Activity of Different Types of Cactus Forage on Testicular Function and Morphology of Sheep Subjected to Environmental Heat Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics
2.2. Experimental Groups
2.3. Body and Testicular Biometry
2.4. Material Processing for Light Microscopy
2.5. Morphometric and Histopathological Analysis of the Testicles
2.6. Oxidative Stress
2.7. Hormonal Dosage
2.8. Statistical Analysis
3. Results
3.1. Body and Testicular Biometry
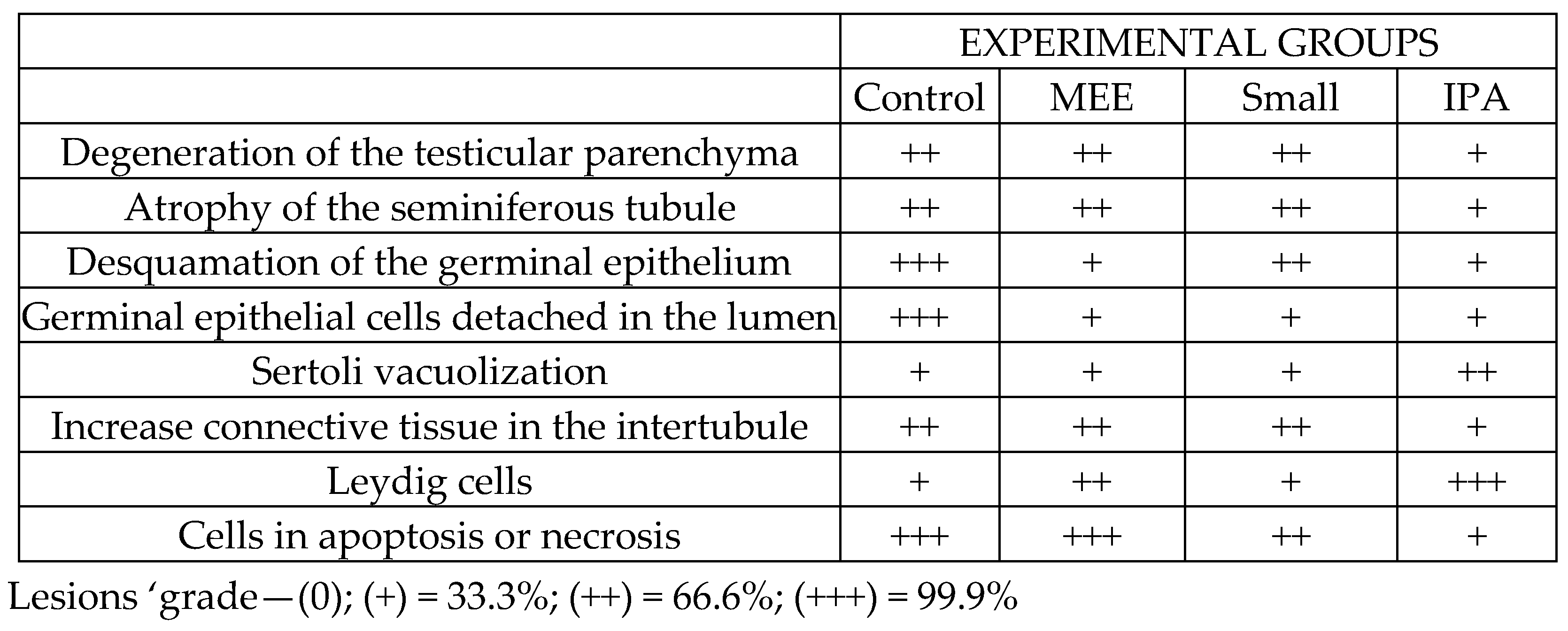
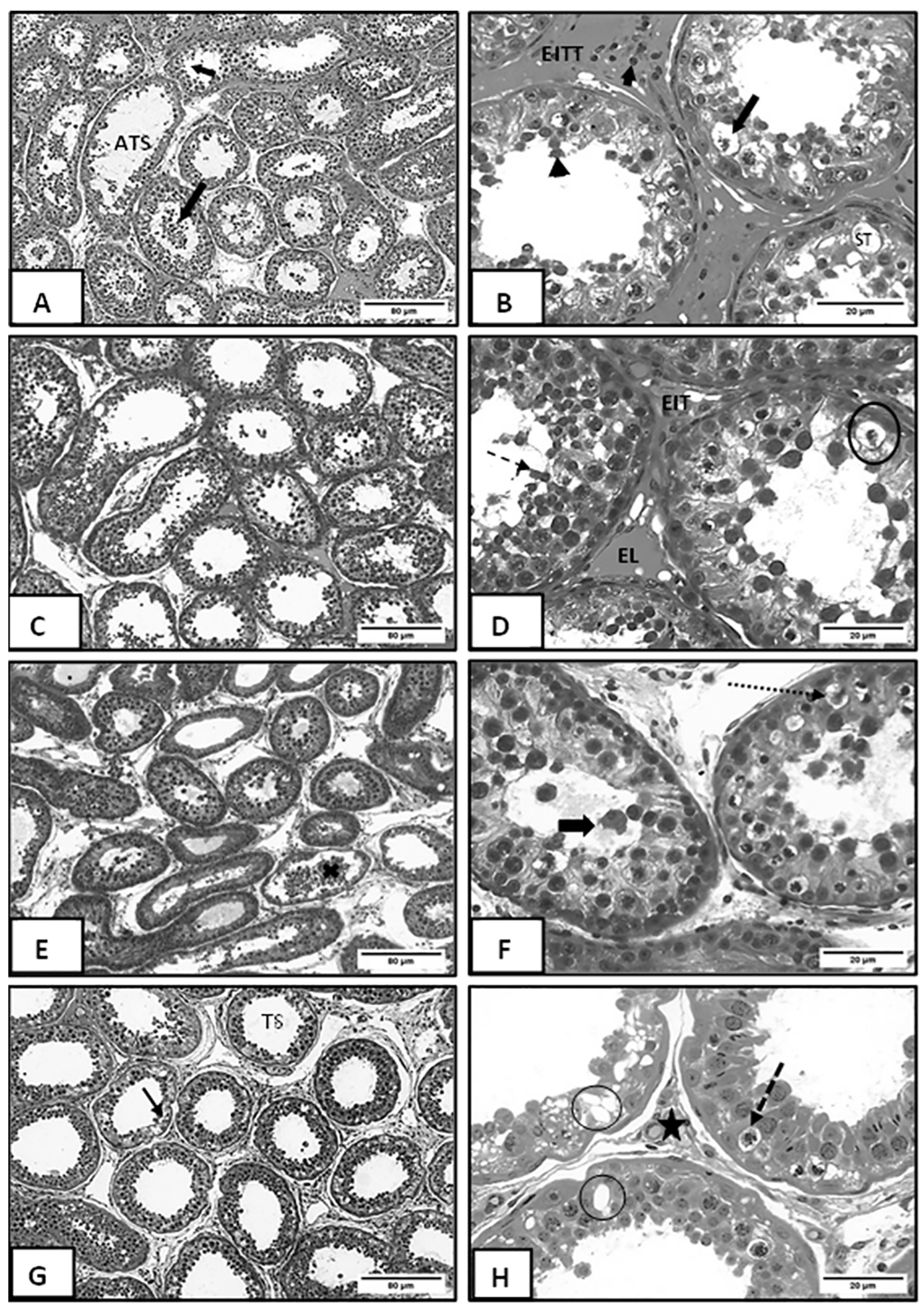
3.2. Morphometric and Histopathological Analyses
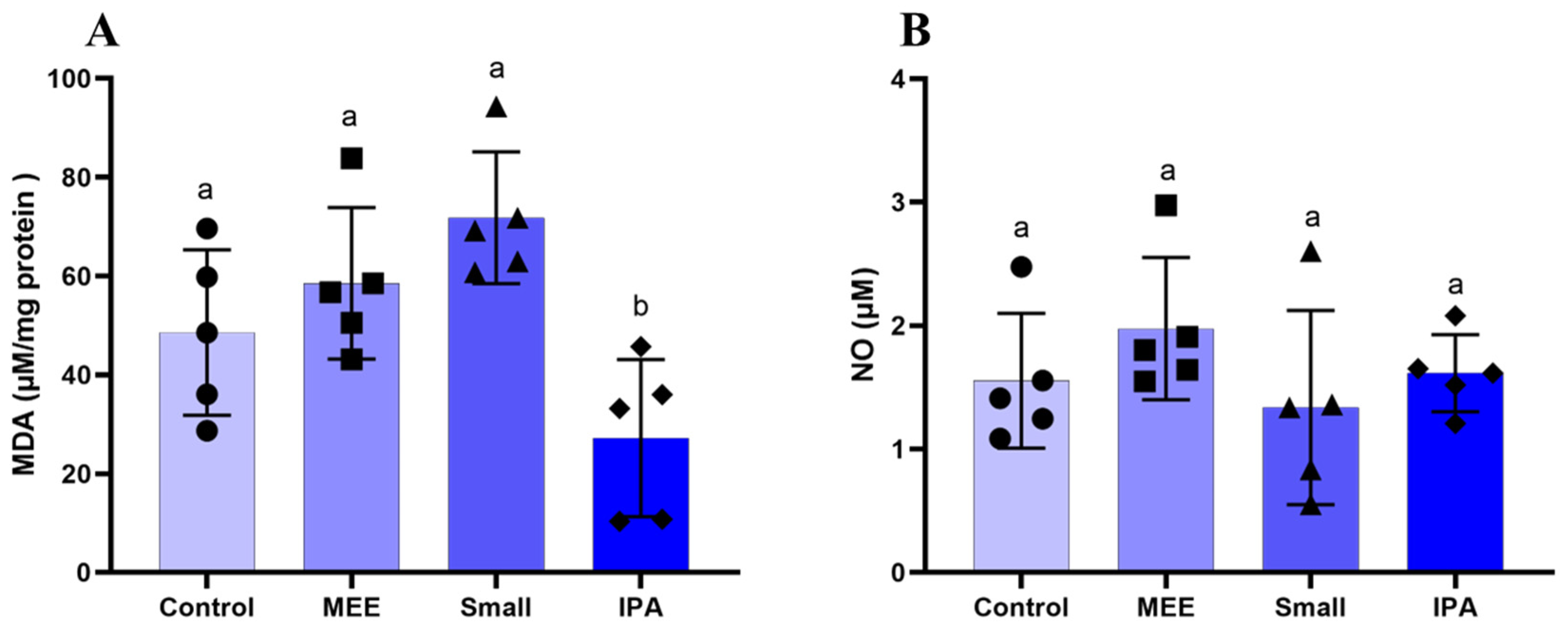
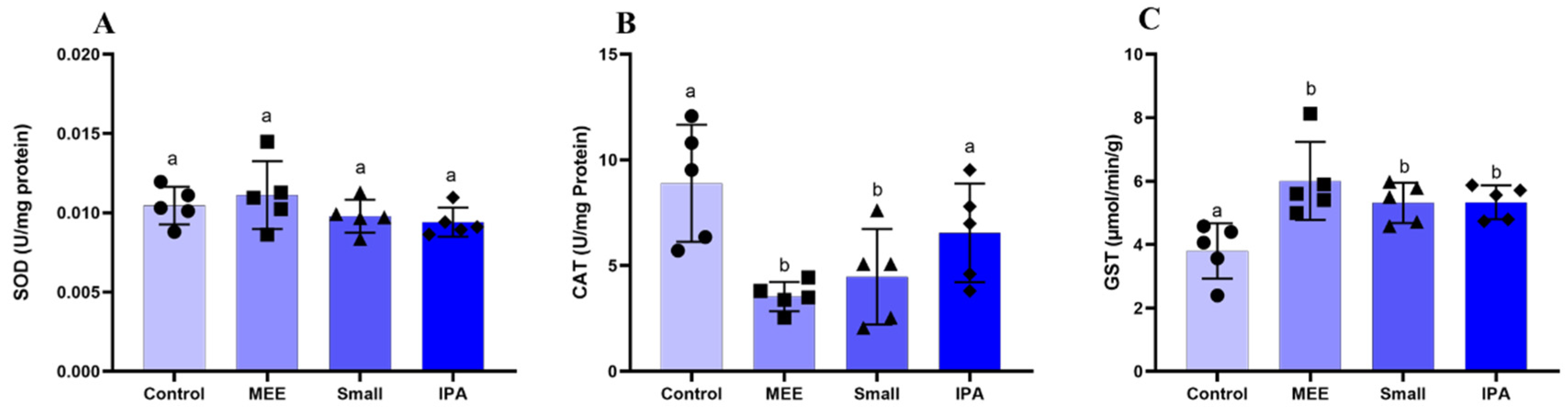
3.3. Oxidative Stress
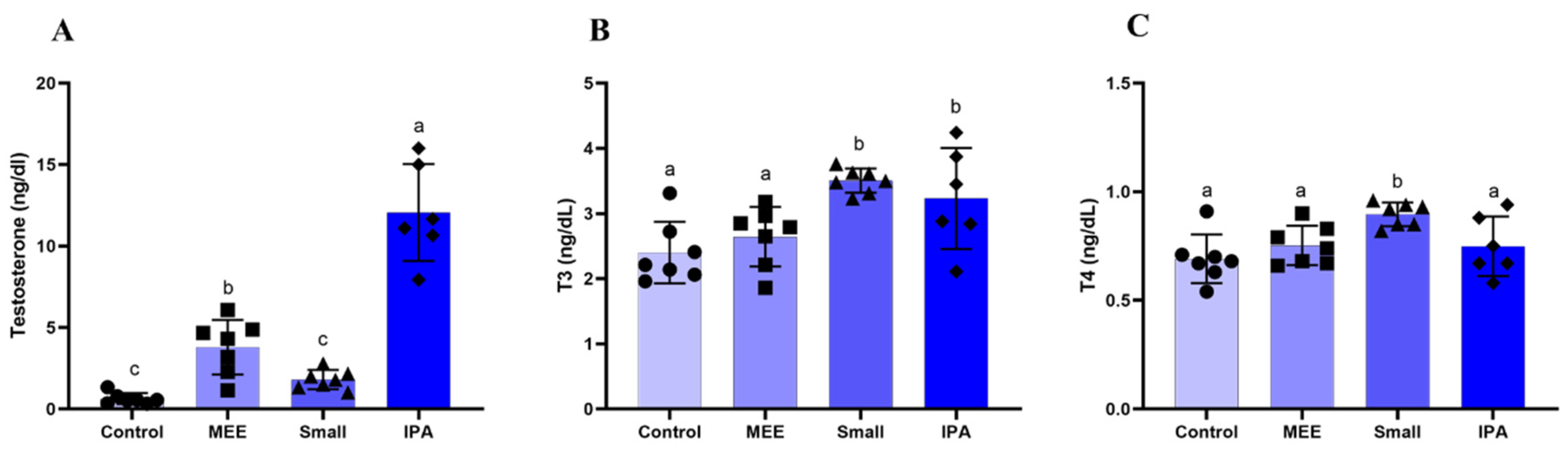
3.4. Hormonal Dosage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannetto, C.; Aragona, F.; Fazio, F.; Piccione, G.; Giudice, E.; Arfuso, F.; Zumbo, A. Investigation of the Impact of Seasonal Climate Conditions on Feed Intake and Body Weight in Horses. Int. J. Biometeorol. 2025, 69, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.C.R.; Andrade, R.R.; Ferreira, L.N. Climate Change Impacts on Livestock in Brazil. Int. J. Biometeorol. 2024, 68, 2693–2704. [Google Scholar] [CrossRef]
- Capela, L.; Leites, I.; Romão, R.; Lopes-Da-Costa, L.; Pereira, R.M.L.N. Impact of Heat Stress on Bovine Sperm Quality and Competence. Animals 2022, 12, 975. [Google Scholar] [CrossRef]
