Circulating ACTH and Cortisol Investigations in Standardbred Racehorses Under Training and Racing Sessions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blood Sampling and Hormone Analyses
2.3. Statistical Analysis
3. Results
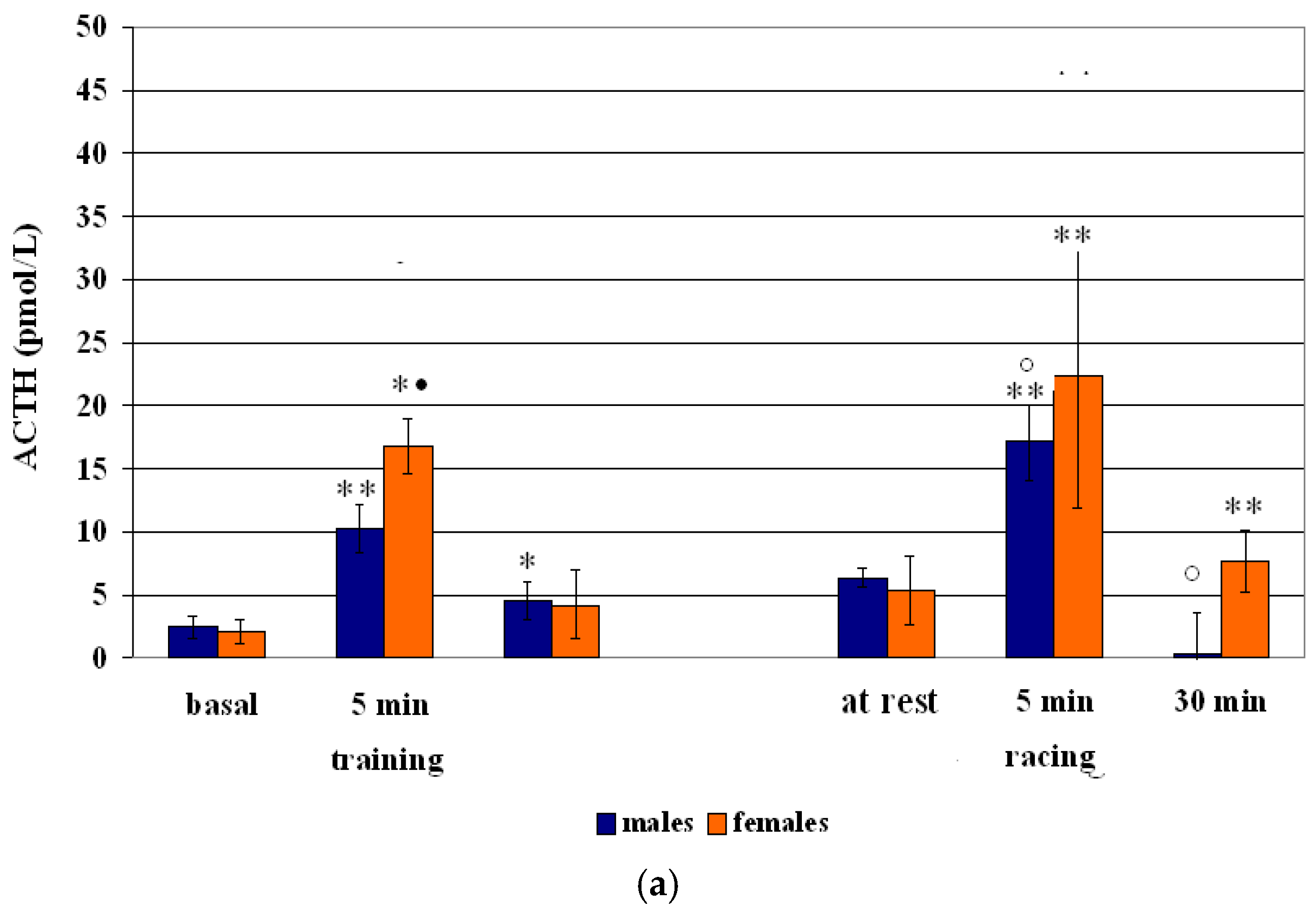
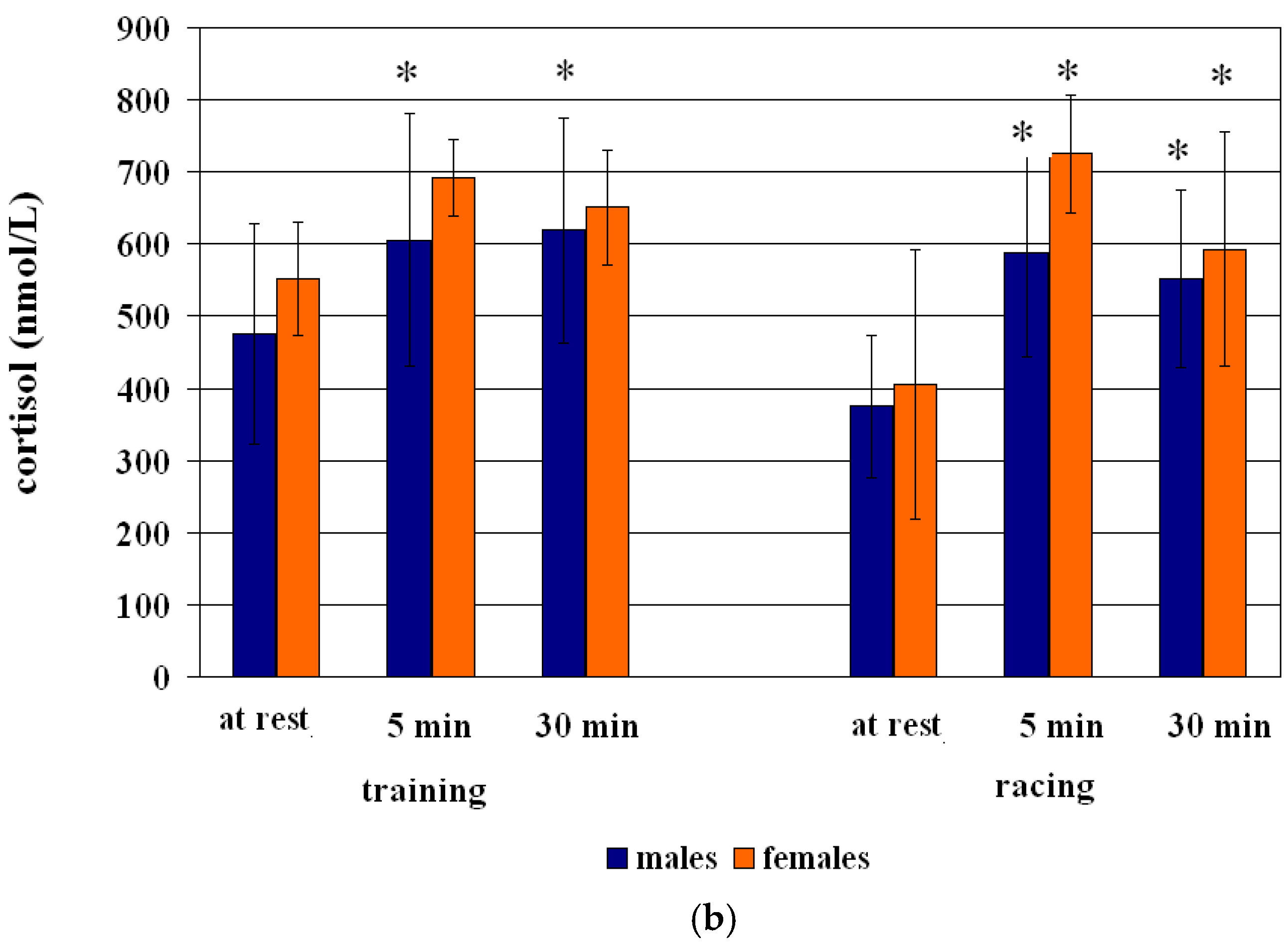
3.1. ACTH-Cortisol Training Session Effect
3.2. ACTH-Cortisol Racing Session Effect
3.3. Racing Session vs. Training Session
3.4. Functional Variables
4. Discussion
Limitations of the Current Investigation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Mastorakos, G.; Pavlatou, M.; Diamanti-Kandarakis, E.; Chrousos, G.P. Exercise and the stress system. Hormones 2005, 4, 73–89. [Google Scholar] [PubMed]
- Hill, E.E.; Zack, E.; Battaglini, C.; Viru, M.; Viru, A.; Hackney, A.C. Exercise and circulating cortisol levels: The intensity threshold effect. J. Endocrinol. Investig. 2008, 31, 587–591. [Google Scholar] [CrossRef]
- Cravana, C.; Medica, P.; Prestopino, M.; Fazio, E.; Ferlazzo, A. Effects of competitive and noncompetitive show jumping on total and free iodothyronines, β-endorphin, ACTH and cortisol levels of horses. Equine Vet. J. 2010, 42, 179–184. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Cravana, C.; Fazio, E.; Medica, P. The different hormonal system during exercise stress coping in horses. Vet. World 2020, 13, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, A.; Fazio, E.; Medica, P. Behavioral features and effects of transport procedures on endocrine variables of horses. J. Vet. Behav. 2020, 39, 21–31. [Google Scholar] [CrossRef]
- Witkowska-Piłaszewicz, O.; Grzędzicka, J.; Sen, J.; Czopowicz, M.; Zmigrodzka, M.; Winnicka, A.; A Cywińska, A.; Carter, C. Stress response after race and endurance training sessions and competitions in Arabian horses. Prev. Vet. Med. 2021, 188, 105265. [Google Scholar] [CrossRef]
- Angle, C.T.; Wakshlag, J.J.; Gillette, R.L.; Stokol, T.; Geske, S.; Adkins, T.O.; Gregor, C. Hematologic, serum biochemical, and cortisol changes associated with anticipation of exercise and short duration high-intensity exercise in sled dogs. Vet. Clin. Pathol. 2009, 38, 370–374. [Google Scholar] [CrossRef]
- Duclos, M.; Tabarin, A. Exercise, training, and the Hypothalamo–Pituitary–Adrenal axis. Front. Horm. Res. 2016, 47, 12–26. [Google Scholar] [CrossRef]
- Negro, S.; Bartolomé, E.; Molina, A.; Solé, M.; Gómez, M.D.; Valera, M. Stress level effects on sport performance during trotting races in Spanish trotter horses. Res. Vet. Sci. 2018, 118, 86–90. [Google Scholar] [CrossRef]
