Vanishing Lung Syndrome in a Dog: Giant Pneumatocele or Giant Pulmonary Bulla Mimicking Tension Pneumothorax—First Report
Simple Summary
Abstract
1. Introduction
2. Case Presentation
2.1. History
2.2. Clinical Examination
2.3. Radiographic Findings
2.4. Diagnosis
2.5. Emergency Treatment
2.6. Etiological Hypotheses
2.7. Additional Examinations
2.8. Follow-Up Radiographs
2.9. Thoracostomy Tube Placement
2.10. Hospitalization Follow-Up
3. Discussion
3.1. Nature of Intrathoracic Air-Filled Cavities
3.2. Distinction Between Blebs and Bullae
3.3. Distinction Between Giant Bulla and Giant Pneumatocele
3.3.1. Epidemiology
3.3.2. Etiopathogenesis
3.3.3. Clinical Presentation: Innumerable Air-Filled Cavities
3.3.4. Evolution
3.4. Originality of This Case
3.4.1. Giant Air-Filled Cavity
3.4.2. Confusion Between a Giant Bulla/Giant Pneumatocele and a Pneumothorax
3.4.3. Vanishing Lung Syndrome
3.4.4. Percutaneous Drainage of the Giant Cavity
3.4.5. Re-Expansion Edema?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipscomb, V.J.; Hardie, R.J.; Dubielzig, R.R. Spontaneous pneumothorax caused by pulmonary blebs and bullae in 12 dogs. J. Am. Anim. Hosp. Assoc. 2003, 39, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Montano, H.; Agthe, P.; Cantatore, M. Traumatic pulmonary pseudocysts in nine dogs and two cats. Vet. Surg. 2023, 52, 607–617. [Google Scholar] [CrossRef]
- Sharma, S.; Kasi, A. Pneumatocele. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Dominguez-Ruiz, M.; Reinero, C.R.; Vientos-Plotts, A.; Grobman, M.E.; Silverstein, D.; Gomes, E.; Le Boedec, K. Association between respiratory clinical signs and respsiratory localization in dogs and cats with abnormal breathing patterns. Vet. J. 2021, 277, 105761. [Google Scholar] [CrossRef]
- Gilday, C.; Odunayo, A.; Hespel, A.M. Spontaneous Pneumothorax: Pathophysiology, Clinical Presentation and Diagnosis. Top Companion. Anim. Med. 2021, 45, 100563. [Google Scholar] [CrossRef]
- Reetz, J.A.; Caceres, A.V.; Suran, J.N.; Oura, T.J.; Zwingenberger, A.L.; Mai, W. Sensitivity, positive predictive value, and interobserver variability of computed tomography in the diagnosis of bullae associated with spontaneous pneumothorax in dogs: 19 cases (2003–2012). J. Am. Vet. Med. Assoc. 2013, 243, 244–251. [Google Scholar] [CrossRef]
- Healy, D.; Ballarini, L.; Agthe, P.; Cantatore, M.; Moores, A.L. Significance of incidentally identified bullae and blebs on thoracic computed tomography and prevalence of subsequent pneumothorax in dogs. Vet. Surg. 2024, 54, 52–58. [Google Scholar] [CrossRef]
