Subclinical Hypocalcemia Across Lactation Stages Reflects Potential Metabolic Vulnerability in Korean Holstein Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Population and Classification
2.3. Blood Sampling
2.4. Complete Blood Count (CBC) Analysis
2.5. Serum Analysis
2.6. Statistical Analysis
3. Results
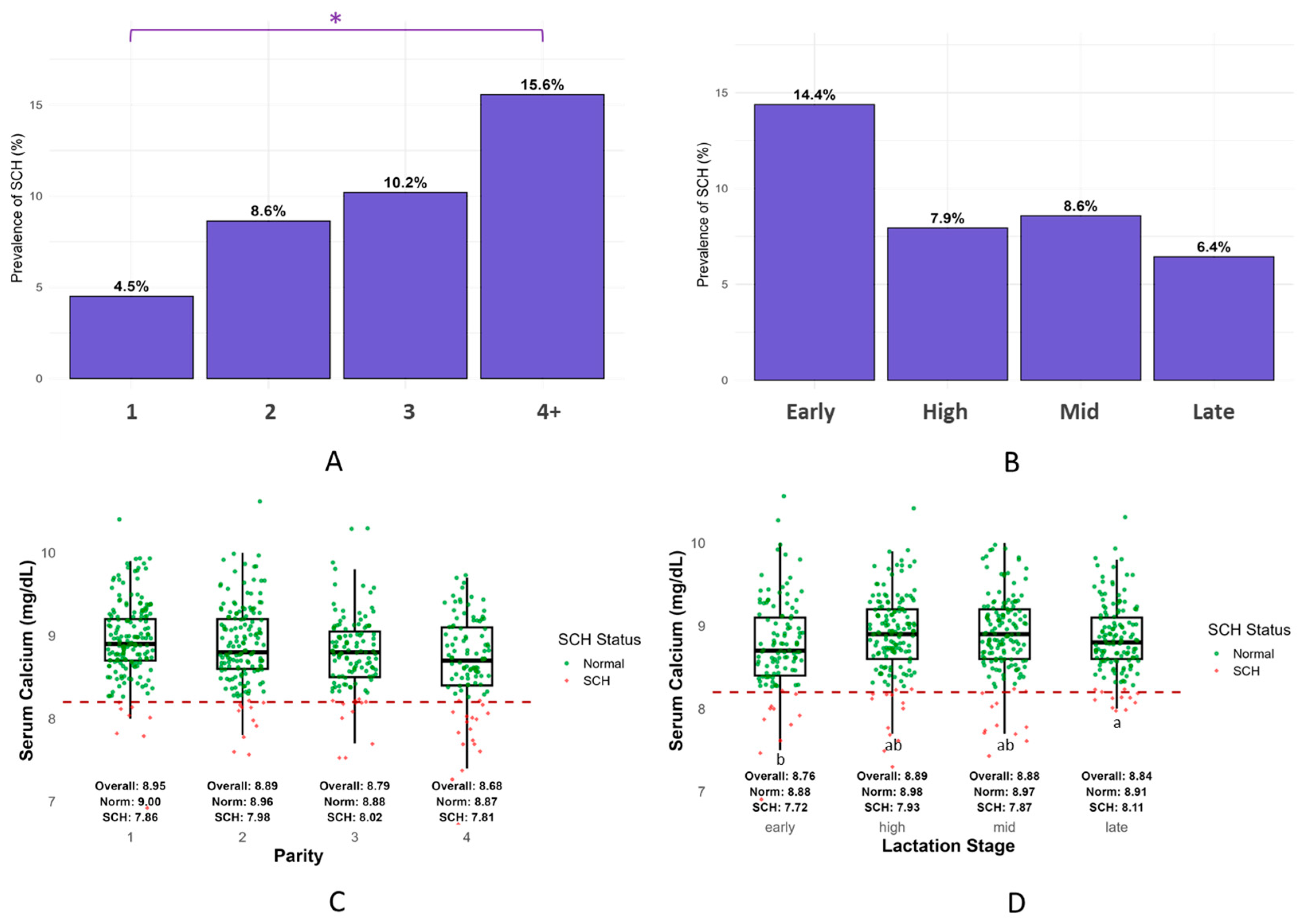
3.1. Prevalence and Severity of Subclinical Hypocalcemia (SCH)
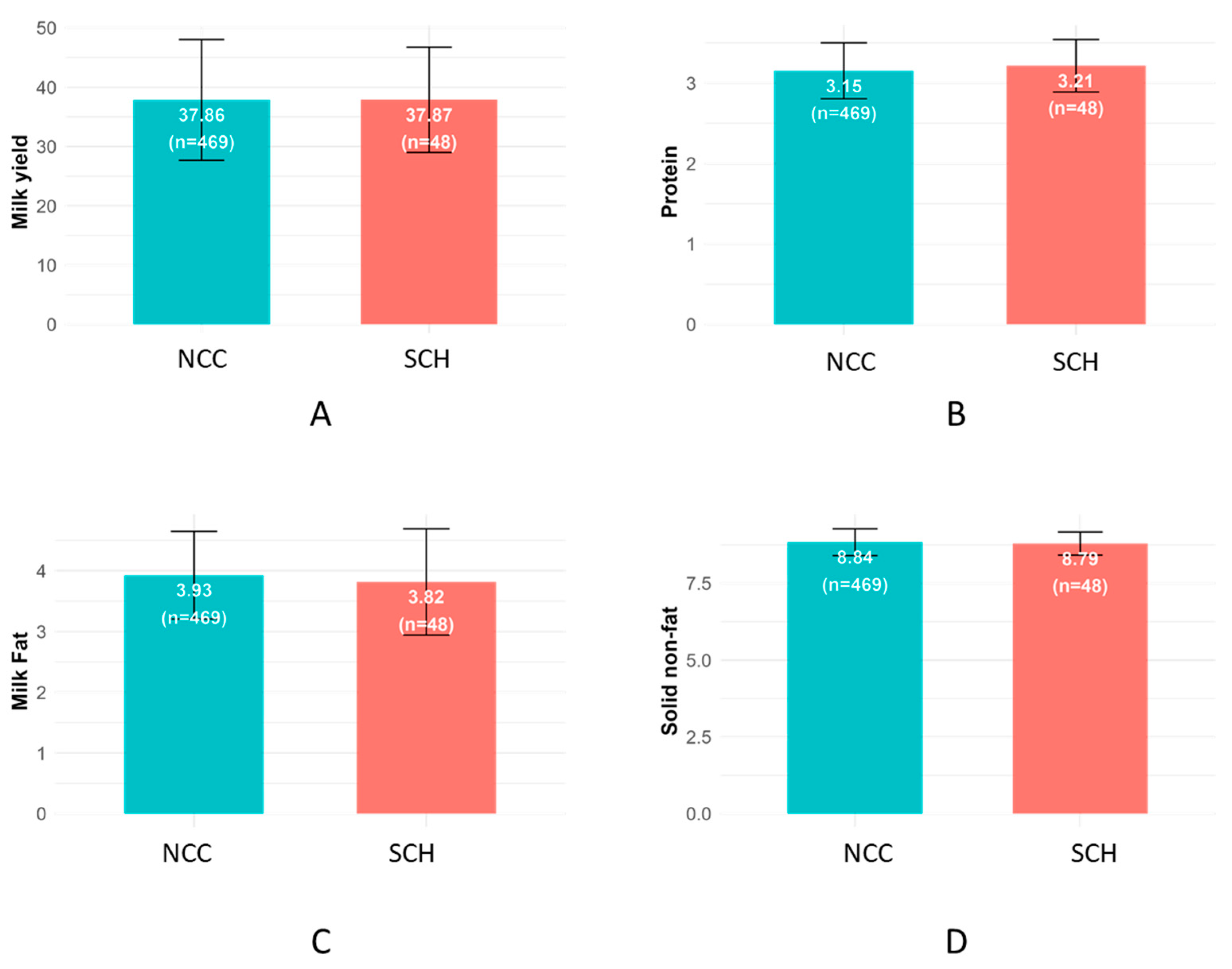
3.2. Milk Yield and Composition
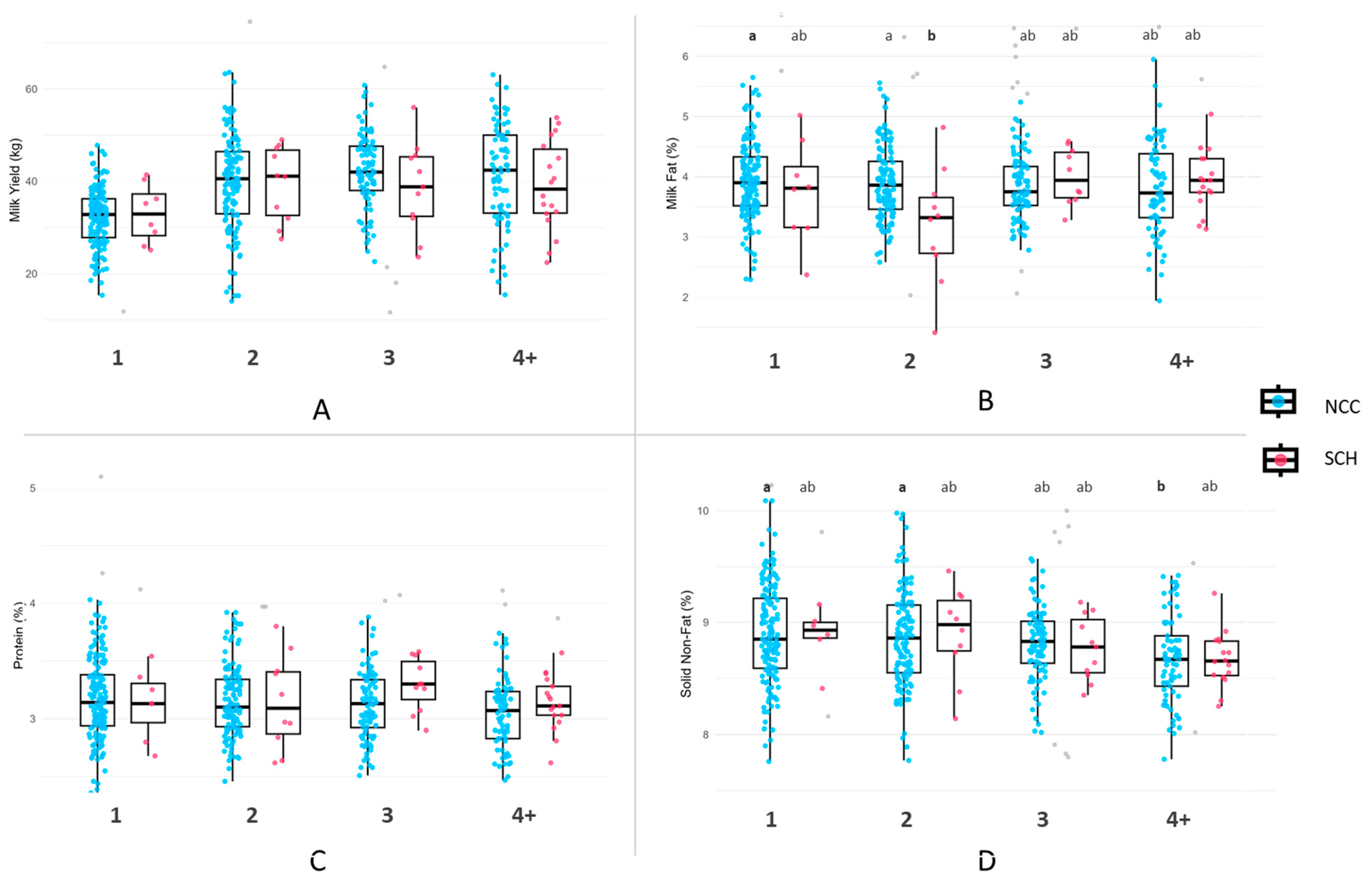
3.3. Milk Yield and Composition by Parity
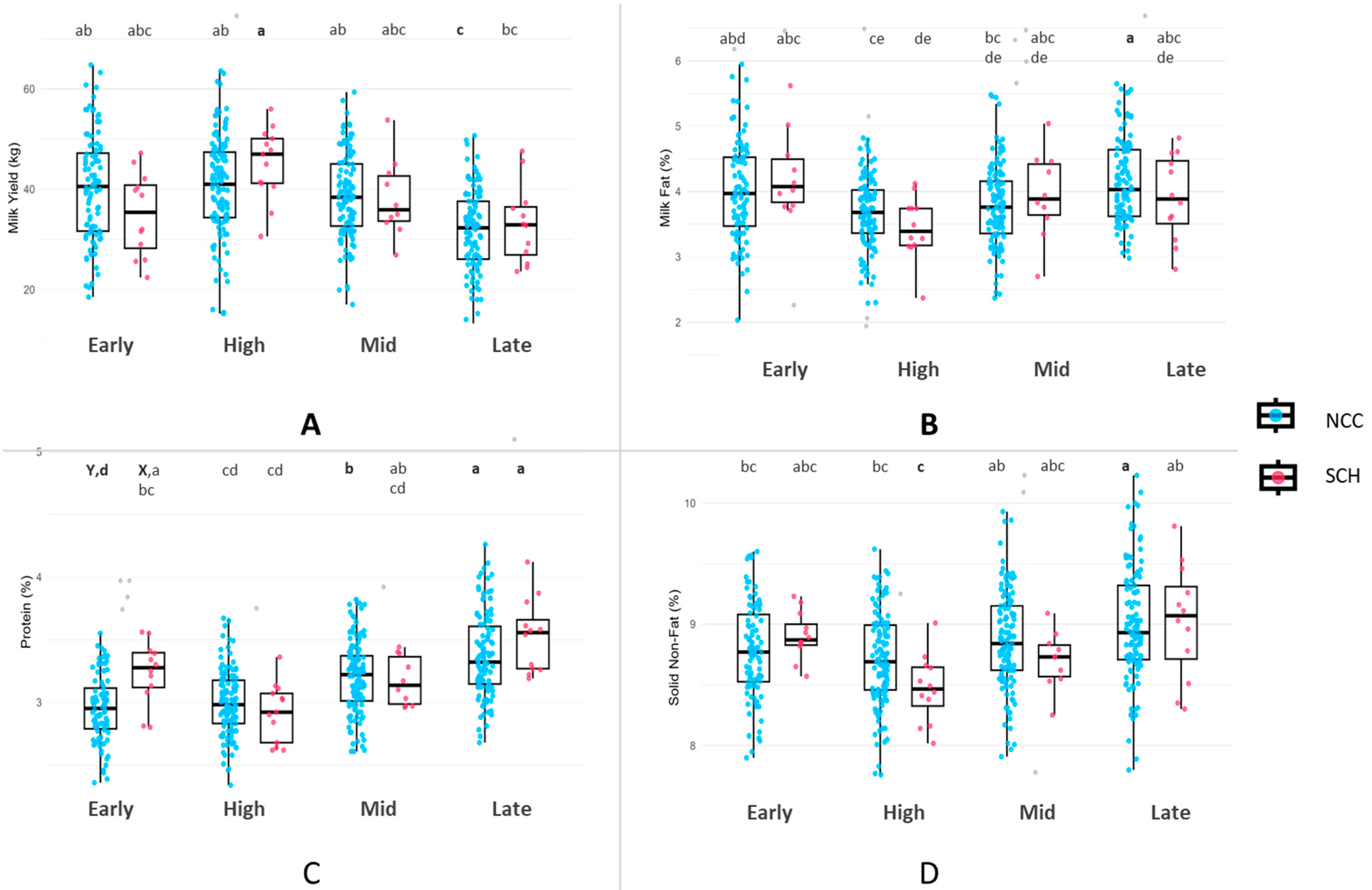
3.4. Milk Yield and Composition by Lactation Stage
3.5. Complete Blood Count (CBC)
3.6. Serum Biochemistry Parameters
4. Discussion
4.1. Prevalence and Group Distribution
4.2. Milk Yield and Production Parameters
4.3. Hematological and Serum Biochemistry Profiles
4.4. Physiological Interpretation of SCH
4.5. Implications and Future Direction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCH | Subclinical Hypocalcemia | Ca | Calcium |
| PTH | Parathyroid Hormone | NEB | Negative Energy Balance |
| BHBA | Beta-Hydroxybutyrate | NEFA | Non-Esterified Fatty Acids |
| CBC | Complete Blood Count | TMR | Total Mixed Ration |
| DIM | Days in Milk | TP | Total Protein |
| ALB | Albumin | GLOB | Globulin |
| AST | Aspartate Aminotransferase | GGT | Gamma-Glutamyl Transferase |
| TBIL | Total Bilirubin | CHOL | Cholesterol |
| BUN | Blood Urea Nitrogen | RBC | Red Blood Cell Count |
| HCT | Hematocrit | HGB | Hemoglobin |
| MCV | Mean Corpuscular Volume | MCH | Mean Corpuscular Hemoglobin |
| MCHC | Mean Corpuscular Hemoglobin Conc. | WBC | White Blood Cell Count |