- Golland, L.C.; Evans, D.L.; Stone, G.M.; Tyler-McGowan, C.M.; Hodgson, D.R.; Rose, R.J. Plasma cortisol and beta-endorphin concentrations in trained and over-trained Standardbred racehorses. Pflug. Arch. 1999, 439, 11–17. [Google Scholar] [CrossRef]
- Malinowski, K.; Shock, E.J.; Rochelle, P.; Kearns, C.F.; Guirnalda, P.D.; McKeever, K.H. Plasma beta-endorphin, cortisol and immune responses to acute exercise are altered by age and exercise training in horses. Equine Vet. J. Suppl. 2006, 36, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Hada, T.; Onaka, T.; Takahashi, T.; Hiraga, A.; Yagi, K. Effects of novelty stress on neuroendocrine activities and running performance in thoroughbred horses. J. Neuroendocrinol. 2003, 36, 638–648. [Google Scholar] [CrossRef]
- Cayado, P.; Muñoz-Escassi, B.; Domínguez, C.; Manley, W.; Olabarri, B.; Sánchez de la Muela, M.; Castejon, F.; Marañon, G.; Vara, E. Hormone response to training and competition in athletic horses. Equine Vet. J. Suppl. 2006, 36, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J.R. Hormonal responses to exercise and training. Vet. Clin. N. Am. Equine Pract. 1985, 1, 477–496. [Google Scholar] [CrossRef]
- Marc, M.; Parvizi, N.; Ellendorff, F.; Kallweit, E.; Elsaesser, F. Plasma cortisol and ACTH concentrations in the warmblood horse in response to a standardized treadmill exercise test as physiological markers for evaluation of training status in response to a standardized treadmill exercise test as physiological markers for evaluation of training status. J. Anim. Sci. 2000, 78, 1936–1946. [Google Scholar] [CrossRef]
- Nogueira de Paula, G.; Barnabe, R.C.; Bedran-de-Castro, J.C.; Moreira, A.F.; Fernandes, W.R.; Mirandola, R.M.S.; How-ard, D.L. Serum cortisol, lactate and creatinine concentrations in Thoroughbreds fillies of different ages and states of training. Braz. J. Vet. Res. Anim. Sci. 2002, 39, 54–57. [Google Scholar] [CrossRef]
- Quaranta, A.; Tateo, A.; Siniscalchi, M.; Padalino, B.; Iacoviello, R.; Centoducati, P. Influence of training on cortisol plasma levels and other hematic parameters in standardbred trotters. Ippologia 2006, 17, 5–10. [Google Scholar]
- Peeters, M.; Joseph Sulon, J.; Didier Serteyn, D.; Vandenheede, M. Assessment of stress level in horses during competi-tion using salivary cortisol: Preliminary study. J. Vet. Behav. 2010, 5, 216. [Google Scholar] [CrossRef]
- McCarthy, R.N.; Jeffcott, L.B.; Funder, J.W.; Fullerton, M.; Clarke, I.J. Plasma beta-endorphin and adrenocorticotrophin in young horses in training. Aust. Vet. J. 1991, 68, 359–361. [Google Scholar] [CrossRef]
