Monovalent Copper Oxide in Broiler Nutrition: Effects on Performance, Intestinal Lesions, and Oocyst Shedding During Mild Eimeria Challenge
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Eimeria Challenge and Biosecurity
2.3. Data and Sample Collection
2.4. Feed Analysis
2.5. Total Oocyst Count
2.6. Cecal Microbiota Analysis
2.7. Bone Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Intestinal Lesion Score and Morphology
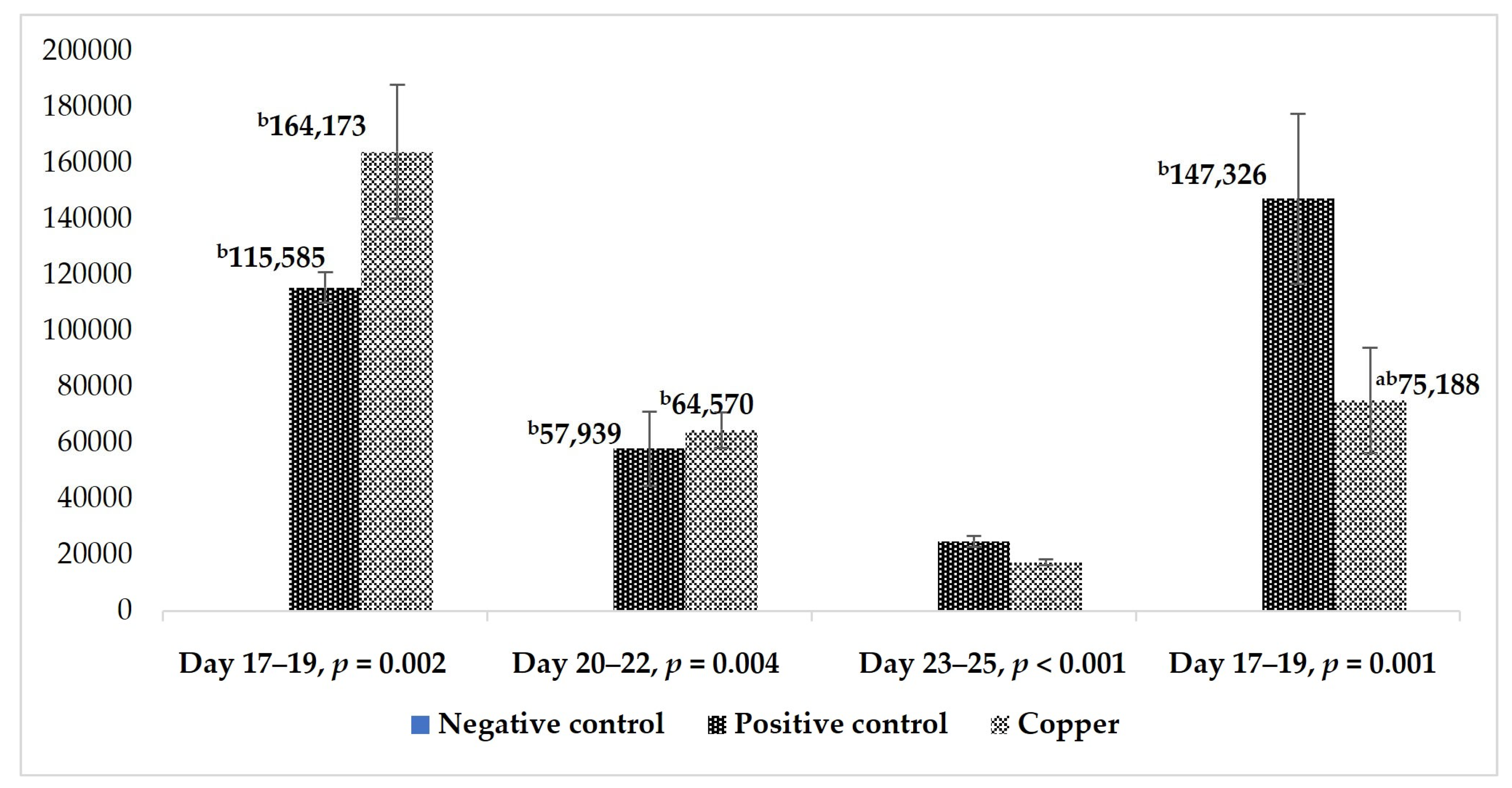
3.3. Fecal Oocyst Count
3.4. Carcass Yield and Gizzard Weight
3.5. Cecal Microbiota Profile
3.6. Tibia Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, P.L. Avian Coccidiosis; Academic Press Inc.: Cambridge, MA, USA, 1993; ISBN 0-12-426014-4. [Google Scholar]
- Blake, D.P.; Marugan-Hernandez, V.; Tomley, F.M. Spotlight on Avian Pathology: Eimeria and the Disease Coccidiosis. Avian Pathol. 2021, 50, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sun, P.; Hu, D.; Tang, X.; Zhang, S.; Shi, F.; Yan, X.; Yan, W.; Shi, T.; Wang, S. Advancements in Understanding Chicken Coccidiosis: From Eimeria Biology to Innovative Control Strategies. One Health Adv. 2024, 2, 6. [Google Scholar] [CrossRef]
- Williams, R. Intercurrent Coccidiosis and Necrotic Enteritis of Chickens: Rational, Integrated Disease Management by Maintenance of Gut Integrity. Avian Pathol. 2005, 34, 159–180. [Google Scholar] [CrossRef]
- da Cunha, A.F.; Santin, E.; Kogut, M. Poultry Coccidiosis: Strategies to Understand and Control. Front. Vet. Sci. 2020, 7, 599322. [Google Scholar] [CrossRef]
- McDougald, L.R. Intestinal Protozoa Important to Poultry. Poult. Sci. 1998, 77, 1156–1158. [Google Scholar] [CrossRef]
- Saif, Y. Diseases of Poultry; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 0-8138-0723-9. [Google Scholar]
- Baba, E.; Wakeshima, H.; Fukui, K.; Fukata, T.; Arakawa, A. Adhesion of Bacteria to the Cecal Mucosal Surface of Conventional and Germ-Free Chickens Infected with Eimeria Tenella. Am. J. Vet. Res. 1992, 53, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D. Re-Calculating the Cost of Coccidiosis in Chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef]
- Chapman, H. Biochemical, Genetic and Applied Aspects of Drug Resistance in Eimeria Parasites of the Fowl. Avian Pathol. 1997, 26, 221–244. [Google Scholar] [CrossRef]
- Williams, R. Anticoccidial Vaccines for Broiler Chickens: Pathways to Success. Avian Pathol. 2002, 31, 317–353. [Google Scholar] [CrossRef]
- Blake, D.P.; Vrba, V.; Xia, D.; Jatau, I.D.; Spiro, S.; Nolan, M.J.; Underwood, G.; Tomley, F.M. Genetic and Biological Characterisation of Three Cryptic Eimeria Operational Taxonomic Units That Infect Chickens (Gallus Gallus Domesticus). Int. J. Parasitol. 2021, 51, 621–634. [Google Scholar] [CrossRef]
- Cantacessi, C.; Riddell, S.; Morris, G.M.; Doran, T.; Woods, W.G.; Otranto, D.; Gasser, R.B. Genetic Characterization of Three Unique Operational Taxonomic Units of Eimeria from Chickens in Australia Based on Nuclear Spacer Ribosomal DNA. Vet. Parasitol. 2008, 152, 226–234. [Google Scholar] [CrossRef]