| NEU | Neutrophils | LYM | Lymphocytes |
| MONO | Monocytes | EOS | Eosinophils |
| BASO | Basophils | PLT | Platelet Count |
References
- Reinhardt, T.A.; Lippolis, J.D.; McCluskey, B.J.; Goff, J.P.; Horst, R.L. Prevalence of Subclinical Hypocalcemia in Dairy Herds. Vet. J. 2011, 188, 122–124. [Google Scholar] [CrossRef]
- Wilkens, M.R.; Nelson, C.D.; Hernandez, L.L.; McArt, J.A.A. Symposium Review: Transition Cow Calcium Homeostasis—Health Effects of Hypocalcemia and Strategies for Prevention. J. Dairy Sci. 2020, 103, 2909–2927. [Google Scholar] [CrossRef] [PubMed]
- Couto Serrenho, R.; DeVries, T.J.; Duffield, T.F.; LeBlanc, S.J. Graduate Student Literature Review: What Do We Know about the Effects of Clinical and Subclinical Hypocalcemia on Health and Performance of Dairy Cows? J. Dairy Sci. 2021, 104, 6304–6326. [Google Scholar] [CrossRef] [PubMed]
- Caixeta, L.S.; Ospina, P.A.; Capel, M.B.; Nydam, D.V. Association between Subclinical Hypocalcemia in the First 3 Days of Lactation and Reproductive Performance of Dairy Cows. Theriogenology 2017, 94, 1–7. [Google Scholar] [CrossRef]
- Kimura, K.; Reinhardt, T.A.; Goff, J.P. Parturition and Hypocalcemia Blunts Calcium Signals in Immune Cells of Dairy Cattle. J. Dairy Sci. 2006, 89, 2588–2595. [Google Scholar] [CrossRef]
- Martinez, N.; Sinedino, L.D.P.; Bisinotto, R.S.; Ribeiro, E.S.; Gomes, G.C.; Lima, F.S.; Greco, L.F.; Risco, C.A.; Galvão, K.N.; Taylor-Rodriguez, D.; et al. Effect of Induced Subclinical Hypocalcemia on Physiological Responses and Neutrophil Function in Dairy Cows. J. Dairy Sci. 2014, 97, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, W.; He, Y.; Ma, X.; Li, M.; Jiang, Q.; Loor, J.J.; Lv, X.; Yang, W.; Xu, C. Store-Operated Ca2+ Entry-Sensitive Glycolysis Regulates Neutrophil Adhesion and Phagocytosis in Dairy Cows with Subclinical Hypocalcemia. J. Dairy Sci. 2023, 106, 7131–7146. [Google Scholar] [CrossRef]
- Mekonnen, S.A.; Alelgn, Z.; Saudik, S.; Molla, W.; Fentie, T.; Jemberu, W.T. Reduced Milk Production, Economic Losses, and Risk Factors Associated to Subclinical Hypocalcemia in Holstein Friesian × Zebu Crossbreed Cows in North-West Ethiopia. Front. Vet. Sci. 2022, 9, 771889. [Google Scholar] [CrossRef]
- Hendriks, S.J.; Huzzey, J.M.; Kuhn-Sherlock, B.; Turner, S.A.; Mueller, K.R.; Phyn, C.V.C.; Donaghy, D.J.; Roche, J.R. Associations between Lying Behavior and Activity and Hypocalcemia in Grazing Dairy Cows during the Transition Period. J. Dairy Sci. 2020, 103, 10530–10546. [Google Scholar] [CrossRef]
- Jawor, P.E.; Huzzey, J.M.; LeBlanc, S.J.; Von Keyserlingk, M.A.G. Associations of Subclinical Hypocalcemia at Calving with Milk Yield, and Feeding, Drinking, and Standing Behaviors around Parturition in Holstein Cows. J. Dairy Sci. 2012, 95, 1240–1248. [Google Scholar] [CrossRef]
- Neves, R.C.; Leno, B.M.; Bach, K.D.; McArt, J.A.A.A. Epidemiology of Subclinical Hypocalcemia in Early-Lactation Holstein Dairy Cows: The Temporal Associations of Plasma Calcium Concentration in the First 4 Days in Milk with Disease and Milk Production. J. Dairy Sci. 2018, 101, 9321–9331. [Google Scholar] [CrossRef]
- McArt, J.A.A.; Nydam, D.V.; Ospina, P.A.; Oetzel, G.R. A Field Trial on the Effect of Propylene Glycol on Milk Yield and Resolution of Ketosis in Fresh Cows Diagnosed with Subclinical Ketosis. J. Dairy Sci. 2011, 94, 6011–6020. [Google Scholar] [CrossRef] [PubMed]