- Jimenez, M.; Hinchcliff, K.W.; Farris, J.W. Cathecolamine and cortisol responses of horses to incremental exertion. Vet. Res. Commun. 1998, 22, 107–118. [Google Scholar] [CrossRef]
- Kurosawa, M.; Nagata, S.; Takeda, F.; Mima, K.; Hiraga, A.; Kai, M.; Taya, K. Plasma catecholamine, adrenocorticotropin and cortisol responses to exhaustive incremental treadmill exercise of the Thoroughbred horse. J. Equine Sci. 1998, 9, 9–18. [Google Scholar] [CrossRef]
- Nagata, S.; Takeda, F.; Kurosawa, M.; Mima, K.; Hiraga, A.; Kai, M.; Taya, K. Plasma adrenocorticotropin, cortisol and catecholamines response to various exercises. Equine Vet. J. Suppl. 1999, 30, 570–574. [Google Scholar] [CrossRef]
- de Graff-Roeflsema, E.; Keizer, H.A.; van Breda, E.; Wijnberg, I.D.; van der Kolk, J.H. Hormonal responses to acute exercise, training and overtraining. A review with emphasis on the horse. Vet. Q. 2007, 29, 82–101. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, A.; Medica, P.; Cravana, C.; Fazio, E. Circulating ß-endorphin, adrenocorticotropin, and cortisol concentrations of horses before and after competitive show jumping with different fence heights. J. Equine Vet. Sci. 2012, 32, 740–746. [Google Scholar] [CrossRef]
- Becker-Bircka, M.; Schmidta, A.; Lasarzika, J.; Aurich, J.; Möstlc, E.; Aurich, C. Cortisol release and heart rate variability in sport horses participating in equestrian competitions. J. Vet. Behav. 2013, 8, 87–94. [Google Scholar] [CrossRef]
- Bartolomé, E.; Cockram, M.S. Potential effects of stress on the performance of sport horses. J. Equine Vet. Sci. 2016, 40, 84–93. [Google Scholar] [CrossRef]
- Fazio, E.; Medica, P.; Aveni, F.; Ferlazzo, A. The potential role of training sessions on the temporal and spatial physio-logical patterns in young Friesian horses. J. Equine Vet. Sci. 2016, 47, 84–89. [Google Scholar] [CrossRef]
- Sokoloff, N.C.; Madhusmita, M.; Ackerman, K.E. Exercise, Training, and the Hypothalamic-Pituitary-Gonadal Axis in Men and Women. Front. Horm. Res. 2016, 47, 27–43. [Google Scholar] [CrossRef]
- Liburt, N.R.; McKeever, K.H.; Malinowski, K.; Smarsh, D.N.; Geor, R.J. Response of the hypothalamic-pituitary-adrenal axis to stimulation tests before and after exercise training in old and young Standardbred mares. J. Anim. Sci. 2013, 91, 5208–5219. [Google Scholar] [CrossRef]
- Bohák, Z.A.; Harnos, A.; Joó, K.; Szenci, O.; Kovács, L. Anticipatory response before competition in Standardbred racehorses. PLoS ONE 2018, 13, e0201691. [Google Scholar] [CrossRef]