- Dickson, R.; Scharf, V.F.; Michael, A.E.; Walker, M.; Thomson, C.; Grimes, J.; Singh, A.; Oblak, M.; Brisson, B.; Case, J.B. Surgical management and outcome of dogs with primary spontaneous pneumothorax: 110 cases (2009–2019). J. Am. Vet. Med. Assoc. 2021, 258, 1229–1235. [Google Scholar] [CrossRef]
- Lipscomb, V.; Brockman, D.; Gregory, S.; Baines, S.; Lamb, C.R. CT scanning of dogs with spontaneous pneumothorax. Vet. Rec. 2004, 154, 344. [Google Scholar]
- Au, J.J.; Weisman, D.L.; Stefanacci, J.D.; Palmisano, M.P. Use of computed tomography for evaluation of lung lesions associated with spontaneous pneumothorax in dogs: 12 cases (1999–2002). J. Am. Vet. Med. Assoc. 2006, 228, 733–737. [Google Scholar] [CrossRef]
- Kim, W.S.; Ward, M.; Huynh, E.; Griffin, L.; Heo, J.; Vinayak, A. Thoracic CT incidental pulmonary bullae in dogs: Characterization, interobserver variability, and general anesthesia risks. Vet. Radiol. Ultrasound 2023, 64, 402–410. [Google Scholar] [CrossRef]
- Puerto, D.A.; Brockman, D.J.; Lindquist, C.; Drobatz, K. Surgical and nonsurgical management of and selected risk factors for spontaneous pneumothorax in dogs: 64 cases (1986–1999). J. Am. Vet. Med. Assoc. 2002, 220, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Kramek, B.A.; Caywood, D.D.; O’Brien, T.D. Bullous emphysema and recurrent pneumothorax in the dog. J. Am. Vet. Med. Assoc. 1985, 186, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Tennant, B.J.; Haywood, S. Congenital bullous emphysema in a dog: A case report. J. Small. Anim. Pract. 1987, 28, 109–116. [Google Scholar] [CrossRef]
- Brissot, H.N.; Dupre, G.P.; Bouvy, B.M.; Paquet, L. Thoracoscopic treatment of bullous emphysema in 3 dogs. Vet. Surg. 2003, 32, 524–529. [Google Scholar] [CrossRef]
- Hamad, A.-M.M.; El-Saka, H.A. Is it a bulla or a pneumatocele? Eur. J. Cardio-Thorac. Surg. 2021, 60, 203. [Google Scholar] [CrossRef]
- Howes, C.L.; Sumner, J.P.; Ahlstrand, K.; Hardie, R.J.; Anderson, D.; Woods, S.; Goh, D.; de la Puerta, B.; Brissot, H.N.; Das, S.; et al. Long-term clinical outcomes following surgery for spontaneous pneumothorax caused by pulmonary blebs and bullae in dogs—A multicentre (AVSTS Research Cooperative) retrospective study. J. Small Anim. Pract. 2020, 61, 436–441. [Google Scholar] [CrossRef]
- Sahn, S.A.; Heffner, J.E. Spontaneous pneumothorax. N. Engl. J. Med. 2000, 342, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Ngoo, A.; Slaney, C.; Mariyappara, B.; Stalewski, H.; Carroll, D. Traumatic pulmonary pseudocysts mimicking a congenital malformation of the lung. J. Pediatr. Surg. Case Rep. 2018, 29, 26–29. [Google Scholar] [CrossRef]