- Alhotan, R.A.; Abudabos, A. Anticoccidial and Antioxidant Effects of Plants Derived Polyphenol in Broilers Exposed to Induced Coccidiosis. Environ. Sci. Pollut. Res. 2019, 26, 14194–14199. [Google Scholar] [CrossRef] [PubMed]
- Muthamilselvan, T.; Kuo, T.-F.; Wu, Y.-C.; Yang, W.-C. Herbal Remedies for Coccidiosis Control: A Review of Plants, Compounds, and Anticoccidial Actions. Evid.-Based Complement. Altern. Med. 2016, 2016, 2657981. [Google Scholar] [CrossRef] [PubMed]
- Peek, H.; Landman, W. Coccidiosis in Poultry: Anticoccidial Products, Vaccines and Other Prevention Strategies. Vet. Q. 2011, 31, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Gangadoo, S.; Stanley, D.; Hughes, R.J.; Moore, R.J.; Chapman, J. Nanoparticles in Feed: Progress and Prospects in Poultry Research. Trends Food Sci. Technol. 2016, 58, 115–126. [Google Scholar] [CrossRef]
- Miles, R.; O’Keefe, S.; Henry, P.; Ammerman, C.; Luo, X. The Effect of Dietary Supplementation with Copper Sulfate or Tribasic Copper Chloride on Broiler Performance, Relative Copper Bioavailability, and Dietary Prooxidant Activity. Poult. Sci. 1998, 77, 416–425. [Google Scholar] [CrossRef]
- El-Kassas, S.; Abdo, S.E.; El-Naggar, K.; Abdo, W.; Kirrella, A.A.K.; Nashar, T.O. Ameliorative Effect of Dietary Supplementation of Copper Oxide Nanoparticles on Inflammatory and Immune Reponses in Commercial Broiler under Normal and Heat-Stress Housing Conditions. J. Therm. Biol. 2018, 78, 235–246. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Vieira, B.S.; Applegate, T.J. Influence of Dietary Zinc, Copper, and Manganese on the Intestinal Health of Broilers under Eimeria Challenge. Front. Vet. Sci. 2020, 7, 13. [Google Scholar] [CrossRef]
- Santos, T.S.d.; Teng, P.-Y.; Yadav, S.; Castro, F.L.d.S.; Gould, R.L.; Craig, S.W.; Chen, C.; Fuller, A.L.; Pazdro, R.; Sartori, J.R. Effects of Inorganic Zn and Cu Supplementation on Gut Health in Broiler Chickens Challenged with Eimeria spp. Front. Vet. Sci. 2020, 7, 230. [Google Scholar] [CrossRef]
- Arias, V.; Koutsos, E. Effects of Copper Source and Level on Intestinal Physiology and Growth of Broiler Chickens. Poult. Sci. 2006, 85, 999–1007. [Google Scholar] [CrossRef]
- Broom, L.J.; Monteiro, A.; Piñon, A. Recent Advances in Understanding the Influence of Zinc, Copper, and Manganese on the Gastrointestinal Environment of Pigs and Poultry. Animals 2021, 11, 1276. [Google Scholar] [CrossRef]
- Forouzandeh, A.; Blavi, L.; Abdelli, N.; Melo-Duran, D.; Vidal, A.; Rodríguez, M.; Monteiro, A.; Pérez, J.; Darwich, L.; Solà-Oriol, D. Effects of Dicopper Oxide and Copper Sulfate on Growth Performance and Gut Microbiota in Broilers. Poult. Sci. 2021, 100, 101224. [Google Scholar] [CrossRef] [PubMed]
- NHMRC. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 8th ed.; Australian Government Publishing Service; The National Health and Medical Research Council: Canberra, Australia, 2013. [Google Scholar]
- Aviagen. Ross Broiler Management Handbook. 2025. Available online: https://Aviagen.Com/Assets/Tech_Center/Ross_Broiler/Aviagen-ROSS-Broiler-Handbook-EN.Pdf (accessed on 15 May 2025).