- Fazio, E.; Lindner, A.; Wegener, J.; Medica, P.; Hartmann, U.; Ferlazzo, A. Plasma cortisol concentration during standardized exercise in Standardbred racehorses within a racing season. Pferdeheilkunde 2023, 39, 151–157. [Google Scholar] [CrossRef]
- Tsai, C.L.; Wang, C.H.; Pan, C.Y.; Chen, F.C.; Huang, T.H.; Chou, F.Y. Executive function and endocrinological responses to acute resistance exercise. Front. Behav. Neurosci. 2014, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.; Krzysztofik, M.; Petr, M.; Zając, A.; Stastny, P. The slow exercise tempo elicits higher glycolytic and muscle dam age but not endocrine response that conventional squat. Neuroendocrinol. Lett. 2020, 41, 101–107. [Google Scholar]
- Gordon, B.A.; Taylor, C.J.; Church, J.E.; Cousins, S.D. A comparison of the gluco-regulatory responses to high-ıntensity ınterval exercise and resistance exercise. Int. J. Environ. Res. Public Health 2021, 18, 287. [Google Scholar] [CrossRef] [PubMed]
- Strzelec, K.; Kankofer, M.; Pietrzak, S. Cortisol concentration in the saliva of horses subjected to different kinds of exercise. Acta Vet. Brno 2011, 80, 101–105. [Google Scholar] [CrossRef]
- Kędzierski, W.; Cywińska, A.; Strzelec, K.; Kowalik, S. Changes in salivary and plasma cortisol levels in Purebred Arabian horses during race training session. Anim. Sci. J. 2014, 85, 313–317. [Google Scholar] [CrossRef]
- Kang, O.D.; Lee, W.S. Changes in Salivary Cortisol Concentration in Horses during Different Types of Exercise. Asian-Australas. J. Anim. Sci. 2016, 29, 747–752. [Google Scholar] [CrossRef]
- Van Der Kolk, J.H.; Nachreiner, R.F.; Scott, H.C.; Refsal, K.R.; Zanella, K.J. Salivary and plasma concentra-tion of cortisol in normal horses and horses with Cushing’s disease. Equine Vet. J. 2001, 33, 211–213. [Google Scholar] [CrossRef]
- Mostl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Peeters, M.; Coline, C.; Becker, J.F.; Ledoux, D.; Vandenheede, M. Comparison between blood serum and salivary cortisol concentrations in horses using an adrenocorticotropic hormone challenge. Equine Vet. J. 2011, 43, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Kędzierski, W.; Strzelec, K.; Cywinska, A.; Kowalik, S. Salivary cortisol concentration in exercised thoroughbred horses. J. Equine Vet. Sci. 2013, 33, 1106–1109. [Google Scholar] [CrossRef]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Fazio, E.; Aronica, V.; Di Majo, R.; Medica, P.; Grasso, L. Circulating concentrations of b-endorphin, ACTH and cortisol in horses after jumping over fences of different size. In Proceedings of the Conference on Equine Sports Medicine and Science, Cordoba, Spain, 24–26 April 1998; pp. 53–56. [Google Scholar]