- Schimpl, G.; Schneider, U. Traumatic pneumatoceles in an infant: Case report and review of the literature. Eur. J. Pediatr. Surg. 1996, 6, 104–106. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Stevenson, G.W. Congenital Lobar Emphysema and Tension Pneumothorax in a Dog. J. Vet. Diagn. Invest. 2007, 19, 322–325. [Google Scholar] [CrossRef]
- Oberhaus, A.; McFadden, M. Use of vessel sealing system for multiple partial lung lobectomies for spontaneous pneumothorax. Can. Vet. J. 2020, 61, 875–879. [Google Scholar] [PubMed]
- Moloney, C.; Puggioni, A.; McKenna, M. Allogenic blood patch pleurodesis for management of pneumothorax in a Cavalier King Charles Spaniel puppy with multiple pulmonary blebs and bullae. J. Vet. Intern. Med. 2022, 36, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Arime, H.; Asai, T.; Fujishiro, A.; Saito, T. Effective use of a supraglottic airway (i-gel) during emergence from anesthesia in a patient with multiple giant bullae. JA Clin. Rep. 2024, 10, 73. [Google Scholar] [CrossRef]
- Chiappetta, M.; Nachira, D.; Porziella, V.; Vita, M.L.; Margaritora, S. Multiple giant bullae of the lung mimicking massive pneumothorax in a patient with osteogenesis imperfecta. Thorax 2016, 71, 577. [Google Scholar] [CrossRef][Green Version]
- Brand, E.M.; Lim, C.K.; Biswell, E.; Jones-Hall, Y.; Heng, H.G. Concurrent bullous emphysema, bronchointerstitial pneumonia with necrosis, and tension pneumothorax in an 8-week-old puppy. Can. Vet. J. 2020, 61, 951–955. [Google Scholar]
- Matuszczak, E.; Oksiuta, M.; Hermanowicz, A.; Debek, W. Traumatic pneumatocele in an 11-year-old boy—Report of a rare case and review of the literature. Kardiochir. Torakochirurgia Pol. 2017, 14, 59–62. [Google Scholar] [CrossRef]
- Mulholland, N.; Keir, I. Traumatic Pulmonary Pseudocysts in a Young Dog Following Non-penetrating Blunt Thoracic Trauma. Front. Vet. Sci. 2019, 6, 237. [Google Scholar] [CrossRef]
- Bertolini, G.; Briola, C.; Angeloni, L.; Costa, A.; Rocchi, P.; Caldin, M. Trauma-Associated Pulmonary Laceration in Dogs—A Cross Sectional Study of 364 Dogs. Vet. Sci. 2020, 7, 41. [Google Scholar] [CrossRef]
- Roberts, L.; Putman, C.; Chen, J.; Goodman, L.; Ravin, C. Vanishing lung syndrome: Upper lobe bullous pneumopathy. Rev. Interam. Radiol. 1987, 12, 249. [Google Scholar]
- Park, J.; Lee, H.B.; Jeong, S.M. Treatment of a giant pulmonary emphysematous cyst with primary bronchoalveolar papillary carcinoma in a Shih Tzu dog. Vet. Surg. 2017, 46, 158–164. [Google Scholar] [CrossRef]
