. PLoS Pathog. 2015, 11, e1005056. [Google Scholar] [CrossRef]
- Batten, C.A.; Maan, S.; Shaw, A.E.; Maan, N.S.; Mertens, P.P. A European field strain of Bluetongue virus derived from two parental vaccine strains by genome segment reassortment. Virus Res. 2008, 137, 56–63. [Google Scholar] [CrossRef]
- Cavany, S.M.; Barbera, C.; Carpenter, M.; Rodgers, C.; Sherman, T.; Stenglein, M.; Mayo, C.; Perkins, T.A. Modeling cellular co-infection and reassortment of Bluetongue virus in Culicoides midges. Virus Evol. 2022, 8, veac094. [Google Scholar] [CrossRef]
- Kopanke, J.; Carpenter, M.; Lee, J.; Reed, K.; Rodgers, C.; Burton, M.; Lovett, K.; Westrich, J.A.; McNulty, E.; McDermott, E.; et al. Bluetongue research at a crossroads: Modern genomics tools can pave the way to new insights. Annu. Rev. Anim. Biosci. 2022, 10, 303–324. [Google Scholar] [CrossRef]
- Voigt, A.; Kampen, H.; Heuser, E.; Zeiske, S.; Hoffmann, B.; Höper, D.; Holsteg, M.; Sick, F.; Ziegler, S.; Wernike, K.; et al. Bluetongue virus serotype 3 and schmallenberg virus in culicoides biting midges, western germany, 2023. Emerg. Infect. Dis. 2024, 30, 1438–1441. [Google Scholar] [CrossRef]
- Subhadra, S.; Sreenivasulu, D.; Pattnaik, R.; Panda, B.K.; Kumar, S. Bluetongue virus: Past, present, and future scope. J. Infect. Dev. Ctries. 2023, 17, 147–156. [Google Scholar] [CrossRef]
- Alkhamis, M.A.; Aguilar-Vega, C.; Fountain-Jones, N.M.; Lin, K.; Perez, A.M.; Sánchez-Vizcaíno, J.M. Global emergence and evolutionary dynamics of Bluetongue virus. Sci. Rep. 2020, 10, 21677. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, S.; Huber, K.; Pagès, N.; Talavera, S.; Burgin, L.E.; Carpenter, S.; Sanders, C.; Dicko, A.H.; Djerbal, M.; Goffredo, M.; et al. Range expansion of the Bluetongue vector, culicoides imicola, in continental France likely due to rare wind-transport events. Sci. Rep. 2016, 6, 27247. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Rana, E.A.; Prodhan, M.A.; Akter, S.H.; Gogoi-Tiwari, J.; Sarker, S.; Annandale, H.; Eagles, D.; Abraham, S.; Uddin, J.M. The global burden of emerging and re-emerging orbiviruses in livestock: An emphasis on Bluetongue virus and epizootic hemorrhagic disease virus. Viruses 2024, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Falconi, C.; López-Olvera, J.R.; Gortázar, C. BTV infection in wild ruminants, with emphasis on red deer: A review. Vet. Microbiol. 2011, 151, 209–219. [Google Scholar] [CrossRef]
- Rivera, N.A.; Varga, C.; Ruder, M.G.; Dorak, S.J.; Roca, A.L.; Novakofski, J.E.; Mateus-Pinilla, N.E. Bluetongue and epizootic hemorrhagic disease in the united states of america at the wildlife-livestock interface. Pathogens 2021, 10, 915. [Google Scholar] [CrossRef]
- Ruder, M.G.; Lysyk, T.J.; Stallknecht, D.E.; Foil, L.D.; Johnson, D.J.; Chase, C.C.; Dargatz, D.A.; Gibbs, E.P.J. Transmission and epidemiology of Bluetongue and epizootic hemorrhagic disease in North America: Current perspectives, research gaps, and future directions. Vector-Borne Zoonotic Dis. 2015, 15, 348–363. [Google Scholar] [CrossRef]