- Binuni Rebez, E.; Sejian, V.; Silpa, M.V.; Kalaignazhal, G.; Devaraj, C.; Nikhil, K.T.; Ninan, J.; Tüfekci, H.; Fonsêca, V.F.C.; Chauhan, S.S.; et al. Feed Additives Supplementation: A Potential Strategy to Ameliorate Heat Stress in Sheep. Ann. Anim. Sci. 2024. [Google Scholar] [CrossRef]
- Gowane, G.R.; Gadekar, Y.P.; Prakash, V.; Kadam, V.; Chopra, A.; Prince, L.L.L. Climate Change Impact on Sheep Production: Growth, Milk, Wool, and Meat. In Sheep Production Adapting to Climate Change; Ejian, V., Sejian, V., Bahatta, R., Gaughan, P., Navqvi, S.M.K., Lal, R., Eds.; Springer: Singapore, 2017. [Google Scholar]
- Godoi, P.F.A.; Magalhães, A.L.R.; de Araújo, G.G.L.; de Melo, A.A.S.; Silva, T.S.; Gois, G.C.; dos Santos, K.C.; do Nascimento, D.B.; da Silva, P.B.; de Oliveira, J.S.; et al. Chemical Properties, Ruminal Fermentation, Gas Production and Digestibility of Silages Composed of Spineless Cactus and Tropical Forage Plants for Sheep Feeding. Animals 2024, 14, 552. [Google Scholar] [CrossRef]
- Bezerra, A.S.; Santos, M.A.S.D.; Lourenco-Junior, J.D.B. Technologies Used in Production Systems for Santa Inês Sheep: A Systematic Review. Front. Vet. Sci. 2022, 9, 896241. [Google Scholar] [CrossRef]
- Carvalho, J.S.; Da Silva, T.R.; De Morais Santos, P.V.; Almeida, F.F.; De Jesus, T.K.S.; Rizzo, H. Characterization of Goat and Sheep Production in the State of Sergipe, Northeast of Brazil. Acta Vet. Bras. 2020, 14, 121–131. [Google Scholar] [CrossRef]