- Roque, C.A.; Lima, B.R.; de Oliveira, G.V.; Nascimento, L.M. Giant and potentially malignant bullae in a dog. Open Vet. J. 2023, 13, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.R.; Kumar, L.; Mitra, S.K. Proteus mirabilis pneumonia with giant pneumatocele. Indian J. Pediatr. 1987, 54, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Paula, S.R.M.d.; Gurgel, M.P.; Macchiaverni, L.; Pereira, F.M.; Ribeiro, M.Â.G.d.O.; Santos, C.I.S. Pneumonia complicada por pneumatocele gigante em criança com síndrome da imunodeficência adquirida: Importância da fisioterapia respiratória. Rev. Paul. De Pediatr. 2010, 28, 244–248. [Google Scholar] [CrossRef]
- Yao, C.W.; Shen, T.C.; Tu, C.Y. Giant pneumatocele with lung herniation. Intern. Med. 2013, 52, 2377–2378. [Google Scholar] [CrossRef]
- Choueiry, E.; Diab, S.; El Haber, C.; Torbey, P.H.; Ghorayeb, Z.; Gerbaka, B. Incomplete treatment of neonatal pneumonia causing giant pneumatocele in a 45-day infant. Int. J. Clin. Pediatr. 2017, 6, 24–27. [Google Scholar] [CrossRef]
- Reyes, I.J.; Bustamante, M.E.M.; Ortega, J.O.; Sánchez, J.R.; Ramírez, A.M. Giant pneumatocele secondary to pulmonary coccidioidomycosis in the immunocompetent patient. A case report and literature review. Rev. Latinoam. De Infectología Pediátrica 2019, 32, 113–118. [Google Scholar]
- Palma, N.Z.; da Cruz, M. Giant Pneumatocele. GaliciaClinica-Off. J. Galician Soc. Intern. Med. SOGAMI 2020, 81, 97. [Google Scholar]
- Rana, M.; Khan, S.; Pervez, M.; Fatimi, S. Giant pneumatocele secondary to Aspergillus nidulans in autosomal dominant hyper-Ige syndrome child. Chest 2020, 157, A33. [Google Scholar] [CrossRef]
- Aujayeb, A. Please do not put a chest drain in my chest! Vanishing lung syndrome. Afr. J. Emerg. Med. 2020, 10, 261–265. [Google Scholar] [CrossRef]
- Burke, R.M. Vanishing Lungs: A Case Report of Bullous Emphysema. Radiology 1937, 28, 367–371. [Google Scholar] [CrossRef]
- Haahti, H.G. Beobachtungen über Einen Fall Mit Ausgedehnten Intrapulmonalen Lufthaltigen Hohlräumen. Acta Radiol. 1932, os-13, 620–638. [Google Scholar] [CrossRef]
- Miller, W.S. A Tuberculous Lung in Which a Large Emphysematous Bulla Was Mistaken for a Cavity. Am. Rev. Tuberc. 1933, 28, 359–369. [Google Scholar]
- Korol, E.; Ensign, C. Bullous emphysema (?) or bilateral pneumothorax (?). Radiology 1934, 23, 223–227. [Google Scholar] [CrossRef]
- Burghard, E. Hochgradige Verlagerung des Mediastinums beim Säugling infolge kongenitaler Bronchiektasie im linken Oberlappen. Fortschr. Röntgenstr. 1926, 34, 308. [Google Scholar]
- Ribadeau-Dumas, L. Chabrun et Weill: Pseudo-pneumothorax chez un enfant presentant une large dilatation des bronches. Presse Méd. 1927, 548. Available online: https://numerabilis.u-paris.fr/medica/bibliotheque-numerique/resultats/index.php?do=page&cote=90130x1927&p=672 (accessed on 19 May 2025).
- Chen, C.K.; Cheng, H.K.; Chang, W.H.; Lai, Y.C.; Su, Y.J. Giant emphysematous bulla mimicking tension pneumothorax. Am. J. Med. Sci. 2009, 337, 205. [Google Scholar] [CrossRef]