- Orth, D.N.; Holscher, M.A.; Wilson, M.G.; Nicholson, W.E.; Plue, R.E.; Mount, C.D. Equine Cushing’s disease: Plasma immunoreactive proopiolipomelanocortin peptide and cortisol levels basally and in response to diagnostic tests. Endocrinology 1982, 110, 1430–1441. [Google Scholar] [CrossRef]
- Hodson, N.P.; Wright, J.A.; Hunt, J. The sympatho-adrenal system and plasma levels of adrenocorticotropic hormone, cortisol and catecholamines in equine grass sickness. Vet. Rec. 1986, 118, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Eades, S.C.; Bounous, D.L. Laboratory Profile of Equine Diseases; Mosby Year Book: Saint Louis, MI, USA, 1997; pp. 15–20. [Google Scholar]
- Cravana, C.; Fazio, E.; Satué, K.; Brancato, G.; Medica, P.; La Fauci, D. ACTH and cortisol dynamics in young Thoroughbred racehorses under competitive and non-competitive sessions. J. Equine Vet. Sci. 2024. submitted. [Google Scholar]
- McBride, S.; Mills, D.S. Psychological factors affecting equine performance. BMC Vet. Res. 2012, 8, 180. [Google Scholar] [CrossRef]
- McKenna, B.; Lembert, M.; Evans, J.A. A study of β-endorphin and cortisol levels in the exercising horse. In Proceedings of the 12th Conference Association for Equine Sports Medicine, Fallbrook, CA, USA, 13–16 March 1993; Foreman, J., Ed.; Veterinary Practice Publishing Comp: Santa Barbara, CA, USA, 1993; pp. 39–43. [Google Scholar]
- Desmecht, D.; Linden, A.; Amory, H.; Art, T.; Lekeux, P. Relationship of plasma lactate production to cortisol release following completion of different types of sporting events in horses. Vet. Res. Commun. 1996, 20, 371–379. [Google Scholar] [CrossRef]
- Cravana, C.; Medica, P.; Fazio, E.; Di Giovanni, F.; Ferlazzo, A. Adrenocorticotropin and cortisol response to competitive and not competitive races in Thoroughbreds of different age. In Management of Lameness Causes in Sport Horses: Muscle, Tendon, Joint and Bone Disorders; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 165–168. [Google Scholar] [CrossRef]
- Lindner, A.; Fazio, E.; Medica, P.; Ferlazzo, A. Effect of age, time record and V4 on plasma cortisol concentration in Standardbred racehorses during exercise. Pferdeheilkunde 2002, 18, 51–56. [Google Scholar] [CrossRef]
- von Lewinski, M.; Biau, S.; Erber, R.; Ille, N.; Aurich, J.; Faure, J.M.; Möstl, E.; Aurich, C. Cortisol release, heart rate and heart rate variability in the horse and its rider: Different responses to training and performance. Vet. J. 2013, 197, 229–232. [Google Scholar] [CrossRef]