- Magro, S.; Costa, A.; Cavallini, D.; Chiarin, E.; De Marchi, M. Phenotypic Variation of Dairy Cows’ Hematic Metabolites and Feasibility of Non-Invasive Monitoring of the Metabolic Status in the Transition Period. Front. Vet. Sci. 2024, 11, 1437352. [Google Scholar] [CrossRef]
- Weaver, S.R.; Laporta, J.; Moore, S.A.E.; Hernandez, L.L. Serotonin and Calcium Homeostasis during the Transition Period. Domest. Anim. Endocrinol. 2016, 56, S147–S154. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gao, C.; Yang, M.; Zhang, B.; Chuang, X.; Yang, W. Characteristics and Prediction of Subclinical Hypocalcemia in Dairy Cows during the Transition Period Using Blood Analytes. Med. Weter. 2022, 78, 31–35. [Google Scholar] [CrossRef]
- Ghasemi, N.; Amanlou, H.; Maheri-Sis, N.; Salamatdoust-Nobar, R.; Jozghasemi, S. Relationship between Hypocalcemia Immediately after Calving with Metabolic Disorders and Body Condition Score in Holstein Cows. Open Vet. J. 2024, 14, 805–813. [Google Scholar] [CrossRef]
- Seely, C.R.; Leno, B.M.; Kerwin, A.L.; Overton, T.R.; McArt, J.A.A. Association of Subclinical Hypocalcemia Dynamics with Dry Matter Intake, Milk Yield, and Blood Minerals during the Periparturient Period. J. Dairy Sci. 2021, 104, 4692–4702. [Google Scholar] [CrossRef]
- McArt, J.A.A.; Neves, R.C. Association of Transient, Persistent, or Delayed Subclinical Hypocalcemia with Early Lactation Disease, Removal, and Milk Yield in Holstein Cows. J. Dairy Sci. 2020, 103, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Pascottini, O.B.; LeBlanc, S.J. Modulation of Immune Function in the Bovine Uterus Peripartum. Theriogenology 2020, 150, 193–200. [Google Scholar] [CrossRef]