- Pang, Y.; Applegate, T. Effects of Dietary Copper Supplementation and Copper Source on Digesta pH, Calcium, Zinc, and Copper Complex Size in the Gastrointestinal Tract of the Broiler Chicken. Poult. Sci. 2007, 86, 531–537. [Google Scholar] [CrossRef]
- Leeson, S. Copper Metabolism and Dietary Needs. Worlds Poult. Sci. J. 2009, 65, 353–366. [Google Scholar] [CrossRef]
- Aviagen. Ross Broiler: Nutrition Specifications. 2022. Available online: Https://Aviagen.Com/Assets/Tech_Center/Ross_Broiler/Ross-BroilerNutritionSpecifications2022-EN.Pdf (accessed on 15 May 2025).
- AOAC. Association of Official Analytical Chemists. Official Methods of Analysis; AOAC: Washington, DC, USA, 1994. [Google Scholar]
- Zanu, H.K.; Kheravii, S.; Morgan, N.; Bedford, M.; Swick, R. Interactive Effect of Dietary Calcium and Phytase on Broilers Challenged with Subclinical Necrotic Enteritis: Part 2. Gut Permeability, Phytate Ester Concentrations, Jejunal Gene Expression, and Intestinal Morphology. Poult. Sci. 2020, 99, 4914–4928. [Google Scholar] [CrossRef] [PubMed]
- Daneshmand, A.; Kumar, A.; Kheravii, S.K.; Pasquali, G.A.M.; Wu, S.-B. Xylanase and Beta-Glucanase Improve Performance Parameters and Footpad Dermatitis and Modulate Intestinal Microbiota in Broilers under an Eimeria Challenge. Poult. Sci. 2023, 102, 103055. [Google Scholar] [CrossRef]
- Godwin, R.M.; Morgan, J.A. A Molecular Survey of Eimeria in Chickens across Australia. Vet. Parasitol. 2015, 214, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.S.; Ruiz-Jimenez, F.; Fletcher, O.J.; Gall, S.; Crespo, R. Image Analysis for Eimeria Oocyst Counts and Classification. J. Appl. Poult. Res. 2022, 31, 100260. [Google Scholar] [CrossRef]
- Jenkins, M.C.; O’Brien, C.N.; Parker, C.; Tucker, M.; Khan, A. Relationship between Eimeria Oocyst Infectivity for Chickens and in Vitro Excystation of E. Acervulina, E. Maxima, and E. Tenella Oocyst during Long-Term Storage. Poult. Sci. 2023, 102, 103133. [Google Scholar] [CrossRef]
- Johnson, J.; Reid, W.M. Anticoccidial Drugs: Lesion Scoring Techniques in Battery and Floor-Pen Experiments with Chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- Dumas, J. Procédés de l’analyse Organique. Ann. Chim. Phys. 1831, 47, 198–205. [Google Scholar]
- Cha, J.O.; Zhao, J.; Yang, M.S.; Kim, W.I.; Cho, H.S.; Lim, C.W.; Kim, B. Oocyst-Shedding Patterns of Three Eimeria Species in Chickens and Shedding Pattern Variation Depending on the Storage Period of Eimeria Tenella Oocysts. J. Parasitol. 2018, 104, 18–22. [Google Scholar] [CrossRef]
- Morris, G.M.; Woods, W.G.; Richards, D.G.; Gasser, R.B. Investigating a Persistent Coccidiosis Problem on a Commercial Broiler–Breeder Farm Utilising PCR-Coupled Capillary Electrophoresis. Parasitol. Res. 2007, 101, 583–589. [Google Scholar] [CrossRef]
- Kheravii, S.K.; Swick, R.A.; Choct, M.; Wu, S.-B. Coarse Particle Inclusion and Lignocellulose-Rich Fiber Addition in Feed Benefit Performance and Health of Broiler Chickens. Poult. Sci. 2017, 96, 3272–3281. [Google Scholar] [CrossRef]
- Abou-Elkhair, R.; Ahmed, H.; Ketkat, S.; Selim, S. Supplementation of a Low-Protein Diet with Tryptophan, Threonine, and Valine and Its Impact on Growth Performance, Blood Biochemical Constituents, Immune Parameters, and Carcass Traits in Broiler Chickens. Vet. World 2020, 13, 1234. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.; McKay, L.; Williams, D.; Garrett, V.; Gentry, R.; Sayler, G. Development of Bacteroides 16S rRNA Gene TaqMan-Based Real-Time PCR Assays for Estimation of Total, Human, and Bovine Fecal Pollution in Water. Appl. Environ. Microbiol. 2006, 72, 4214–4224. [Google Scholar] [CrossRef] [PubMed]
- Requena, T.; Burton, J.; Matsuki, T.; Munro, K.; Simon, M.A.; Tanaka, R.; Watanabe, K.; Tannock, G.W. Identification, Detection, and Enumeration of Human Bifidobacterium Species by PCR Targeting the Transaldolase Gene. Appl. Environ. Microbiol. 2002, 68, 2420–2427. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, S.; Fite, A.; Macfarlane, G.T.; McMurdo, M.E. Characterization of Bacterial Communities in Feces from Healthy Elderly Volunteers and Hospitalized Elderly Patients by Using Real-Time PCR and Effects of Antibiotic Treatment on the Fecal Microbiota. Appl. Environ. Microbiol. 2004, 70, 3575–3581. [Google Scholar] [CrossRef]