- de Farias, A.E.M.; Alves, J.R.A.; Alves, F.S.F.; Pinheiro, R.R.; Faccioli-Martins, P.Y.; Lima, A.M.C.; Azevedo, S.S.; Alves, C.J. Characterization of Goat Production Systems in Five States of Northeastern Brazil. Semin. Cienc. Agrar. 2019, 40, 3691–3708. [Google Scholar] [CrossRef]
- Moghaddam, A.; Panah, M.; Souri, M. Improved Early Postnatal Nutrition and Its Effect on Histomorphological Parameters in the Testes of Sanjabi Ram Lambs. Trop. Anim. Heal. Prod. 2019, 51, 1539–1544. [Google Scholar] [CrossRef]
- Shirazinia, R.; Rahimi, V.B.; Kehkhaie, A.R.; Sahebkar, A.; Rakhshandeh, H.; Askari, V.R. Opuntia Dillenii: A Forgotten Plant with Promising Pharmacological Properties. J. Pharmacopunct. 2019, 22, 16–27. [Google Scholar] [CrossRef]
- Silva, C.d.S.; de Araújo, G.G.L.; Santos, E.M.; de Oliveira, J.S.; da Silva, T.G.F.; Araújo, C.d.A.; Novaes, J.J.d.S.; de Macedo, A.; de Araújo, J.S.; Lima, D.O.; et al. Fermentative Characteristics, Nutritional Aspects, Aerobic Stability, and Microbial Populations of Total Mixed Ration Silages Based on Relocated Sorghum Silage and Cactus Pear for Sheep Diets. Agronomy 2025, 15, 506. [Google Scholar] [CrossRef]
- Vila Nova, S.D.R.M.; Cassia, A.R.; Santos, M.D.F.R. Potencial Econômico de Patentes Expiradas: Análise Do Caso Da Palma Forrageira (Opuntia Ficus-Indica); Engema: Mechelen, Belgium, 2018. [Google Scholar]
- Bomfim, M.A.D.; Henrique Melo Andrade Rodrigues de Albuquerque, F.; Teixeira de Sousa, R. Papel da Nutrição Sobre a Reprodução Ovina [The Role of Nutrition on Reproduction of Sheep]. Acta Vet. Bras. 2014, 8, 372–379. [Google Scholar]