- Espiritu, H.; Al Faruk, M.S.; Lee, G.; Lopez, B.I.; Lee, S.; Cho, Y. Assessment of Bovine Blood Sample Stability for Complete Blood Count and Blood Gases and Electrolytes Analysis during Storage. Korean J. Vet. Serv. 2019, 42, 265–274. [Google Scholar] [CrossRef]
- Venjakob, P.L.; Borchardt, S.; Heuwieser, W. Hypocalcemia—Cow-Level Prevalence and Preventive Strategies in German Dairy Herds. J. Dairy Sci. 2017, 100, 9258–9266. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.C.; Fernandes, B.D.; dos Santos Lima, J.M.; Rodrigues, G.P.; Dias, D.L.B.; de Oliveira Souza, E.J.; Filho, M.A.M. Prevalence of Subclinical Hypocalcemia in Dairy Cows in the Sousa City Micro-Region, Paraíba State. Trop. Anim. Health Prod. 2019, 51, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.I.; McDougall, S. Risk Factors for Subclinical Hypocalcaemia, and Associations between Subclinical Hypocalcaemia and Reproductive Performance, in Pasture-Based Dairy Herds in New Zealand. N. Z. Vet. J. 2019, 67, 12–19. [Google Scholar] [CrossRef]
- Seifi, H.; Kia, S. Subclinical Hypocalcemia in Dairy Cows: Pathophysiology, Consequences and Monitoring; Ferdowsi University of Mashhad: Mashhad, Iran, 2017; Volume 9, pp. 1–15. [Google Scholar]
- Leno, B.; Williams, S.; Ryan, C.; Briggs, D.; Crombie, M.; Overton, T. Effect of Peripartum Source of Magnesium and Calcium, and Postpartum Feeding Rate of Magnesium, on Intake, Performance and Mineral and Energy Status of Multiparous Holstein Cows. 2016. Available online: https://ecommons.cornell.edu/bitstream/1813/44742/1/12Leno_manuscript.pdf (accessed on 31 March 2025).