- Wise, M.; Siragusa, G. Quantitative Analysis of the Intestinal Bacterial Community in One-to Three-week-old Commercially Reared Broiler Chickens Fed Conventional or Antibiotic-free Vegetable-based Diets. J. Appl. Microbiol. 2007, 102, 1138–1149. [Google Scholar] [CrossRef]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of Inulin on the Human Gut Microbiota: Stimulation of Bifidobacterium Adolescentis and Faecalibacterium Prausnitzii. Br. J. Nutr. 2008, 101, 541–550. [Google Scholar] [CrossRef]
- Lee, D.-H.; Zo, Y.-G.; Kim, S.-J. Nonradioactive Method to Study Genetic Profiles of Natural Bacterial Communities by PCR-Single-Strand-Conformation Polymorphism. Appl. Environ. Microbiol. 1996, 62, 3112–3120. [Google Scholar] [CrossRef] [PubMed]
- Seedor, J.G.; Quartuccio, H.A.; Thompson, D.D. The Bisphosphonate Alendronate (MK-217) Inhibits Bone Loss Due to Ovariectomy in Rats. J. Bone Miner. Res. 1991, 6, 339–346. [Google Scholar] [CrossRef]
- Nabian, S.; Arabkhazaeli, F.; Seifouri, P.; Farahani, A. Morphometric Analysis of the Intestine in Experimental Coccidiosis in Broilers Treated with Anticoccidial Drugs. Iran. J. Parasitol. 2018, 13, 493. [Google Scholar] [PubMed]
- Madlala, T.; Okpeku, M.; Adeleke, M.A. Understanding the Interactions between Eimeria Infection and Gut Microbiota, towards the Control of Chicken Coccidiosis: A Review. Parasite 2021, 28, 48. [Google Scholar] [CrossRef]
- McDonald, V.; Shirley, M. Past and Future: Vaccination against Eimeria. Parasitology 2009, 136, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.P.; Tomley, F.M. Securing Poultry Production from the Ever-Present Eimeria Challenge. Trends Parasitol. 2014, 30, 12–19. [Google Scholar] [CrossRef]
- Zaghari, M.; Pouraghaali, S.; Zhandi, M.; Abbasi, M. Effect of Monovalent Copper Oxide and Potentiated Zinc Oxide on Growth Performance and Gut Morphology of Broiler Chickens Challenged with Coccidiosis. Biol. Trace Elem. Res. 2023, 201, 2524–2535. [Google Scholar] [CrossRef]
- Pesti, G.M.; Bakalli, R.I. Studies on the Feeding of Cupric Sulfate Pentahydrate and Cupric Citrate to Broiler Chickens. Poult. Sci. 1996, 75, 1086–1091. [Google Scholar] [CrossRef]
- Ewing, H.P.; Pesti, G.M.; Bakalli, R.I.; Menten, J. Studies on the Feeding of Cupric Sulfate Pentahydrate, Cupric Citrate, and Copper Oxychloride to Broiler Chickens. Poult. Sci. 1998, 77, 445–448. [Google Scholar] [CrossRef]
- Pang, Y.; Patterson, J.; Applegate, T. The Influence of Copper Concentration and Source on Ileal Microbiota. Poult. Sci. 2009, 88, 586–592. [Google Scholar] [CrossRef]
- Hamdi, M.; Solà, D.; Franco, R.; Durosoy, S.; Roméo, A.; Pérez, J. Including Copper Sulphate or Dicopper Oxide in the Diet of Broiler Chickens Affects Performance and Copper Content in the Liver. Anim. Feed Sci. Technol. 2018, 237, 89–97. [Google Scholar] [CrossRef]
- Olukosi, O.A.; van Kuijk, S.; Han, Y. Copper and Zinc Sources and Levels of Zinc Inclusion Influence Growth Performance, Tissue Trace Mineral Content, and Carcass Yield of Broiler Chickens. Poult. Sci. 2018, 97, 3891–3898. [Google Scholar] [CrossRef]
- López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Overview of Poultry Eimeria Life Cycle and Host-Parasite Interactions. Front. Vet. Sci. 2020, 7, 384. [Google Scholar] [CrossRef]
- Anissimova, M.; Koinaski, V.; Gabrashanska, M.; Vladov, I.; Ermakov, V.; Danailova, V. The Effect of Tribasic Copper Chloride on Broiler Chickens Experimentally Infected with Eimeria Tenella (Protozoa). Wildl. Res. Kyrg. 2013, 1, 66–70. [Google Scholar]
- Mesa-Pineda, C.; Navarro-Ruíz, J.L.; López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Chicken Coccidiosis: From the Parasite Lifecycle to Control of the Disease. Front. Vet. Sci. 2021, 8, 787653. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H. Milestones in Avian Coccidiosis Research: A Review. Poult. Sci. 2014, 93, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.; Lillehoj, H.; Lillehoj, E. Intestinal Immune Responses to Coccidiosis. Dev. Comp. Immunol. 2000, 24, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Dao, H.T.; Sharma, N.K.; Bradbury, E.J.; Swick, R.A. Response of Meat Chickens to Different Sources of Arginine in Low-protein Diets. J. Anim. Physiol. Anim. Nutr. 2021, 105, 731–746. [Google Scholar] [CrossRef]