- Elmahi, M.M.; Hussien, M.O.; Karrar, A.R.E.; Elhassan, A.M.; El Hussein, A.R.M. Sero-epidemiological survey of Bluetongue disease in one-humped camel (camelus dromedarius) in kassala state, eastern sudan. Ir. Vet. J. 2021, 74, 9. [Google Scholar] [CrossRef]
- Selim, A.; Alsubki, R.A.; Albohairy, F.M.; Attia, K.A.; Kimiko, I. A survey of Bluetongue infection in one-humped camels (Camelus Dromedarius); seroprevalence and risk factors analysis. BMC Vet. Res. 2022, 18, 322. [Google Scholar] [CrossRef]
- Ortega, J.; Crossley, B.; Dechant, J.E.; Drew, C.P.; MacLachlan, N.J. Fatal Bluetongue virus infection in an alpaca (vicugna pacos) in California. J. Vet. Diagn. Investig. 2010, 22, 134–136. [Google Scholar] [CrossRef]
- Caballero-Gómez, J.; Sánchez-Sánchez, M.; Lorca-Oró, C.; de Mera, I.G.F.; Zorrilla, I.; López, G.; Rosell, R.; Grande-Gómez, R.; Montoya-Oliver, J.I.; Salcedo, J.; et al. Bluetongue virus in the iberian lynx (lynx pardinus), 2010–2022. Emerg. Infect. Dis. 2024, 30, 2169–2173. [Google Scholar] [CrossRef]
- Jauniaux, T.P.; De Clercq, K.E.; Cassart, D.E.; Kennedy, S.; Vandenbussche, F.E.; Vandemeulebroucke, E.L.; Vanbinst, T.M.; Verheyden, B.I.; Goris, N.E.; Coignoul, F.L. Bluetongue in Eurasian Lynx. Emerg. Infect. Dis. 2008, 14, 1496–1498. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Animal Health and Welfare (AHAW); More, S.; Bicout, D.; Bøtner, A.; Butterworth, A.; Depner, K.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Schmidt, C.G.; et al. Assessment of listing and categorisation of animal diseases within the framework of the animal health law (regulation (eu) no 2016/429): Bluetongue. EFSA J. 2017, 15, e04957. [Google Scholar] [CrossRef] [PubMed]
- Thabet, S.; Lajnef, R. Potential mechanisms underlying Bluetongue virus emergence and spread. Front. Virol. 2024, 4, 1448192. [Google Scholar] [CrossRef]
- Purse, B.V.; Carpenter, S.; Venter, G.J.; Bellis, G.; Mullens, B.A. Bionomics of temperate and tropical culicoides midges: Knowledge gaps and consequences for transmission of culicoides-borne viruses. Annu. Rev. Entomol. 2015, 60, 373–392. [Google Scholar] [CrossRef]
- Mayo, C.; McDermott, E.; Kopanke, J.; Stenglein, M.; Lee, J.; Mathiason, C.; Carpenter, M.; Reed, K.; Perkins, T.A. Ecological dynamics impacting Bluetongue virus transmission in North America. Front. Vet. Sci. 2020, 7, 186. [Google Scholar] [CrossRef]
- Wilson, A.J.; Mellor, P.S. Bluetongue in Europe: Vectors, epidemiology and climate change. Parasitol. Res. 2008, 103 (Suppl. S1), S69–S77. [Google Scholar] [CrossRef] [PubMed]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector--borne diseases. Ann. N. Y. Acad. Sci. 2018, 1436, 157–173. [Google Scholar] [CrossRef]
- Purse, B.V.; Rogers, D.J. Chapter 16—Bluetongue virus and climate change. In Bluetongue; Mellor, P.S., Baylis, M., Mertens, P.P.C., Eds.; Academic Press: London, UK, 2009; pp. 343–364. [Google Scholar]
- World Organisation for Animal Health. Bluetongue in Europe: How Climate Change Is Shifting Disease Patterns; World Organisation for Animal Health: Paris, France, 2024; Available online: https://www.woah.org/en/article/bluetongue-in-europe-how-climate-change-is-shifting-disease-patterns/ (accessed on 5 March 2025).