- Rodríguez, E.M.; Arís, A.; Bach, A. Associations between Subclinical Hypocalcemia and Postparturient Diseases in Dairy Cows. J. Dairy Sci. 2017, 100, 7427–7434. [Google Scholar] [CrossRef]
- Neves, R.C.; Leno, B.M.; Stokol, T.; Overton, T.R.; McArt, J.A.A. Risk Factors Associated with Postpartum Subclinical Hypocalcemia in Dairy Cows. J. Dairy Sci. 2017, 100, 3796–3804. [Google Scholar] [CrossRef]
- Taylor, M.S.; Knowlton, K.F.; McGilliard, M.L.; Swecker, W.S.; Ferguson, J.D.; Wu, Z.; Hanigan, M.D. Dietary Calcium Has Little Effect on Mineral Balance and Bone Mineral Metabolism through Twenty Weeks of Lactation in Holstein Cows. J. Dairy Sci. 2009, 92, 223–237. [Google Scholar] [CrossRef]
- Gaignon, P.; Le Grand, K.; Laza-Knoerr, A.L.; Hurtaud, C.; Boudon, A. Effect of Calcium Intake and the Dietary Cation-Anion Difference during Early Lactation on the Bone Mobilization Dynamics throughout Lactation in Dairy Cows. PLoS ONE 2019, 14, e0218979. [Google Scholar] [CrossRef]
- Hove, K. Cyclic Changes in Plasma Calcium and the Calcium Homeostatic Endocrine System of the Postparturient Dairy Cow. J. Dairy Sci. 1986, 69, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.P.; Liesegang, A.; Horst, R.L. Diet-Induced Pseudohypoparathyroidism: A Hypocalcemia and Milk Fever Risk Factor. J.Diary Sci. 2014, 97, 1520–1528. [Google Scholar] [CrossRef]
- Sweeney, B.; Martens, E.; Felippe, M.; Overton, T. Impacts and Evaluation of Subclinical Hypocalcemia in Dairy Cattle. 2014. Available online: https://ecommons.cornell.edu/server/api/core/bitstreams/04889c4a-e158-4870-bfcb-a7b26fab4f71/content (accessed on 17 October 2024).