- Popov, S.; Saphier, O.; Popov, M.; Shenker, M.; Entus, S.; Shotland, Y.; Saphier, M. Factors Enhancing the Antibacterial Effect of Monovalent Copper Ions. Curr. Microbiol. 2020, 77, 361–368. [Google Scholar] [CrossRef]
- Govind, V.; Bharadwaj, S.; Sai Ganesh, M.; Vishnu, J.; Shankar, K.V.; Shankar, B.; Rajesh, R. Antiviral Properties of Copper and Its Alloys to Inactivate Covid-19 Virus: A Review. Biometals 2021, 34, 1217–1235. [Google Scholar] [CrossRef]
- Augustine, P.C. Cellular Invasion by Avian Eimeria Species. Poult. Avian Biol. Rev. 2000, 11, 113–122. [Google Scholar]
- Bortoluzzi, C.; Vieira, B.; Lumpkins, B.; Mathis, G.; King, W.; Graugnard, D.; Dawson, K.; Applegate, T. Can Dietary Zinc Diminish the Impact of Necrotic Enteritis on Growth Performance of Broiler Chickens by Modulating the Intestinal Immune-System and Microbiota? Poult. Sci. 2019, 98, 3181–3193. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.; Yulong, M.; Peng, G.; Zirong, X. Antibacterial Effects of the Cu (II)-Exchanged Montmorillonite on Escherichia Coli K88 and Salmonella Choleraesuis. Vet. Microbiol. 2005, 105, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ladomersky, E.; Petris, M.J. Copper Tolerance and Virulence in Bacteria. Metallomics 2015, 7, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Reyna, P.S.; McDougald, L.R.; Mathis, G.F. Survival of Coccidia in Poultry Litter and Reservoirs of Infection. Avian Dis. 1983, 27, 464–473. [Google Scholar] [CrossRef]
- Georgieva, N.V.; Gabrashanska, M.; Koinarski, V.; Yaneva, Z. Zinc Supplementation against Eimeria Acervulina-Induced Oxidative Damage in Broiler Chickens. Vet. Med. Int. 2011, 2011, 647124. [Google Scholar] [CrossRef]
- Teng, P.-Y.; Yadav, S.; Shi, H.; Kim, W.K. Evaluating Endogenous Loss and Standard Ileal Digestibility of Amino Acids in Response to the Graded Severity Levels of E. Maxima Infection. Poult. Sci. 2021, 100, 101426. [Google Scholar] [CrossRef]
- Dozier III, W.; Kidd, M.; Corzo, A. Dietary Amino Acid Responses of Broiler Chickens. J. Appl. Poult. Res. 2008, 17, 157–167. [Google Scholar] [CrossRef]
- Laika, M.; Jahanian, R. Increase in Dietary Arginine Level Could Ameliorate Detrimental Impacts of Coccidial Infection in Broiler Chickens. Livest. Sci. 2017, 195, 38–44. [Google Scholar] [CrossRef]
- Poudel, S.; Tabler, G.T.; Lin, J.; Zhai, W.; Zhang, L. Riboflavin and Bacillus Subtilis Effects on Growth Performance and Woody-Breast of Ross 708 Broilers with or without Eimeria spp. Challenge. J. Anim. Sci. Technol. 2022, 64, 443. [Google Scholar] [CrossRef]
- Choi, J.; Kong, B.; Bowker, B.C.; Zhuang, H.; Kim, W.K. Nutritional Strategies to Improve Meat Quality and Composition in the Challenging Conditions of Broiler Production: A Review. Animals 2023, 13, 1386. [Google Scholar] [CrossRef]
- Awad, W.; Ghareeb, K.; Abdel-Raheem, S.; Böhm, J. Effects of Dietary Inclusion of Probiotic and Synbiotic on Growth Performance, Organ Weights, and Intestinal Histomorphology of Broiler Chickens. Poult. Sci. 2009, 88, 49–56. [Google Scholar] [CrossRef]
- Teng, P.-Y.; Yadav, S.; de Souza Castro, F.L.; Tompkins, Y.H.; Fuller, A.L.; Kim, W.K. Graded Eimeria Challenge Linearly Regulated Growth Performance, Dynamic Change of Gastrointestinal Permeability, Apparent Ileal Digestibility, Intestinal Morphology, and Tight Junctions of Broiler Chickens. Poult. Sci. 2020, 99, 4203–4216. [Google Scholar] [CrossRef]
- Philpot, S.; Perryman, K.; Dozier III, W. Growth Performance and Carcass Characteristics of Broilers Fed Diets Varying in Supplemental Copper Concentrations from 29 to 53 Days of Age. J. Appl. Poult. Res. 2020, 29, 289–300. [Google Scholar] [CrossRef]