- Nesse, L.; Johansen, G.I.; Blom, A.K. Effect of racing of lymphocyte proliferation in horses. Am. J. Vet. Res. 2002, 63, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Rivera, E.; Benjamin, S.; Nielsen, B.; Shelle, J.; Zanella, A.J. Behavioural and physiological responses of horses to initial training: The comparison between pastured versus stalled horses. Appl. Anim. Behav. Sci. 2002, 78, 235–252. [Google Scholar] [CrossRef]
- Bohák, Z.; Szabó, F.; Beckers, J.-F.; Melo de Sousa, N.; Kutasi, O.; Nagy, K.; Szenci, O. Monitoring the circadian rhythm of serum and salivary cortisol concentrations in the horse. Domest. Anim. Endocrinol. 2013, 45, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Duclos, M.; Tabarin, A. Activation of the HPA Axis During an Acute Bout of Exercise Chapter 2 Exercise, Training, and the Hypothalamo–Pituitary–Adrenal Axis; Ghigo, E., Lanfranco, F., Strasburger, J., Eds.; Hormone Use and Abuse by Athletes, Endocrine Updates 29, 9; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2011; pp. 9–11. [Google Scholar] [CrossRef]
- Leleu, C.; Gloria, G.; Renault, G.; Barrey, E. Analysis of trotter gait on the track by accelerometry and image analysis. Equine Vet. J. Suppl. 2002, 34, 344–348. [Google Scholar] [CrossRef]
- Traustadóttir, T.; Bosch, P.R.; Cantu, T.; Matt, K.S. Hypothalamic-pituitary-adrenal axis response and recovery from high-intensity exercise in women: Effects of aging and fitness. J. Clin. Endocrinol. Metab. 2004, 89, 3248–3254. [Google Scholar] [CrossRef]




| Training | Racing |
|---|---|
| Two rounds: strenuous training: velocity 8–10 m/s; duration 5 min | Two rounds: strenuous training: velocity 8–10 m/s; duration 5 min |
| 1600 m: sprint training: velocity 10–12 m/s; duration 2.40 min | 1600 m: sprint training: velocity 15–17 m/s; duration 1.58 min |
| Two rounds: basic training: velocity 5–8 m/s; duration 10 min | One round: basic training: velocity 5–8 m/s; duration 10 min |
| Cool down at the pass: duration 10 min | Cool down at the pass: duration 10 min |
| ACTH (pmol/L) | |||||
|---|---|---|---|---|---|
| at Rest | 5 min | Δ% | 30 min | Δ% | |
| Total | 2.50 ± 0.89 | 12.91 ± 3.43 b | +416 | 4.40 ± 8.66 b | +76 |
| 2-year-old | 2.78 ± 0.95 | 12.13 ± 3.66 a | +336 | 5.10 ± 1.80 | +83 |
| 3-year-old | 2.31 ± 0.78 | 13.40 ± 4.06 a | +480 | 4.04 ± 1.83 a | +75 |
| males | 2.48 ± 0.89 | 10.28 ± 1.89 b | +314 | 4.51 ± 1.51 a | +82 |
| females | 2.11 ± 0.94 | 16.81 ± 2.21 aB | +697 | 4.19 ± 2.72 | +98 |
| cortisol (nmol/L) | |||||
| Total | 498 ± 136 | 632 ± 151 b | +27 | 629 ± 133 b | +26 |
| 2-year-old | 555 ± 82 | 678 ± 59 A | +22 | 659 ± 76 A | +19 |
| 3-year-old | 460 ± 158 | 601 ± 190 a | +31 | 608 ± 165 a | +32 |