- Bouwknegt, C.; van Rijn, P.A.; Schipper, J.J.M.; Hölzel, D.; Boonstra, J.; Nijhof, A.M.; van Rooij, E.M.A.; Jongejan, F. Potential role of ticks as vectors of Bluetongue virus. Exp. Appl. Acarol. 2010, 52, 183–192. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Jasperson, D.C.; Dubovi, E.J.; Johnson, D.J.; Ostlund, E.N.; Wilson, W.C. Whole genome sequence analysis of circulating Bluetongue virus serotype 11 strains from the United States including two domestic canine isolates. J. Vet. Diagn. Investig. 2015, 27, 442–448. [Google Scholar] [CrossRef]
- van der Sluijs, M.T.W.; de Smit, A.J.; Moormann, R.J.M. Vector independent transmission of the vector-borne Bluetongue virus. Crit. Rev. Microbiol. 2014, 42, 57–64. [Google Scholar] [CrossRef]
- Batten, C.; Darpel, K.; Henstock, M.; Fay, P.; Veronesi, E.; Gubbins, S.; Graves, S.; Frost, L.; Oura, C. Evidence for transmission of Bluetongue virus serotype 26 through direct contact. PLoS ONE 2014, 9, e96049. [Google Scholar] [CrossRef]
- Breckon, R.D.; Luedke, A.J.; Walton, T.E. Bluetongue virus in bovine semen: Viral isolation. Am. J. Vet. Res. 1980, 41, 439–442. [Google Scholar] [CrossRef]
- De Clercq, K.; Vandaele, L.; Vanbinst, T.; Riou, M.; Deblauwe, I.; Wesselingh, W.; Pinard, A.; Van Eetvelde, M.; Boulesteix, O.; Leemans, B.; et al. Transmission of Bluetongue virus serotype 8 by artificial insemination with frozen-thawed semen from naturally infected bulls. Viruses 2021, 13, 652. [Google Scholar] [CrossRef] [PubMed]
- Kirschvink, N.; Raes, M.; Saegerman, C. Impact of a natural Bluetongue serotype 8 infection on semen quality of belgian rams in 2007. Vet. J. 2009, 182, 244–251. [Google Scholar] [CrossRef]
- Mayo, C.E.; Crossley, B.M.; Hietala, S.K.; Gardner, I.A.; Breitmeyer, R.E.; MacLachlan, N.J. Colostral transmission of Bluetongue virus nucleic acid among newborn dairy calves in California. Transbound. Emerg. Dis. 2010, 57, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Darpel, K.E.; Barber, J.; Hope, A.; Wilson, A.J.; Gubbins, S.; Henstock, M.; Frost, L.; Batten, C.; Veronesi, E.; Moffat, K.; et al. Using shared needles for subcutaneous inoculation can transmit Bluetongue virus mechanically between ruminant hosts. Sci. Rep. 2016, 6, 20627. [Google Scholar] [CrossRef]
- Evermann, J.F. Letter to the editor, regarding Bluetongue virus and canine abortions. J. Vet. Diagn. Investig. 2013, 25, 670. [Google Scholar] [CrossRef]
- Cappai, S.; Loi, F.; Coccollone, A.; Contu, M.; Capece, P.; Fiori, M.; Canu, S.; Foxi, C.; Rolesu, S. Retrospective analysis of Bluetongue farm risk profile definition, based on biology, farm management practices and climatic data. Prev. Vet. Med. 2018, 155, 75–85. [Google Scholar] [CrossRef]
- Flannery, J.; Frost, L.; Fay, P.; Hicks, H.; Henstock, M.; Smreczak, M.; Orłowska, A.; Rajko-Nenow, P.; Darpel, K.; Batten, C. Btv-14 infection in sheep elicits viraemia with mild clinical symptoms. Microorganisms 2020, 8, 892. [Google Scholar] [CrossRef]
- Niedbalski, W. Bluetongue in Europe and the role of wildlife in the epidemiology of disease. Pol. J. Vet. Sci. 2015, 18, 455–461. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare Bluetongue: Control, surveillance and safe movement of animals. EFSA J. 2017, 15, e04698. [CrossRef]