- Horst, R.L. Regulation of Calcium and Phosphorus Homeostasis in the Dairy Cow. J. Dairy Sci. 1986, 69, 604–616. [Google Scholar] [CrossRef]
- Beitz, D.C.; Burkhart, D.J.; Jacobson, N.L. Effects of Calcium to Phosphorus Ratio in the Diet of Dairy Cows on Incidence of Parturient Paresis. J. Dairy Sci. 1974, 57, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M. Calcium and Phosphorus Metabolism in Jersey and Holstein Cows During Early Lactation. 2007. Available online: https://vtechworks.lib.vt.edu/items/c26ef0f3-503f-4003-8c47-1a6c22b5f0ea (accessed on 1 April 2025).
- Chamberlin, W.G.; Middleton, J.R.; Spain, J.N.; Johnson, G.C.; Ellersieck, M.R.; Pithua, P. Subclinical Hypocalcemia, Plasma Biochemical Parameters, Lipid Metabolism, Postpartum Disease, and Fertility in Postparturient Dairy Cows. J. Dairy Sci. 2013, 96, 7001–7013. [Google Scholar] [CrossRef]
- Gross, J.J.; Schwinn, A.C.; Müller, E.; Münger, A.; Dohme-Meier, F.; Bruckmaier, R.M. Plasma Cholesterol Levels and Short-Term Adaptations of Metabolism and Milk Production during Feed Restriction in Early Lactating Dairy Cows on Pasture. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Huf, L.F.; Guyoti, V.M.; Bragança, J.F.M.; Da Rocha, J.F.X.; Bennemann, P.E.; Da Rocha, R.X. COLESTEROL E BETA-HIDROXIBUTIRATO SÉRICO EM VACAS COM HIPOCALCEMIA SUBCLÍNICA (Serum Cholesterol and Beta-Hydroxybutyrate in Cows with Hypocalcemia Subclinical). Arch. Vet. Sci. 2015, 20, 1–5. [Google Scholar] [CrossRef]
- Goff, J.P.; Hohman, A.; Timms, L.L. Effect of Subclinical and Clinical Hypocalcemia and Dietary Cation-Anion Difference on Rumination Activity in Periparturient Dairy Cows. J. Dairy Sci. 2020, 103, 2591–2601. [Google Scholar] [CrossRef]
- Caixeta, L.S.; Ospina, P.A.; Capel, M.B.; Nydam, D.V. Relationship between Subclinical Hypocalcemia and Negative Energy Balance on the Return of Ovarian Function during the Voluntary Waiting Period of Dairy Cows. Am. Assoc. Bov. Pract. Conf. Proc. 2014, 47, 129. [Google Scholar] [CrossRef]
- Seely, C.R.; McArt, J.A.A. The Association of Subclinical Hypocalcemia at 4 Days in Milk with Reproductive Outcomes in Multiparous Holstein Cows. JDS Commun. 2023, 4, 111–115. [Google Scholar] [CrossRef]
- Abuelo, A. Hypocalcemia in Dairy Cattle. In Encyclopedia of Livestock Medicine for Large Animal and Poultry Production; Living Edition; Springer: Cham, Switzerland, 2024; pp. 1–7. [Google Scholar] [CrossRef]
- Leal Yepes, F.A.; Behling-Kelly, E.; Caixeta, L.S.; Tikofsky, L.; Parrish, L.; Heaton, K.N. Evaluation of a Point-of-Care Calcium Device in Bovine Plasma and Serum. JDS Commun. 2023, 4, 390–393. [Google Scholar] [CrossRef]

| CBC Parameters | n (SCH/NCC) | Normal Ranges | NCC (Mean ± SD) | SCH (Mean ± SD) | p-Value |
|---|---|---|---|---|---|
| RBC (M/μL) | 74/688 | 4.5–9.4 | 6.05 ± 0.85 | 5.92 ± 0.75 | 0.167 |
| HCT (%) | 74/688 | 22.5–39.9 | 0.3 ± 0.04 | 0.29 ± 0.03 | 0.101 |
| HGB (g/dL) | 74/688 | 7.4–12.8 | 9.94 ± 1.19 | 9.75 ± 1.02 | 0.14 |