| males | 475 ± 153 | 606 ± 175 a | +27 | 619 ± 156 a | +30 |
| females | 552 ± 79 | 692 ± 54 | +25 | 651 ± 79 | +18 |
| ACTH (pmol/L) | |||||
|---|---|---|---|---|---|
| at Rest | Δ% | 5 min | Δ% | 30 min | |
| Total | 6.13 ± 7.79 C | +203 | 18.61 ± 26.82 Cb | +23 | 7.52 ± 1.54 CD |
| 2-year-old | 6.43 ± 6.13 | +243 | 22.05 ± 8.10 Ca | +29 | 8.29 ± 2.0 C |
| 3-year-old | 5.92 ± 8.70 | +176 | 16.32 ± 2.74 C | +31 | 7.74 ± 3.28 C |
| males | 6.30 ± 3.52 | +172 | 17.15 ± 3.05 bC | +32 | 8.35 ± 3.25 C |
| females | 5.34 ± 12.35 | +68 | 22.29 ± 10.41 b | +44 | 7.67 ± 2.41 b |
| cortisol (nmol/L) | |||||
| Total | 383 ± 121 C | +64 | 629 ± 141 b | +47 | 565 ± 126 a |
| 2-year-old | 375 ± 118 | +48 | 57 ± 215 a | +32 | 494 ± 165 a |
| 3-year-old | 389 ± 132 | +74 | 678 ± 77 | +57 | 612 ± 77 |
| males | 375 ± 99 | +56 | 587 ± 143 a | +47 | 552 ± 123 a |
| females | 405 ± 187 | +79 | 725 ± 82 a | +46 | 593 ± 162 a |
| Training | ||||
|---|---|---|---|---|
| At Rest | End | 15 min | 30 min | |
| HR (beats/min) | 38 ± 10 | 180 ± 20 a | 80 ± 15 a | 65 ± 5 a |
| RR (breaths/min) | 18 ± 8 | 80 ± 5 a | 75 ± 10 a | 40 ± 10 a |
| RT °C | 37.5 ± 0.3 | 38.6 ± 0.3 a | 38.5 ± 0.2 a | 37.8 ± 0.4 |
| racing | ||||
| HR (beats/min) | 39 ± 11 | 189 ± 30 a | 84 ± 10 a | 55 ± 5 a |
| RR (breaths/min) | 18 ± 3 | 80 ± 5 a | 78 ± 10 a | 45 ± 8 a |
| RT °C | 37.6 ± 0.3 | 39.2 ± 0.4 a | 38.6 ± 0.3 a | 37.8 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cravana, C.; Medica, P.; Fazio, E.; Satué, K.; Brancato, G.; La Fauci, D.; Bruschetta, G. Circulating ACTH and Cortisol Investigations in Standardbred Racehorses Under Training and Racing Sessions. Vet. Sci. 2025, 12, 493. https://doi.org/10.3390/vetsci12050493
Cravana C, Medica P, Fazio E, Satué K, Brancato G, La Fauci D, Bruschetta G. Circulating ACTH and Cortisol Investigations in Standardbred Racehorses Under Training and Racing Sessions. Veterinary Sciences. 2025; 12(5):493. https://doi.org/10.3390/vetsci12050493
Chicago/Turabian StyleCravana, Cristina, Pietro Medica, Esterina Fazio, Katiuska Satué, Giacoma Brancato, Deborah La Fauci, and Giuseppe Bruschetta. 2025. "Circulating ACTH and Cortisol Investigations in Standardbred Racehorses Under Training and Racing Sessions" Veterinary Sciences 12, no. 5: 493. https://doi.org/10.3390/vetsci12050493
APA StyleCravana, C., Medica, P., Fazio, E., Satué, K., Brancato, G., La Fauci, D., & Bruschetta, G. (2025). Circulating ACTH and Cortisol Investigations in Standardbred Racehorses Under Training and Racing Sessions. Veterinary Sciences, 12(5), 493. https://doi.org/10.3390/vetsci12050493