- Torok, V.A.; Allison, G.E.; Percy, N.J.; Ophel-Keller, K.; Hughes, R.J. Influence of Antimicrobial Feed Additives on Broiler Commensal Posthatch Gut Microbiota Development and Performance. Appl. Environ. Microbiol. 2011, 77, 3380–3390. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.D.; Hernandez-Velasco, X.; Kuttappan, V.A.; Wolfenden, R.E.; Vicente, J.L.; Wolfenden, A.D.; Bielke, L.R.; Prado-Rebolledo, O.F.; Morales, E.; Hargis, B.M. Selection of Bacillus Spp. for Cellulase and Xylanase Production as Direct-Fed Microbials to Reduce Digesta Viscosity and Clostridium Perfringens Proliferation Using an in Vitro Digestive Model in Different Poultry Diets. Front. Vet. Sci. 2015, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.D.; Hernandez-Velasco, X.; Wolfenden, R.E.; Vicente, J.L.; Wolfenden, A.D.; Menconi, A.; Bielke, L.R.; Hargis, B.M.; Tellez, G. Evaluation and Selection of Bacillus Species Based on Enzyme Production, Antimicrobial Activity, and Biofilm Synthesis as Direct-Fed Microbial Candidates for Poultry. Front. Vet. Sci. 2016, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Faber, T.; Dilger, R.; Hopkins, A.; Price, N.; Fahey, G., Jr. The Effects of a Galactoglucomannan Oligosaccharide-Arabinoxylan (GGMO-AX) Complex in Broiler Chicks Challenged with Eimeria Acervulina. Poult. Sci. 2012, 91, 1089–1096. [Google Scholar] [CrossRef]
- Moraes, P.d.O.; Cardinal, K.M.; Gouvêa, F.L.; Schroeder, B.; Ceron, M.S.; Lunedo, R.; Frazzon, A.P.G.; Frazzon, J.; Ribeiro, A.M.L. Comparison between a Commercial Blend of Functional Oils and Monensin on the Performance and Microbiota of Coccidiosis-Challenged Broilers. Poult. Sci. 2019, 98, 5456–5464. [Google Scholar] [CrossRef]
- Collier, C.; Hofacre, C.; Payne, A.; Anderson, D.; Kaiser, P.; Mackie, R.; Gaskins, H. Coccidia-Induced Mucogenesis Promotes the Onset of Necrotic Enteritis by Supporting Clostridium Perfringens Growth. Vet. Immunol. Immunopathol. 2008, 122, 104–115. [Google Scholar] [CrossRef]
- Deplancke, B.; Gaskins, H.R. Microbial Modulation of Innate Defense: Goblet Cells and the Intestinal Mucus Layer. Am. J. Clin. Nutr. 2001, 73, 1131S–1141S. [Google Scholar] [CrossRef]
- Wang, X.; Farnell, Y.Z.; Kiess, A.S.; Peebles, E.D.; Wamsley, K.G.; Zhai, W. Effects of Bacillus Subtilis and Coccidial Vaccination on Cecal Microbial Diversity and Composition of Eimeria-Challenged Male Broilers. Poult. Sci. 2019, 98, 3839–3849. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.T.; Kheravii, S.K.; Wu, S.; Roberts, J.R.; Swick, R.A.; Toghyani, M. Sources and Levels of Copper Affect Liver Copper Profile, Intestinal Morphology and Cecal Microbiota Population of Broiler Chickens Fed Wheat-Soybean Meal Diets. Sci. Rep. 2022, 12, 2249. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Williams, B.; Kwakkel, R.; Li, H.; Li, X.; Luo, J.; Li, W.; Verstegen, M. Effects of Mushroom and Herb Polysaccharides, as Alternatives for an Antibiotic, on the Cecal Microbial Ecosystem in Broiler Chickens. Poult. Sci. 2004, 83, 175–182. [Google Scholar] [CrossRef]
- Rosen, G.D. Antibacterials in Poultry and Pig Nutrition. Biotechnol. Anim. Feeds Anim. Feed. 1995, 172, 143. [Google Scholar]
- Sahraei, M.; Janmmohamdi, H.; Taghizadeh, A.; Cheraghi, S. Effect of Different Zinc Sources on Tibia Bone Morphology and Ash Content of Broiler Chickens. Adv. Biol. Res. 2012, 6, 128–132. [Google Scholar]
- Van der Klis, J.; Verstegen, M.; De Wit, W. Absorption of Minerals and Retention Time of Dry Matter in the Gastrointestinal Tract of Broilers. Poult. Sci. 1990, 69, 2185–2194. [Google Scholar] [CrossRef]
- Kakhki, R.A.M.; Lu, Z.; Thanabalan, A.; Leung, H.; Mohammadigheisar, M.; Kiarie, E. Eimeria Challenge Adversely Affected Long Bone Attributes Linked to Increased Resorption in 14-Day-Old Broiler Chickens. Poult. Sci. 2019, 98, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.; Van Ginkel, F.; Macklin, K.; Blake, J. Effects of Phytase Supplementation in Broiler Diets on a Natural Eimeria Challenge in Naive and Vaccinated Birds. Poult. Sci. 2011, 90, 781–790. [Google Scholar] [CrossRef]
- Mireles, A.; Kim, S.; Klasing, K. An Acute Inflammatory Response Alters Bone Homeostasis, Body Composition, and the Humoral Immune Response of Broiler Chickens. Poult. Sci. 2005, 84, 553–560. [Google Scholar] [CrossRef]
- Banks, K.; Thompson, K.; Rush, J.; Applegate, T. Effects of Copper Source on Phosphorus Retention in Broiler Chicks and Laying Hens. Poult. Sci. 2004, 83, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Masood, S.; Zaneb, H.; Rabbani, I.; Akbar, J.; Kuthu, Z.; Masood, A.; Vargas-Bello-Pérez, E. Effects of Copper Nanoparticles on Performance, Muscle and Bone Characteristics and Serum Metabolites in Broilers. Braz. J. Biol. 2022, 84, e261578. [Google Scholar] [CrossRef] [PubMed]