- Ruiz-Fons, F.; Sánchez-Matamoros, A.; Gortázar, C.; Sánchez-Vizcaíno, J.M. The role of wildlife in Bluetongue virus maintenance in Europe: Lessons learned after the natural infection in Spain. Virus Res. 2014, 182, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Backx, A.; Heutink, C.G.; Van Rooij, E.M.A.; Van Rijn, P.A. Clinical signs of Bluetongue virus serotype 8 infection in sheep and goats. Vet. Rec. 2007, 161, 591–592. [Google Scholar] [CrossRef]
- Veldhuis, A.; Brouwer-Middelesch, H.; Marceau, A.; Madouasse, A.; Van der Stede, Y.; Fourichon, C.; Welby, S.; Wever, P.; van Schaik, G. Application of syndromic surveillance on routinely collected cattle reproduction and milk production data for the early detection of outbreaks of Bluetongue and Schmallenberg viruses. Prev. Vet. Med. 2016, 124, 15–24. [Google Scholar] [CrossRef]
- Santman-Berends, I.; Hage, J.; van Rijn, P.; Stegeman, J.; van Schaik, G. Bluetongue virus serotype 8 (BTV-8) infection reduces fertility of dutch dairy cattle and is vertically transmitted to offspring. Theriogenology 2010, 74, 1377–1384. [Google Scholar] [CrossRef]
- Brink, K.v.D.; Brouwer-Middelesch, H.; van Schaik, G.; Lam, T.; Stegeman, J.; Brom, R.v.D.; Spierenburg, M.; Santman-Berends, I. The impact of Bluetongue serotype 3 on cattle mortality, abortions and premature births in The Netherlands in the first year of the epidemic. Prev. Vet. Med. 2025, 239, 106493. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare. Scientific opinion on Bluetongue Serotype 8. EFSA J. 2011, 9, 2189. [Google Scholar] [CrossRef]
- Ries, C.; Beer, M.; Hoffmann, B. Bluetongue virus infection of goats: Re-emerged European serotype 8 vs. Two atypical serotypes. Viruses 2022, 14, 1034. [Google Scholar] [CrossRef]
- García-Bocanegra, I.; Arenas-Montes, A.; Lorca-Oró, C.; Pujols, J.; González, M.Á.; Napp, S.; Gómez-Guillamón, F.; Zorrilla, I.; Miguel, E.S.; Arenas, A. Role of wild ruminants in the epidemiology of Bluetongue virus serotypes 1, 4 and 8 in Spain. Vet. Res. 2011, 42, 88. [Google Scholar] [CrossRef]
- Di Rubbo, A.; Agnihotri, K.; Bowden, T.R.; Giles, M.; Newberry, K.; Peck, G.R.; Shiell, B.J.; Zamanipereshkaft, M.; White, J.R. Challenges of btv-group specific serology testing: No one test fits all. Viruses 2024, 16, 1810. [Google Scholar] [CrossRef]
- Holwerda, M.; Santman-Berends, I.M.; Harders, F.; Engelsma, M.; Vloet, R.P.; Dijkstra, E.; van Gennip, R.G.; Mars, M.H.; Spierenburg, M.; Roos, L.; et al. Emergence of Bluetongue virus Serotype 3, The Netherlands, September 2023. Emerg. Infect. Dis. 2024, 30, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, K.M.J.A.; Santman-Berends, I.M.G.A.; Harkema, L.; Scherpenzeel, C.G.M.; Dijkstra, E.; Bisschop, P.I.H.; Peterson, K.; van de Burgwal, N.S.; Waldeck, H.W.F.; Dijkstra, T.; et al. Bluetongue virus serotype 3 in ruminants in The Netherlands: Clinical signs, seroprevalence and pathological findings. Vet. Rec. 2024, 195, e4533. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, N.J.; Osburn, B.I. Teratogenic Bluetongue and related orbivirus infections in pregnant ruminant livestock: Timing and pathogen genetics are critical. Curr. Opin. Virol. 2017, 27, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.E.; Healy, S.; Forbes, W.; Roberts, J.; LaCour, J.; Foil, L.D. Postmortem detection of Bluetongue and epizootic hemorrhagic disease viruses in the bone marrow of white-tailed deer. J. Wildl. Dis. 2020, 56, 58–65. [Google Scholar] [CrossRef]