- AL-Janabi, Q.A.R.; Shaban, R.K.; AL-Kraie, N.I.H. The Effectiveness of the Protective Role of Beetroot Juice for the Male Reproductive System of Albino Rats against the Toxicity of Cadmium Chloride. Tikrit J. Pure Sci. 2019, 24, 43–51. [Google Scholar] [CrossRef]
- Eustáquio Filho, A.; Martins Teodoro, S.; Antônio Chaves, M.; Eduardo Ferreira dos Santos, P.; Welber Ribeiro da Silva, M.; Mendes Murta, R.; Giordano Pinto de Carvalho, G.; Eduardo Barreto de Souza, L. Zona de Conforto Térmico de Ovinos Da Raça Santa Inês Com Base Nas Respostas Fisiológicas. Rev. Bras. Zootec. 2011, 40, 1087–1814. [Google Scholar] [CrossRef]
- Giannetto, C.; Aragona, F.; Arfuso, F.; Piccione, G.; De Caro, S.; Fazio, F. Diurnal Variation in Rectal and Cutaneous Temperatures of Horses Housed under Different Management Conditions. Int. J. Biometeorol. 2022, 66, 1601–1611. [Google Scholar] [CrossRef]
- Karnovsky, M.J. A Formaldehyde-Glutaraldehyde Fixative of High Osmolaty for Use in Electron Microscopy. J. Cell Biol. 1965, 27, 137–138. [Google Scholar]
- Amann, R.P. The Male Rabbit. IV. Quantitative Testicular Histology and Comparisons between Daily Sperm Production as Determined Histologically and Daily Sperm Output. Fertil Steril 1970, 21, 662–672. [Google Scholar] [CrossRef]
- Dias, F.C.R.; Martins, A.L.P.; de Melo, F.C.S.A.; Cupertino, M.D.C.; Gomes, M.d.L.M.; de Oliveira, J.M.; Damasceno, E.M.; Silva, J.; Otoni, W.C.; da Matta, S.L.P. Hydroalcoholic Extract of Pfaffia Glomerata Alters the Organization of the Seminiferous Tubules by Modulating the Oxidative State and the Microstructural Reorganization of the Mice Testes. J. Ethnopharmacol. 2019, 233, 179–189. [Google Scholar] [CrossRef]