- Gokce, M.; Saydam, O.; Altin, R.; Kart, L. Giant bulla mimicking tension pneumothorax. Tuberk Toraks 2009, 57, 435–438. [Google Scholar]
- Yalcinkaya, S.; Vural, A.H.; Ozal, H. An adult case of giant bronchogenic cyst mimicking tension pneumothorax. Asian Cardiovasc. Thorac. Ann. 2010, 18, 476–478. [Google Scholar] [CrossRef]
- Vega, M.E.; Civic, B. Images in clinical medicine: A tension bulla mimicking tension pneumothorax. N. Engl. J. Med. 2011, 365, 1915. [Google Scholar] [CrossRef]
- Tam, J.K.; Lim, K.S. Massive pulmonary tuberculosis cavity misdiagnosed as pneumothorax. Respirol. Case Rep. 2013, 1, 23–25. [Google Scholar] [CrossRef]
- Tanaka, R.; Hino, H.; Okabe, A.; Utsumi, T.; Maru, N.; Matsui, H.; Taniguchi, Y.; Saito, T.; Tsuta, K.; Murakawa, T. Giant Bullae Misdiagnosed as Pneumothorax:Report of a Case. Kyobu Geka 2023, 76, 331–334. [Google Scholar]
- Ascano, M.P.; Kramer, N.; Le, K. Differentiating Giant Bullous Emphysema From Tension Pneumothorax: A Case Report. Cureus 2024, 16, e55988. [Google Scholar] [CrossRef]
- Ye, Y.; Zhan, Y. Giant pulmonary bullae mistaken for pneumothorax. Am. J. Emerg. Med. 2024, 83, 162.e1–162.e3. [Google Scholar] [CrossRef]
- Stern, E.J.; Webb, W.R.; Weinacker, A.; Muller, N.L. Idiopathic giant bullous emphysema (vanishing lung syndrome): Imaging findings in nine patients. AJR Am. J. Roentgenol. 1994, 162, 279–282. [Google Scholar] [CrossRef]
- Waseem, M.; Jones, J.; Brutus, S.; Munyak, J.; Kapoor, R.; Gernsheimer, J. Giant bulla mimicking pneumothorax. J. Emerg. Med. 2005, 29, 155–158. [Google Scholar] [CrossRef]
- Khasawneh, F.A.; Nakhla, E.N.; Karim, A.; Halloush, R.A. Vanishing lung syndrome mistaken for bilateral spontaneous pneumothorax. BMJ Case Rep. 2013, 2013, A302. [Google Scholar] [CrossRef]
- Lai, C.C.; Huang, S.H.; Wu, T.T.; Lin, S.H. Vanishing lung syndrome mimicking pneumothorax. Postgrad. Med. J. 2013, 89, 427–428. [Google Scholar] [CrossRef]
- Aramini, B.; Ruggiero, C.; Stefani, A.; Morandi, U. Giant bulla or pneumothorax: How to distinguish. Int. J. Surg. Case Rep. 2019, 62, 21–23. [Google Scholar] [CrossRef]
- Muhamad, N.I.; Mohd Nawi, S.N.; Yusoff, B.M.; Ab Halim, N.A.; Mohammad, N.; Wan Ghazali, W.S. Vanishing lung syndrome Masquerading as bilateral pneumothorax: A case report. Respir. Med. Case Rep. 2020, 31, 101276. [Google Scholar] [CrossRef]