- Brown, C.C.; Rhyan, J.C.; Grubman, M.J.; Wilbur, L.A. Distribution of Bluetongue virus in tissues of experimentally infected pregnant dogs as determined by in situ hybridization. Vet. Pathol. 1996, 33, 337–340. [Google Scholar] [CrossRef]
- Mellor, P.S.; Boorman, J. The transmission and geographical spread of African horse sickness and Bluetongue viruses. Ann. Trop. Med. Parasitol. 1995, 89, 1–15. [Google Scholar] [CrossRef]
- Baylis, M.; Caminade, C.F.; Turner, J.C.C.F.; Jones, A.E.T.J.F.; Jones, A.E. The role of climate change in a developing threat: The case of Bluetongue in europe. Rev. Sci. Tech. 2017, 36, 467–478. [Google Scholar] [CrossRef]
- Gondard, M.; Postic, L.; Garin, E.; Turpaud, M.; Vorimore, F.; Ngwa-Mbot, D.; Tran, M.-L.; Hoffmann, B.; Warembourg, C.; Savini, G.; et al. Exceptional Bluetongue virus (BTV) and epizootic hemorrhagic disease virus (EHDV) circulation in France in 2023. Virus Res. 2024, 350, 199489. [Google Scholar] [CrossRef]
- Belbis, G.; Zientara, S.; Bréard, E.; Sailleau, C.; Caignard, G.; Vitour, D.; Attoui, H. Bluetongue virus: From btv-1 to btv-27. Adv. Virus Res. 2017, 99, 161–197. [Google Scholar] [CrossRef]
- Ganter, M. Bluetongue disease—Global overview and future risks. Small Rumin. Res. 2014, 118, 79–85. [Google Scholar] [CrossRef]
- Kundlacz, C.; Caignard, G.; Sailleau, C.; Viarouge, C.; Postic, L.; Vitour, D.; Zientara, S.; Breard, E. Bluetongue virus in france: An illustration of the european and mediterranean context since the 2000s. Viruses 2019, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Howerth, E.W.; Dorminy, M.; Dreesen, D.W.; Spires, E.A.; Stallknecht, D.E. Low prevalence of antibodies to Bluetongue and epizootic hemorrhagic disease viruses in dogs from Southern Georgia. J. Vet. Diagn. Investig. 1995, 7, 393–394. [Google Scholar] [CrossRef]
- Hemati, B.; Contreras, V.; Urien, C.; Bonneau, M.; Takamatsu, H.-H.; Mertens, P.P.C.; Bréard, E.; Sailleau, C.; Zientara, S. Bluetongue virus targets conventional dendritic cells in skin lymph. J. Virol. 2009, 83, 8789–8799. [Google Scholar] [CrossRef] [PubMed]
- Darpel, K.E.; Monaghan, P.; Simpson, J.; Anthony, S.J.; Veronesi, E.; Brooks, H.W.; Elliott, H.; Brownlie, J.; Takamatsu, H.-H.; Mellor, P.S.; et al. Involvement of the skin during Bluetongue virus infection and replication in the ruminant host. Vet. Res. 2012, 43, 705–740. [Google Scholar] [CrossRef]
- Rojas, J.M.; Avia, M.; Martín, V.; Sevilla, N. Inhibition of the IFN response by Bluetongue virus: The story so far. Front. Microbiol. 2021, 12, 692069. [Google Scholar] [CrossRef]
- Maclachlan, N.J.; Henderson, C.; Schwartz-Cornil, I.; Zientara, S. The immune response of ruminant livestock to Bluetongue virus: From type I interferon to antibody. Virus Res. 2014, 182, 71–77. [Google Scholar] [CrossRef]
- Marin-Lopez, A.; Bermúdez, R.; Calvo-Pinilla, E.; Moreno, S.; Brun, A.; Ortego, J. Pathological characterization of ifnar(-/-) mice infected with Bluetongue virus serotype 4. Int. J. Biol. Sci. 2016, 12, 1448–1460. [Google Scholar] [CrossRef]
- Schwartz-Cornil, I.; Mertens, P.P.; Contreras, V.; Hemati, B.; Pascale, F.; Bréard, E.; Mellor, P.S.; MacLachlan, N.J.; Zientara, S. Bluetongue virus: Virology, pathogenesis and immunity. Vet. Res. 2008, 39, 46. [Google Scholar] [CrossRef]