| MCV (fL) | 74/688 | 40.4–56.4 | 49.91 ± 4.06 | 49.92 ± 3.53 | 0.974 |
| MCH (pg) | 74/688 | 11.5–18.5 | 16.57 ± 2.32 | 16.54 ± 1.12 | 0.859 |
| MCHC (g/dL) | 74/688 | 30.2–33.5 | 33.29 ± 5.36 | 33.15 ± 1.06 | 0.576 |
| WBC (K/μL) | 74/688 | 2.7–17.8 | 12.21 ± 6.04 | 12.32 ± 6.69 | 0.893 |
| NEU (K/μL) | 74/676 | 0.7–7.0 | 2.66 ± 1.94 | 2.55 ± 2.05 | 0.643 |
| LYM (K/μL) | 74/676 | 1.2–10.6 | 6.14 ± 4.13 | 5.96 ± 3.01 | 0.644 |
| MONO (K/μL) | 74/676 | 0.02–2.2 | 3.01 ± 2.21 | 3.45 ± 3.48 | 0.301 |
| EOS (K/μL) | 74/687 | 0.0–1.2 | 0.36 ± 0.33 | 0.35 ± 0.45 | 0.772 |
| BASO (K/μL) | 74/687 | 0.0–0.04 | 0.01 ± 0.02 | 0.02 ± 0.02 | 0.473 |
| PLT (K/μL) | 74/682 | 147.0–663.0 | 247.73 ± 143 | 245.64 ± 125 | 0.893 |
| Biochemical Marker | n (SCH/ Normal) | Reference Range (SI) | NCC (Mean ± SD) | SCH (Mean ± SD) | p-Value |
|---|---|---|---|---|---|
| GLU (mmol/L) | 76/726 | 2.50–4.20 | 2.74 ± 0.75 | 2.61 ± 0.67 | 0.114 |
| BHBA (mmol/L) | 81/778 | <1.00 | 1.07 ± 0.74 | 1.15 ± 0.94 | 0.461 |
| PHOS (mmol/L) | 81/778 | 1.29–2.78 | 2.10 ± 0.30 | 2.28 ± 0.46 | 0.001 |
| Mg (mmol/L) | 81/777 | 0.74–1.23 | 0.97 ± 0.00 | 0.96 ± 0.01 | 0.493 |
| BUN (mmol/L) | 81/778 | 3.60–8.93 | 5.10 ± 1.75 | 5.23 ± 1.58 | 0.500 |
| TP (g/L) | 81/778 | 62.00–80.00 | 76.30 ± 6.50 | 74.00 ± 6.60 | 0.002 |
| ALB (g/L) | 81/778 | 25.00–35.00 | 29.50 ± 2.60 | 28.20 ± 3.00 | <0.001 |
| GLOB (g/L) | 81/777 | 30.00–49.00 | 46.90 ± 5.00 | 45.70 ± 5.80 | 0.063 |
| ALB/GLOB Ratio | 81/777 | 0.80–1.20 | 0.63 ± 0.09 | 0.63 ± 0.10 | 0.802 |
| AST (U/L) | 81/778 | 50.00–150.00 | 112.66 ± 38.91 | 116.10 ± 35.79 | 0.416 |
| GGT (U/L) | 81/778 | 0.00–87.00 | 37.94 ± 23.49 | 36.14 ± 10.94 | 0.224 |
| TBIL (μmol/L) | 78/704 | 0.00–12.00 | 7.01 ± 5.65 | 6.67 ± 3.77 | 0.395 |
| CHOL (mmol/L) | 80/778 | 1.16–5.18 | 6.16 ± 2.02 | 5.22 ± 2.29 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espiritu, H.M.; Al Faruk, M.S.; Lee, H.-W.; Pioquinto, J.M.; Lee, S.-S.; Cho, Y.-I. Subclinical Hypocalcemia Across Lactation Stages Reflects Potential Metabolic Vulnerability in Korean Holstein Cows. Vet. Sci. 2025, 12, 495. https://doi.org/10.3390/vetsci12050495
Espiritu HM, Al Faruk MS, Lee H-W, Pioquinto JM, Lee S-S, Cho Y-I. Subclinical Hypocalcemia Across Lactation Stages Reflects Potential Metabolic Vulnerability in Korean Holstein Cows. Veterinary Sciences. 2025; 12(5):495. https://doi.org/10.3390/vetsci12050495
Chicago/Turabian StyleEspiritu, Hector M., Md. Shohel Al Faruk, Hee-Woon Lee, Jaylord M. Pioquinto, Sang-Suk Lee, and Yong-Il Cho. 2025. "Subclinical Hypocalcemia Across Lactation Stages Reflects Potential Metabolic Vulnerability in Korean Holstein Cows" Veterinary Sciences 12, no. 5: 495. https://doi.org/10.3390/vetsci12050495
APA StyleEspiritu, H. M., Al Faruk, M. S., Lee, H.-W., Pioquinto, J. M., Lee, S.-S., & Cho, Y.-I. (2025). Subclinical Hypocalcemia Across Lactation Stages Reflects Potential Metabolic Vulnerability in Korean Holstein Cows. Veterinary Sciences, 12(5), 495. https://doi.org/10.3390/vetsci12050495