- Yousaf, M.N.; Chan, N.N.; Janvier, A. Vanishing Lung Syndrome: An Idiopathic Bullous Emphysema Mimicking Pneumothorax. Cureus 2020, 12, e9596. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.; Park, J.H.; Lee, J.Y.; Cho, E.J. Cardiovascular Collapse after the Induction of Anesthesia Due to the MASS Effect of Unruptured Giant Bullae. Medicina 2023, 59, 1689. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Nunes, N.; Milhomem, P.; Macedo, B.; Moutinho, T.; Vieira, W.; Tavares, M. Giant Pneumatocele as Late Evolution Mimics Pneumothorax. In Proceedings of the Abstracts from CIPP XVI Meeting—Pediatric Pulmonology, Lisbon, Portugal, 22–25 June 2017; pp. S173–S174. [Google Scholar]
- Hsieh, M.-S.; Chen, C.-K.; Wong, W.-W.; Huang, C.-S. Rapid-growth pneumatocele mimics massive pneumothorax in a HIV-positive patient. Thorax 2013, 68, 307. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hariri, E.; Benson, R.; Navia, J.; Gordon, S. Pseudo-vanishing lung syndrome in a patient with tricuspid valve bacterial endocarditis. J. Cardiol. Cases 2018, 17, 215–219. [Google Scholar] [CrossRef]
- Khan, Q.; Batool, A.; Haider, M.A.; Hanif, M.; Ali, M.J.; Abdul Sattar, S.B.; Khan, S.J. Large emphysematous bullae mimicking as a pneumothorax leading to unnecessary chest tube insertion and iatrogenic pneumothorax. J. Ayub. Med. Coll. Abbottabad 2021, 33, 526–528. [Google Scholar]
- Heo, J.; Bak, S.H.; Ryu, S.M.; Hong, Y. Tuberculosis-Infected Giant Bulla Treated by Percutaneous Drainage Followed by Obliteration of the Pulmonary Cavity Using Talc: Case Report. J. Chest Surg. 2021, 54, 408–411. [Google Scholar] [CrossRef]
- Im, Y.; Jeong, B.H.; Park, H.Y.; Kim, T.S.; Kim, H. Expeditious Resolution of Giant Bullae with Endobronchial Valves and Percutaneous Catheter Insertion. Yonsei Med. J. 2022, 63, 195–198. [Google Scholar] [CrossRef]
- Lodro, M.S.; Mazcuri, M.; Ahmad, T.; Abid, A. Clinical Results After Intra-Cavitary Drainage Of Giant Bullae In Patients With Poor Pulmonary Reserves. J. Ayub Med. Coll. Abbottabad 2023, 35, 21–26. [Google Scholar] [CrossRef]
- Lu, H.; Li, Z.; Yu, Y.; Li, G.; Liang, T.; Zhang, H. Efficacy and safety of medical thoracoscopic bulla volume reduction in the treatment of chronic obstructive pulmonary disease combined with giant emphysematous bullae. Sichuan Da Xue Xue Bao Yi Xue Ban 2024, 55, 403–410. [Google Scholar] [CrossRef]
- Zuhdi, M.K.; Spear, R.M.; Worthen, H.M.; Peterson, B.M. Percutaneous catheter drainage of tension pneumatocele, secondarily infected pneumatocele, and lung abscess in children. Crit. Care Med. 1996, 24, 330–333. [Google Scholar] [CrossRef]
- Kogutt, M.S.; Lutrell, C.A.; Puyau, F.A.; Tieman, E.K. Decompression of pneumatocele in a neonate by percutaneous catheter placement. Pediatr. Radiol. 1999, 29, 488–489. [Google Scholar] [CrossRef]