- Howerth, E.W. Cytokine release and endothelial dysfunction: A perfect storm in orbivirus pathogenesis. Vet. Ital. 2015, 51, 275–281. [Google Scholar] [CrossRef]
- Herder, V.; Caporale, M.; MacLean, O.A.; Pintus, D.; Huang, X.; Nomikou, K.; Palmalux, N.; Nichols, J.; Scivoli, R.; Boutell, C.; et al. Correlates of disease severity in Bluetongue as a model of acute arbovirus infection. PLoS Pathog. 2024, 20, e1012466. [Google Scholar] [CrossRef]
- Hardy, A.; Bakshi, S.; Furnon, W.; MacLean, O.; Gu, Q.; Varjak, M.; Varela, M.; Aziz, M.A.; Shaw, A.E.; Pinto, R.M.; et al. The timing and magnitude of the type i interferon response are correlated with disease tolerance in arbovirus infection. mBio 2023, 14, e0010123. [Google Scholar] [CrossRef] [PubMed]
- Newbrook, K.; Khan, N.; Fisher, A.; Chong, K.; Gubbins, S.; Davies, W.C.; Sanders, C.; Busquets, M.G.; Cooke, L.; Corla, A.; et al. Specific T-cell subsets have a role in anti-viral immunity and pathogenesis but not viral dynamics or onwards vector transmission of an important livestock arbovirus. Front. Immunol. 2024, 15, 1328820. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, H.; Mellor, P.S.; Mertens, P.P.C.; Kirkham, P.A.; Burroughs, J.N.; Parkhouse, R.M.E. A possible overwintering mechanism for Bluetongue virus in the absence of the insect vector FN1. J. Gen. Virol. 2003, 84, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martín, D.; Louloudes-Lázaro, A.; Avia, M.; Martín, V.; Rojas, J.M.; Sevilla, N. The interplay between Bluetongue virus infections and adaptive immunity. Viruses 2021, 13, 1511. [Google Scholar] [CrossRef]
- Darpel, K.E.; Monaghan, P.; Anthony, S.J.; Takamatsu, H.-H.; Mertens, P.P. Bluetongue virus in the mammalian host and the induced immune response. In Bluetongue; Elsevier: Amsterdam, The Netherlands, 2009; pp. 265–284. [Google Scholar]
- DeMaula, C.D.; Leutenegger, C.M.; Bonneau, K.R.; MacLachlan, N. The role of endothelial cell-derived inflammatory and vasoactive mediators in the pathogenesis of Bluetongue. Virology 2002, 296, 330–337. [Google Scholar] [CrossRef]
- Clercq, D.; De Leeuw, I.; Verheyden, B.; Vandemeulebroucke, E.; Vanbinst, T.; Herr, C.; Méroc, E.; Bertels, G.; Steurbaut, N.; Miry, C.; et al. Transplacental infection and apparently immunotolerance induced by a wild-type Bluetongue virus serotype 8 natural infection. Transbound. Emerg. Dis. 2008, 55, 352–359. [Google Scholar] [CrossRef]
- van der Sluijs, M.T.; Schroer-Joosten, D.P.; Fid-Fourkour, A.; Vrijenhoek, M.P.; Debyser, I.; Moulin, V.; Moormann, R.J.; De Smit, A.J. Transplacental transmission of Bluetongue virus serotype 1 and serotype 8 in sheep: Virological and pathological findings. PLoS ONE 2013, 8, e81429. [Google Scholar] [CrossRef]
- Saegerman, C.; Bolkaerts, B.; Baricalla, C.; Raes, M.; Wiggers, L.; de Leeuw, I.; Vandenbussche, F.; Zimmer, J.-Y.; Haubruge, E.; Cassart, D.; et al. The impact of naturally-occurring, trans-placental Bluetongue virus serotype-8 infection on reproductive performance in sheep. Vet. J. 2011, 187, 72–80. [Google Scholar] [CrossRef]
- Menzies, F.D.; McCullough, S.J.; McKeown, I.M.; Forster, J.; Jess, S.; Batten, C.; Murchie, A.K.; Gloster, J.; Fallows, J.G.; Pelgrim, W.; et al. Evidence for transplacental and contact transmission of Bluetongue virus in cattle. Vet. Rec. 2008, 163, 203–209. [Google Scholar] [CrossRef]