| Ingredients, % | Starter (Days 0–10) | Grower (Days 10–21) | Finisher (Days 21–35) |
|---|---|---|---|
| Soybean meal | 33.47 | 25.88 | 20.76 |
| Canola oil | 2.89 | 4.41 | 5.18 |
| Wheat | 60.14 | 61.75 | 66.48 |
| Canola meal | 0.00 | 5.00 | 5.00 |
| Limestone | 1.52 | 1.35 | 1.24 |
| Salt | 0.173 | 0.185 | 0.193 |
| Mono-dicalcium phosphate | 0.562 | 0.334 | 0.137 |
| Sodium bicarbonate | 0.130 | 0.112 | 0.097 |
| L-lysine | 0.275 | 0.258 | 0.249 |
| DL-methionine | 0.336 | 0.264 | 0.233 |
| L-threonine | 0.135 | 0.097 | 0.077 |
| L-valine | 0.011 | 0.000 | 0.000 |
| Choline chloride 75% | 0.025 | 0.025 | 0.020 |
| 2 Vitamin and mineral premix | 0.175 | 0.175 | 0.175 |
| Filler (bentonite) | 0.100 | 0.100 | 0.100 |
| 3 Xylanase | 0.025 | 0.025 | 0.025 |
| 4 Phytase | 0.030 | 0.030 | 0.030 |
| Total | 100 | 100 | 100 |
| Calculated nutrient, % (Otherwise, as stated) | |||
| Dry matter | 90.5 | 90.6 | 90.6 |
| AME (Kcal/kg) | 3005 | 3109 | 3198 |
| Crude protein | 23.39 | 21.77 | 19.84 |
| Cude fibre | 2.62 | 2.93 | 2.84 |
| Crude fat | 4.17 | 5.75 | 6.53 |
| Ash | 5.14 | 4.62 | 4.07 |
| 5 Dig lysine | 1.28 | 1.150 | 1.020 |
| Dig methionine | 0.623 | 0.549 | 0.497 |
| Dig methionine + cystine | 0.950 | 0.870 | 0.800 |
| Dig cysteine | 0.323 | 0.316 | 0.298 |
| Dig threonine | 0.860 | 0.770 | 0.680 |
| Dig tryptophan | 0.300 | 0.278 | 0.253 |
| Dig glycine | 0.778 | 0.739 | 0.671 |
| Dig arginine | 1.370 | 1.230 | 1.090 |
| Dig serine | 0.778 | 0.739 | 0.671 |
| Dig histidine | 0.507 | 0.466 | 0.419 |
| Dig isoleucine | 0.877 | 0.796 | 0.710 |
| Dig leucine | 1.465 | 1.343 | 1.207 |
| Dig phenylalanine | 0.979 | 0.893 | 0.804 |
| Dig tyrosine | 0.823 | 0.743 | 0.672 |
| Dig valine | 0.960 | 0.882 | 0.796 |
| Calcium | 0.960 | 0.870 | 0.780 |
| Available phosphorus | 0.480 | 0.435 | 0.390 |
| Sodium | 0.160 | 0.160 | 0.160 |
| Chloride | 0.230 | 0.230 | 0.230 |
| Potassium | 0.944 | 0.856 | 0.767 |
| Linoleic acid | 1.215 | 1.422 | 1.550 |
| Choline (mg/kg) | 1867 | 1963 | 1810 |
| Dietary electrolyte balance (mEq/kg) | 246 | 224 | 201 |
| Feeding Phase | Treatment | Dry Matter (%) | Gross Energy (kcal/kg) | Crude Protein (%) | Cu (mg/kg) |
|---|---|---|---|---|---|
| Starter | Control | 87.08 | 3948 | 22.52 | 17.95 |
| Copper | 86.56 | 3946 | 23.34 | 84.03 | |
| Grower | Control | 86.47 | 4008 | 21.17 | 17.39 |
| Copper | 86.83 | 4006 | 20.73 | 90.12 | |
| Finisher | Control | 87.14 | 4065 | 20.74 | 16.48 |
| Copper | 86.72 | 4061 | 20.18 | 113.23 |
| Target Group or Organism | Primer Sequence (5′–3′) | Annealing Temperature (°C) | Reference |
|---|---|---|---|
| Bacillus sp. | F-GCA ACG AGC GCA ACC CTT GA R-TCA TCC CCA CCT TCC TCC GGT | 63 | Abou-Elkhair et al. [41] |
| Bacteroides sp. | F-GAG AGG AAG GTC CCC CAC R-CGC TAC TTG GCT GGT TCA G | 63 | Layton et al. [42] |
| Bifidobacterium sp. | F-GCG TCC GCT GTG GGC R-CTT CTC CGG CAT GGT GTT G | 63 | Requena et al. [43] |
| Enterobacteriaceae | F-CAT TGA CGT TAC CCG CAG AAG AAG C R-CTC TAC GAG ACT CAA GCT TGC | 63 | Bartosch et al. [44] |
| Lactobacillus sp. | F-CAC CGC TAC ACA TGG AG R-AGC AGT AGG GAA TCT TCC A | 63 | Wise and Siragusa [45] |
| Ruminococcus sp. | F-GGC GGC YTR CTG GGC TTT R-CCA GGT GGA TWA CTT ATT GTG TTA A | 63 | Ramirez-Farias et al. [46] |
| Total bacteria | F-CGG YCC AGA CTC CTA CGG G R-TTA CCG CGG CTG CTG GCA C | 63 | Lee et al. [47] |
| Treatment | Weight Gain (g) | Feed Intake (g) | FCR | Mortality (%) | |
|---|---|---|---|---|---|
| Starter | Control | 258 | 352 | 1.367 | 0.00 |
| Copper | 254 | 345 | 1.362 | 0.00 | |
| 1 SEM | 4.12 | 7.17 | 0.03 | 0.00 | |
| p-value | 0.548 | 0.508 | 0.904 | 1.00 | |
| Grower | Negative control | 739 | 1079 | 1.463 | 1.39 |
| Positive control | 747 | 1096 | 1.469 | 1.39 | |
| Copper | 752 | 1055 | 1.403 | 1.39 | |
| SEM | 12.50 | 12.35 | 0.02 | 1.38 | |
| p-value | 0.746 | 0.095 | 0.057 | 1.00 | |
| Finisher | Negative control | 1443 | 2315 | 1.606 | 2.78 |
| Positive control | 1399 | 2422 | 1.735 | 0.00 | |
| Copper | 1400 | 2421 | 1.730 | 1.39 | |
| SEM | 16.43 | 26.69 | 0.03 | 1.29 | |
| p-value | 0.498 | 0.189 | 0.071 | 0.342 | |
| Overall | Negative control | 2431 | 3721 | 1.532 | 4.17 |