- Fujii, A.M.; Moulton, S. Percutaneous catheter evacuation of a pneumatocele in an extremely premature infant with respiratory failure. J. Perinatol. 2003, 23, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Mukhopadhyay, K.; Bhatia, A. Successful percutaneous drainage of pneumatoceles in an extremely low-birthweight infant. Case Rep. 2018, 2018, bcr-2017-222630. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.; Moores, D.C.; Khan, F.A.; Baerg, J.; Radulescu, A. Successful treatment of post-infectious pneumatocele via percutaneous drainage in a premature infant. J. Pediatr. Surg. Case Rep. 2019, 47, 101235. [Google Scholar] [CrossRef]
- Muniraman, H.; Chintala, S.; Richardson, R.; Duarte, A. Successful ultrasound guided percutaneous drainage of pneumatocele in an extremely preterm infant. Radiol. Case Rep. 2021, 16, 607–611. [Google Scholar] [CrossRef]
- Grant, M.J. Acute unilateral pulmonary oedema following re-expansion of a spontaneous pneumothorax: Case report. N. Z. Med. J. 1971, 74, 250–251. [Google Scholar]
- Rozenman, J.; Yellin, A.; Simansky, D.A.; Shiner, R.J. Re-expansion pulmonary oedema following spontaneous pneumothorax. Respir. Med. 1996, 90, 235–238. [Google Scholar] [CrossRef]
- Papakonstantinou, D.K.; Gatzioufas, Z.I.; Tzegas, G.I.; Stergiopoulos, P.I.; Tsokantaridis, C.G.; Chalikias, G.K.; Tziakas, D.N. Unilateral pulmonary oedema due to lung re-expansion following pleurocentesis for spontaneous pneumothorax. The role of non-invasive continuous positive airway pressure ventilation. Int. J. Cardiol. 2007, 114, 398–400. [Google Scholar] [CrossRef]
- Sanchez Lopez, J.D.; Nunez, O.S.; Ferrero, E.; Garcia-Sancho, L.; Picardo, A.L. Re-expansion pulmonary oedema following spontaneous pneumothorax. Postgrad. Med. J. 2019, 95, 452. [Google Scholar] [CrossRef]
- Abaunza-Camacho, M.P.; Galindo, J.L.; Carrillo, J.A. Localised re-expansion pulmonary oedema following spontaneous pneumothorax. Lancet 2024, 403, 567. [Google Scholar] [CrossRef]
- McCoskey, E.H.; McKinney, L.M.; Byrd, R.P., Jr.; Roy, T.M. Re-expansion pulmonary edema following puncture of a giant bulla. J. Am. Osteopath. Assoc. 2000, 100, 788–791. [Google Scholar]
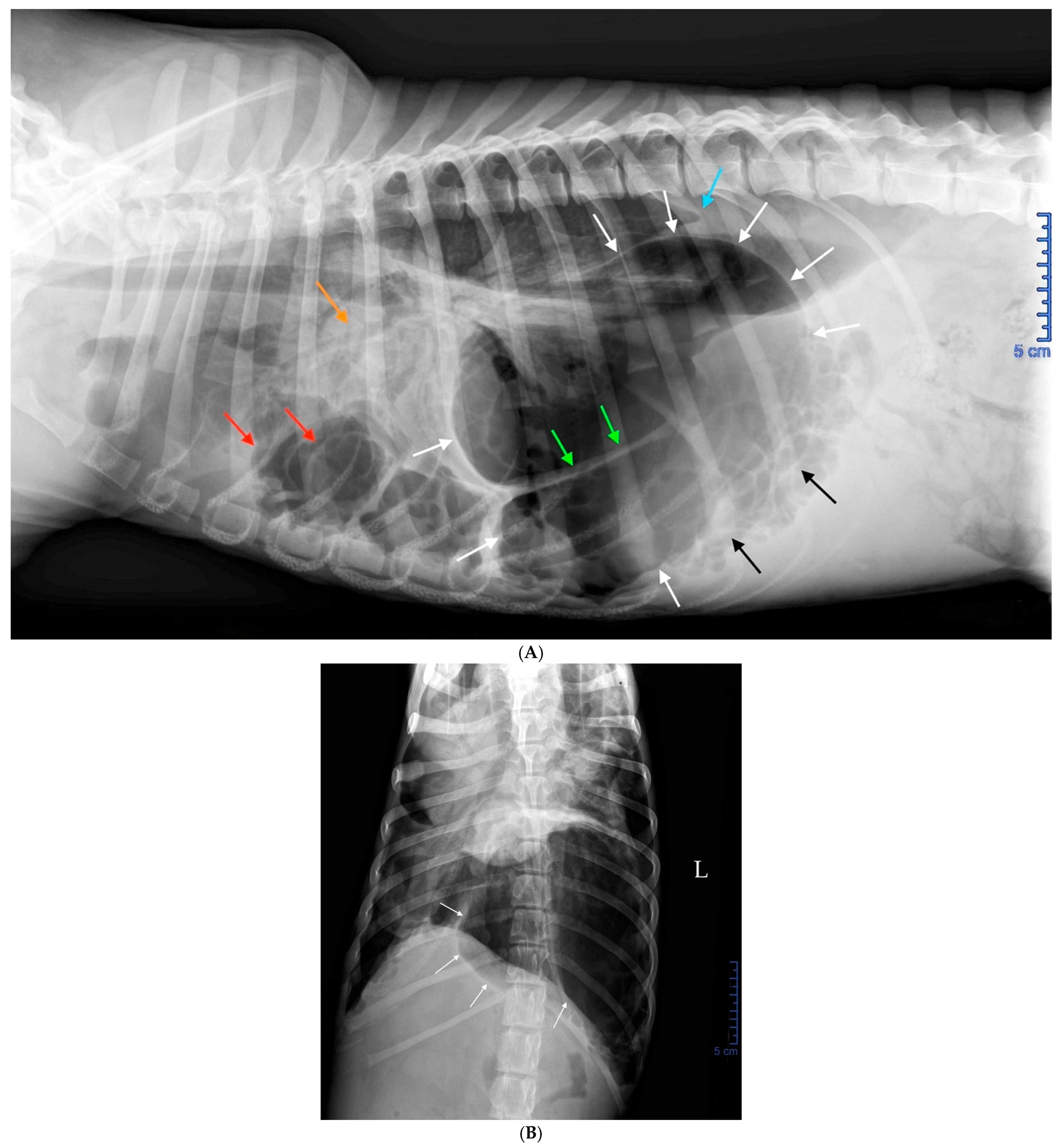

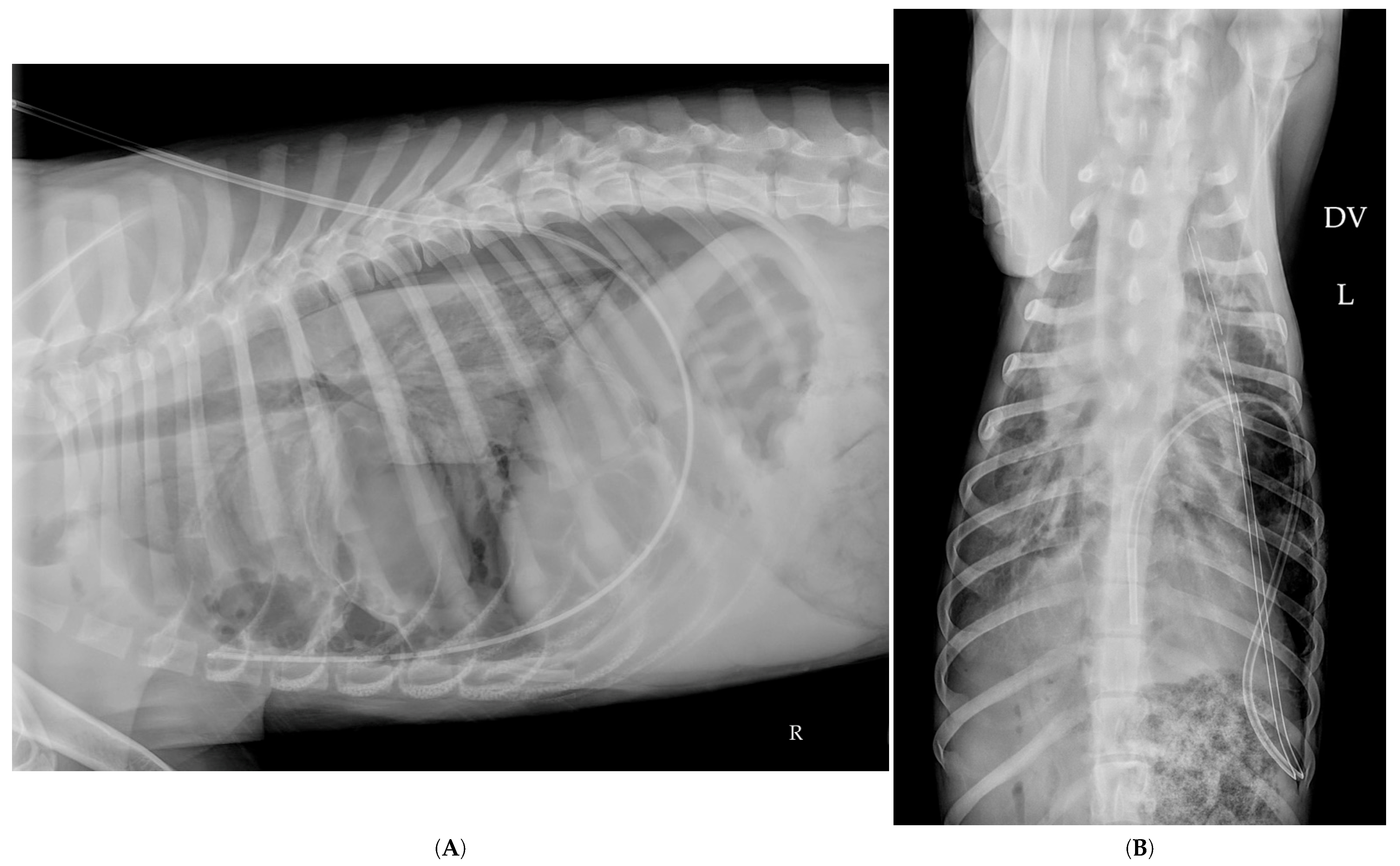
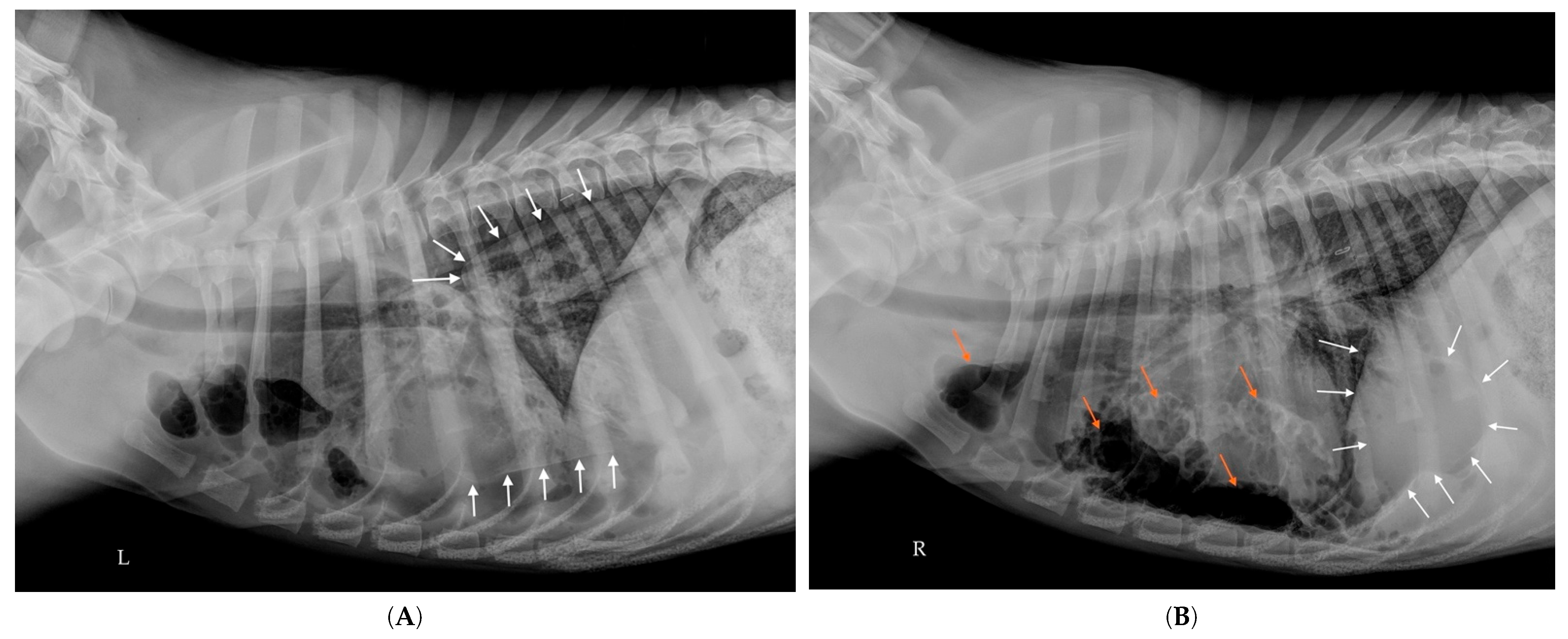

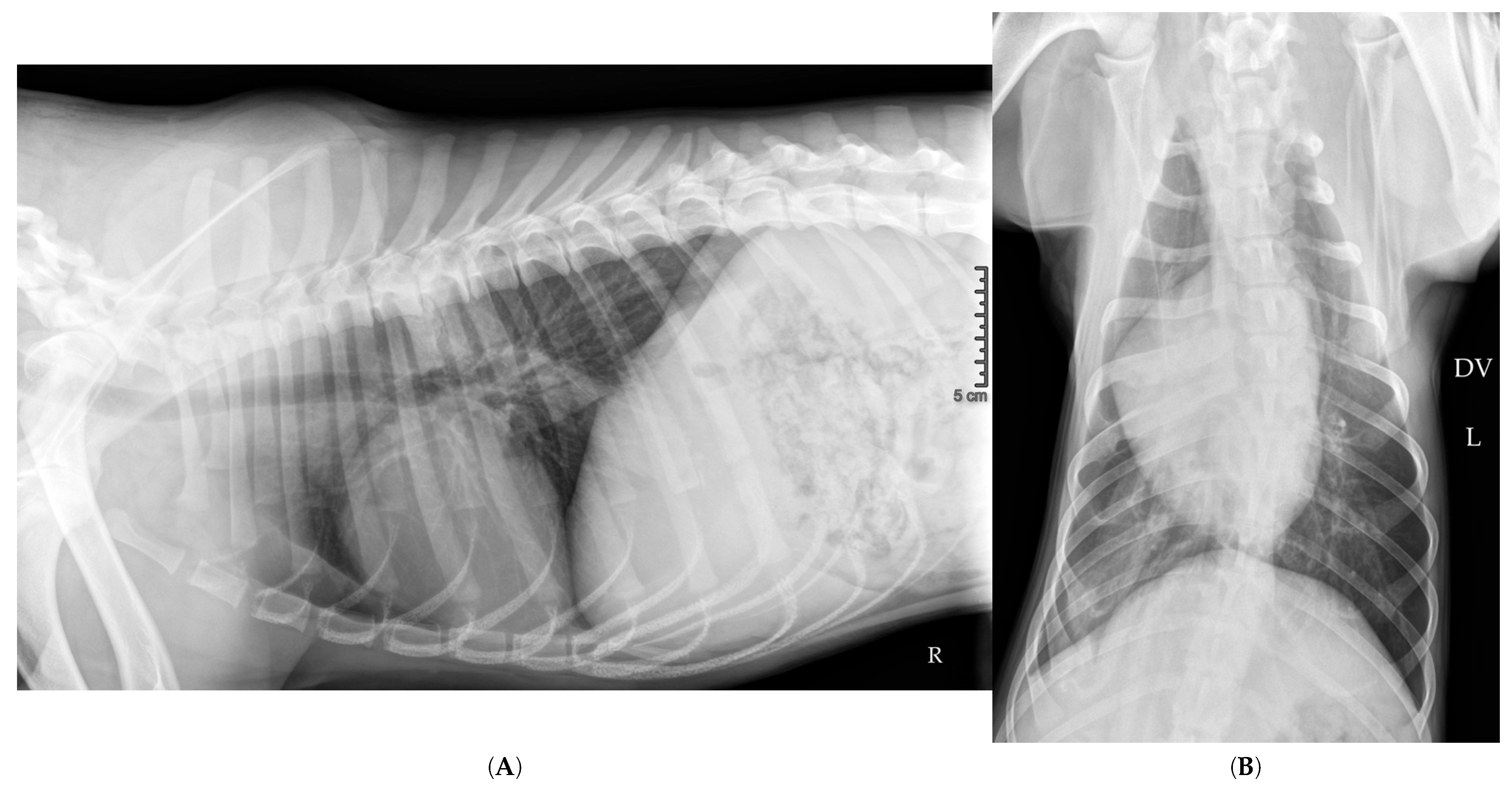
| Feature | Pulmonary Bulla | Pneumatocele |
|---|---|---|
| Etiology | Congenital or acquired: associated with emphysema, smoking | Acquired: post-infectious, trauma, barotrauma (mechanical ventilation) |
| Age of Onset | Typically affects young thin adults, especially smokers | More common in infants, children, and young adults |
| Clinical Context | Often incidental; may present with spontaneous pneumothorax | Occurs after pneumonia, chest trauma, or mechanical ventilation |