- Backx, A.; Heutink, R.; van Rooij, E.; van Rijn, P. Transplacental and oral transmission of wild-type Bluetongue virus serotype 8 in cattle after experimental infection. Vet. Microbiol. 2009, 138, 235–243. [Google Scholar] [CrossRef]
- Desmecht, D.; Bergh, R.V.; Sartelet, A.; Leclerc, M.; Mignot, C.; Misse, F.; Sudraud, C.; Berthemin, S.; Jolly, S.; Mousset, B.; et al. Evidence for transplacental transmission of the current wild-type strain of Bluetongue virus serotype 8 in cattle. Vet. Rec. 2008, 163, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Worwa, G.; Hilbe, M.; Chaignat, V.; Hofmann, M.A.; Griot, C.; Ehrensperger, F.; Doherr, M.G.; Thür, B. Virological and pathological findings in Bluetongue virus serotype 8 infected sheep. Vet. Microbiol. 2010, 144, 264–273. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.; Rodríguez-Sánchez, B.; Risalde, M.; Molina, V.; Pedrera, M.; Sánchez-Vizcaíno, J.; Gómez-Villamandos, J. Immunohistochemical detection of Bluetongue virus in fixed tissue. J. Comp. Pathol. 2010, 143, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.D.; Savini, G.; Lorusso, A.; Bellacicco, A.; Palmarini, M.; Caporale, M.; Rasmussen, T.B.; Belsham, G.J.; Bøtner, A. Transplacental transmission of field and rescued strains of BTV-2 and BTV-8 in experimentally infected sheep. Vet. Res. 2013, 44, 75. [Google Scholar] [CrossRef]
- Saminathan, M.; Vineetha, S.; Biswas, S.; Milton, A.; Pavulraj, S.; Dhama, K.; Singh, K. Effect of Bluetongue virus serotype 1 infection during late-stage of gestation in ifnar1-blocked immunocompetent mouse model. J. Pure Appl. Microbiol. 2024, 18, 2895–2910. [Google Scholar] [CrossRef]
- Rojas, J.M.; Martín, V.; Sevilla, N. Vaccination as a strategy to prevent Bluetongue virus vertical transmission. Pathogens 2021, 10, 1528. [Google Scholar] [CrossRef]
- Nusinovici, S.; Seegers, H.; Joly, A.; Beaudeau, F.; Fourichon, C. Increase in the occurrence of abortions associated with exposure to the Bluetongue virus serotype 8 in naïve dairy herds. Theriogenology 2012, 78, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.A.; Howard, T.H. Transmission of Bluetongue virus by intrauterine inoculation or insemination of virus-containing bovine semen. Am. J. Vet. Res. 1984, 45, 1386–1388. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Ko, H.-J.; Kweon, M.-N. Mucosal dendritic cells shape mucosal immunity. Exp. Mol. Med. 2014, 46, e84. [Google Scholar] [CrossRef]
- Rescigno, M. Intestinal dendritic cells. Adv. Immunol. 2010, 107, 109–138. [Google Scholar] [CrossRef]
- Lombardi, V.C.; Khaiboullina, S.F. Plasmacytoid dendritic cells of the gut: Relevance to immunity and pathology. Clin. Immunol. 2014, 153, 165–177. [Google Scholar] [CrossRef]
- Léger, A.; De Nardi, M.; Simons, R.; Adkin, A.; Ru, G.; Estrada-Peña, A.; Stärk, K.D. Assessment of biosecurity and control measures to prevent incursion and to limit spread of emerging transboundary animal diseases in Europe: An expert survey. Vaccine 2017, 35, 5956–5966. [Google Scholar] [CrossRef] [PubMed]