| Positive control | 2402 | 3887 | 1.620 | 2.78 | |
| Copper | 2403 | 3871 | 1.610 | 2.77 | |
| SEM | 18.67 | 35.74 | 0.02 | 2.18 | |
| p-value | 0.807 | 0.125 | 0.085 | 0.874 | |
| Treatment | Duodenum | Jejunum | Ileum | |||
|---|---|---|---|---|---|---|
| Length | Diameter | Length | Diameter | Length | Diameter | |
| Day 21 | ||||||
| Negative control | 26.1 | 1.83 | a 58.8 | a 1.90 | a 60.5 | a 1.59 |
| Positive control | 27.0 | 1.80 | b 67.8 | b 2.23 | b 75.0 | b 1.79 |
| Copper | 27.3 | 1.82 | b 67.7 | ab 2.15 | b 70.7 | b 1.81 |
| SEM | 0.52 | 0.10 | 1.46 | 0.08 | 1.52 | 0.04 |
| p-value | 0.290 | 0.977 | <0.001 | 0.029 | <0.001 | 0.005 |
| Day 35 | ||||||
| Negative control | a 28.5 | 2.30 | a 68.0 | 2.27 | a 68.9 | 1.89 |
| Positive control | b 31.3 | 2.25 | b 80.7 | 2.50 | b 75.6 | 1.92 |
| Copper | b 32.9 | 2.36 | b 77.0 | 2.38 | c 82.1 | 2.05 |
| SEM | 0.66 | 0.06 | 1.68 | 0.09 | 1.70 | 0.05 |
| p-value | 0.001 | 0.516 | <0.001 | 0.210 | <0.001 | 0.098 |
| Treatment | Breast | Thigh | Drumstick | Abdominal Fat |
|---|---|---|---|---|
| Negative control | b 179 | 101 | 87.0 | 10.43 |
| Positive control | a 173 | 101 | 87.1 | 9.14 |
| Copper | a 171 | 103 | 87.2 | 11.04 |
| SEM | 1.60 | 1.27 | 0.84 | 0.62 |
| p-value | 0.006 | 0.430 | 0.992 | 0.123 |
| Treatment | Day 21 | Day 35 | ||
|---|---|---|---|---|
| Gizzard Full | Gizzard Empty | Gizzard Full | Gizzard Empty | |
| Negative control | 25.0 | b 19.0 | 16.6 | 12.6 |
| Positive control | 27.1 | ab 18.4 | 16.8 | 12.6 |
| Copper | 24.7 | a 17.0 | 17.3 | 12.7 |
| SEM | 0.81 | 0.51 | 0.70 | 0.38 |
| p-value | 0.098 | 0.037 | 0.751 | 0.996 |
| Treatment | Lactobacillus sp. | Ruminococcus sp. | Bacteroides sp. | Bacillus sp. | Bifidobacterium sp. | Enterobacteriaceae | Total Bacteria |
|---|---|---|---|---|---|---|---|
| Day 21 | |||||||
| Negative control | 9.18 | 10.09 | 7.70 | ab 8.32 | 9.31 | 8.96 | 11.36 |
| Positive control | 9.33 | 10.14 | 7.59 | b 8.54 | 9.74 | 8.84 | 11.45 |
| Copper | 9.35 | 10.08 | 7.56 | a 7.98 | 9.13 | 8.89 | 11.44 |
| SEM | 0.04 | 0.02 | 0.03 | 0.08 | 0.11 | 0.06 | 0.02 |
| p-value | 0.119 | 0.630 | 0.106 | 0.010 | 0.073 | 0.796 | 0.238 |
| Day 35 | |||||||
| Negative control | 9.15 | 10.00 | 7.62 | 8.65 | 10.28 | 8.66 | 11.44 |
| Positive control | 9.17 | 9.99 | 7.51 | 8.47 | 10.30 | 8.70 | 11.45 |
| Copper | 9.17 | 9.95 | 7.52 | 8.66 | 10.36 | 8.62 | 11.45 |
| SEM | 0.02 | 0.02 | 0.03 | 0.04 | 0.05 | 0.07 | 0.02 |
| p-value | 0.943 | 0.391 | 0.160 | 0.098 | 0.853 | 0.884 | 0.977 |
| Treatment | Fresh Weight (g) | Air-Dry Weight (g) | Length (mm) | Seedor Index | Diameter (mm) | Breaking Strength (N) | Ash as Is (%) |
|---|---|---|---|---|---|---|---|
| Negative control | 13.1 | 7.01 | 92.2 | 75.8 | 7.97 | 400 | 39.8 |
| Positive control | 11.9 | 6.23 | 91.8 | 70.5 | 7.13 | 371 | 38.9 |
| Copper | 12.9 | 6.82 | 91.8 | 74.3 | 7.34 | 405 | 40.1 |
| SEM | 0.60 | 0.32 | 0.62 | 3.34 | 0.38 | 21.56 | 0.42 |
| p-value | 0.380 | 0.282 | 0.827 | 0.534 | 0.301 | 0.494 | 0.188 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, N.; Dao, T.H.; Kumar, A.; Cadogan, D.; Crowley, T.M.; Moss, A.F. Monovalent Copper Oxide in Broiler Nutrition: Effects on Performance, Intestinal Lesions, and Oocyst Shedding During Mild Eimeria Challenge. Vet. Sci. 2025, 12, 494. https://doi.org/10.3390/vetsci12050494
Akter N, Dao TH, Kumar A, Cadogan D, Crowley TM, Moss AF. Monovalent Copper Oxide in Broiler Nutrition: Effects on Performance, Intestinal Lesions, and Oocyst Shedding During Mild Eimeria Challenge. Veterinary Sciences. 2025; 12(5):494. https://doi.org/10.3390/vetsci12050494
Chicago/Turabian StyleAkter, Nasima, Thi Hiep Dao, Alip Kumar, David Cadogan, Tamsyn M. Crowley, and Amy F. Moss. 2025. "Monovalent Copper Oxide in Broiler Nutrition: Effects on Performance, Intestinal Lesions, and Oocyst Shedding During Mild Eimeria Challenge" Veterinary Sciences 12, no. 5: 494. https://doi.org/10.3390/vetsci12050494
APA StyleAkter, N., Dao, T. H., Kumar, A., Cadogan, D., Crowley, T. M., & Moss, A. F. (2025). Monovalent Copper Oxide in Broiler Nutrition: Effects on Performance, Intestinal Lesions, and Oocyst Shedding During Mild Eimeria Challenge. Veterinary Sciences, 12(5), 494. https://doi.org/10.3390/vetsci12050494