| Clinical Evolution | Progressive; may remain stable or enlarge over time | Generally resolves spontaneously or with conservative treatment |
| Symptoms | Often asymptomatic until rupture; large bullae can cause dyspnea | May be asymptomatic or cause mild respiratory distress |
| Imaging: Number of Lesions | Usually solitary or few in number | Multiple; can be solitary or numerous |
| Imaging: Distribution of Lesions | Typically subpleural, especially in upper lobes | Variable; can be unilateral or bilateral, often in areas of prior infection or trauma |
| Imaging: Wall Characteristics | Very thin-walled, often imperceptible on imaging | Thin-walled, well-defined air-filled cavities |
| Imaging: Shape | Well-circumscribed, round | Round or oval |
| Imaging: Size | Typically > 1 cm; giant bullae may occupy more than 30% of hemithorax | Varies; can be small or large, sometimes termed “giant” if very large |
| Association with Pneumothorax | Frequent; rupture is a common cause of spontaneous pneumothorax | Can be associated, especially if ruptured |
| Response to Conservative Treatment | Rarely regresses; may require surgical intervention if symptomatic | Often resolves without intervention |
| Prognosis | Variable; depends on size, number, and associated complications | Generally favorable with resolution of underlying cause |
| Histopathology | Air-filled space lined by attenuated epithelium; associated with alveolar wall destruction | Air-filled cavity without epithelial lining; may show organizing fibrosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deschamps, J.-Y.; Abboud, N.; Penaud, P.; Roux, F.A. Vanishing Lung Syndrome in a Dog: Giant Pneumatocele or Giant Pulmonary Bulla Mimicking Tension Pneumothorax—First Report. Vet. Sci. 2025, 12, 501. https://doi.org/10.3390/vetsci12050501
Deschamps J-Y, Abboud N, Penaud P, Roux FA. Vanishing Lung Syndrome in a Dog: Giant Pneumatocele or Giant Pulmonary Bulla Mimicking Tension Pneumothorax—First Report. Veterinary Sciences. 2025; 12(5):501. https://doi.org/10.3390/vetsci12050501
Chicago/Turabian StyleDeschamps, Jack-Yves, Nour Abboud, Pierre Penaud, and Françoise A. Roux. 2025. "Vanishing Lung Syndrome in a Dog: Giant Pneumatocele or Giant Pulmonary Bulla Mimicking Tension Pneumothorax—First Report" Veterinary Sciences 12, no. 5: 501. https://doi.org/10.3390/vetsci12050501
APA StyleDeschamps, J.-Y., Abboud, N., Penaud, P., & Roux, F. A. (2025). Vanishing Lung Syndrome in a Dog: Giant Pneumatocele or Giant Pulmonary Bulla Mimicking Tension Pneumothorax—First Report. Veterinary Sciences, 12(5), 501. https://doi.org/10.3390/vetsci12050501