- Bibard, A.; Martinetti, D.; Giraud, A.; Picado, A.; Chalvet-Monfray, K.; Porphyre, T. Quantitative risk assessment for the introduction of Bluetongue virus into mainland Europe by long-distance wind dispersal of Culicoides spp.: A case study from Sardinia. Risk Anal. 2024, 45, 108–127. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.R.; Horigan, V.; Ip, S.; Taylor, R.A.; Crescio, M.I.; Maurella, C.; Mastrantonio, G.; Bertolini, S.; Ru, G.; Cook, C.; et al. A spatial risk assessment model framework for incursion of exotic animal disease into the European Union member states. Microb. Risk Anal. 2019, 13, 100075. [Google Scholar] [CrossRef]
- Animal and Plant Health Agency (APHA). Risk Assessment for Bluetongue Virus (btv-3 and btv-8): Risk Assessment of Entry into Great Britain; Department for Environment, Food and Rural Affairs: London, UK, 2024. [Google Scholar]
- Nelson, E.; Thurston, W.; Pearce-Kelly, P.; Jenkins, H.; Cameron, M.; Carpenter, S.; Guthrie, A.; England, M. A qualitative risk assessment for Bluetongue disease and african horse sickness: The risk of entry and exposure at a uk zoo. Viruses 2022, 14, 502. [Google Scholar] [CrossRef]
- Omazic, A.; Bylund, H.; Boqvist, S.; Högberg, A.; Björkman, C.; Tryland, M.; Evengård, B.; Koch, A.; Berggren, C.; Malogolovkin, A.; et al. Identifying climate-sensitive infectious diseases in animals and humans in Northern regions. Acta Vet. Scand. 2019, 61, 53. [Google Scholar] [CrossRef]
- Burgin, L.E.; Gloster, J.; Sanders, C.; Mellor, P.S.; Gubbins, S.; Carpenter, S. Investigating incursions of Bluetongue virus using a model of long-distance culicoides biting midge dispersal. Transbound. Emerg. Dis. 2012, 60, 263–272. [Google Scholar] [CrossRef]
- Mahefarisoa, K.; Delso, N.S.; Zaninotto, V.; Colin, M.; Bonmatin, J. The threat of veterinary medicinal products and biocides on pollinators: A One Health perspective. One Health 2021, 12, 100237. [Google Scholar] [CrossRef]
- Dórea, F.C.; Swanenburg, M.; Horigan, V.; Han, S.; Young, B.; Costa, E.d.F.; Santos, M.A.d.S.; Evans, D.; Royall, E.; Aznar, I.; et al. Data collection for risk assessments on animal health: Review protocol 2021. EFSA Support. Publ. 2022, 19, 7086E. [Google Scholar] [CrossRef]
- van Rijn, P.A. Prospects of next-generation vaccines for Bluetongue. Front. Vet. Sci. 2019, 6, 407. [Google Scholar] [CrossRef]
- van Rijn, P.A.; Maris-Veldhuis, M.A.; Spedicato, M.; Savini, G.; van Gennip, R.G.P. Pentavalent disabled infectious single animal (disa)/diva vaccine provides protection in sheep and cattle against different serotypes of Bluetongue virus. Vaccines 2021, 9, 1150. [Google Scholar] [CrossRef] [PubMed]
- Morales, R.; Weber-Vintzel, L.; Awada, L.; Cãceres, P.; Tizzani, P.; Meske, M. The world animal health information system as a tool to support decision-making and research in animal health. Rev. Sci. Tech. 2023, 42, 242–251. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payan-Carreira, R.; Simões, M. Bluetongue’s New Frontier—Are Dogs at Risk? Vet. Sci. 2025, 12, 505. https://doi.org/10.3390/vetsci12050505
Payan-Carreira R, Simões M. Bluetongue’s New Frontier—Are Dogs at Risk? Veterinary Sciences. 2025; 12(5):505. https://doi.org/10.3390/vetsci12050505
Chicago/Turabian StylePayan-Carreira, Rita, and Margarida Simões. 2025. "Bluetongue’s New Frontier—Are Dogs at Risk?" Veterinary Sciences 12, no. 5: 505. https://doi.org/10.3390/vetsci12050505
APA StylePayan-Carreira, R., & Simões, M. (2025). Bluetongue’s New Frontier—Are Dogs at Risk? Veterinary Sciences, 12(5), 505. https://doi.org/10.3390/vetsci12050505