- Venâncio, A.K.L.P.; Dias, F.C.R.; Nascimento, A.S.; Ramos, F.F.; Oliveira, F.; Torres, S.M.; Carvalho, F.F.R.; da Silva Júnior, V.A. Effects of Dietary Crude Glycerin Concentration on Testicular Morphology and Oxidative Stress Markers and on Plasma Testosterone Concentrations. J. Comp. Pathol. 2021, 185, 72–81. [Google Scholar] [CrossRef]
- Sarban, S.; Kocyigit, A.; Yazar, M.; Isikan, U.E. Plasma Total Antioxidant Capacity, Lipid Peroxidation, and Erythrocyte Antioxidant Enzyme Activities in Patients with Rheumatoid Arthritis and Osteoarthritis. Clin. Biochem. 2005, 38, 981–986. [Google Scholar] [CrossRef]
- Dieterich, S.; Bieligk, U.; Beulich, K.; Hasenfuss, G.; Prestle, J. Gene Expression of Antioxidative Enzymes in the Human Heart: Increased Expression of Catalase in the End-Stage Failing Heart. Circulation 2000, 101, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7140. [Google Scholar] [CrossRef]
- Wallin, B.; Rosengren, B.; Shertzer, H.G.; Camejo, G. Lipoprotein Oxidation and Measurement of Thiobarbituric Acid Reacting Substances Formation in a Single Microtiter Plate: Its Use for Evaluation of Antioxidants. Anal. Biochem. 1993, 208, 10–15. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, L.; Rabdall, R.J. Protein Measurement with the Folin Phenol Reagent. Anal. Biochem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dias, F.C.R.; Gomes, M.L.M.; Melo, F.C.S.A.; Menezes, T.P.; Martins, A.L.P.; Cupertino, M.C.; Otoni, W.C.; MAtta, S.L.P. Pfaffia Glomerata Hydroalcoholic Extract Stimulates Penile Tissue in Adult Swiss Mice Pfaffia Glomerata Hydroalcoholic Extract Stimulates Penile Tissue in Adult Swiss Mice. J. Ethnopharmacol. 2020, 261, 113182. [Google Scholar] [CrossRef]
- Kazerouni, F.; Amirrasouli, H. Performance Characteristics of Three Automated Immunoassays for Thyroid Hormones. Casp. J. Intern. Med. 2012, 3, 400. [Google Scholar]
- Dornelles, J. Avaliação da Frequência de Coletas de Sêmen em Machos Reprodutores Suínos No Início da vida Reprodutiva. 2019. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/vtt-212762 (accessed on 8 February 2025).
- Wagner, M.S.; Wajner, S.M.; Maia, A.L. The Role of Thyroid Hormone in Testicular Development and Function. J. Endocrinol. 2008, 199, 351–365. [Google Scholar] [CrossRef]
- Thomas, J.; D, V.; Kalra, P. A Comparative Study of Sensory-Motor Coordination, Executive Function, and Testosterone Levels in Hypothyroid and Euthyroid Males. Natl. J. Physiol. Pharm. Pharmacol. 2017, 1, 391–395. [Google Scholar] [CrossRef]
- Foster, R. Sistema Reprodutivo Do Macho. In Bases da Patologia em Veterinária; Editorial Elsevier: Rio de Janeiro, Brazil, 2009; pp. 1317–1348. [Google Scholar]
- Araújo, M.S. Avaliação Do Cruzamento Racial Sobre a Estrutura Testicular e Espermatogênese em Ovinos da Raça Santa Inês e Mestiços de Santa Inês e Dorper. 2016. Available online: http://repositorio.ufpi.br:8080/xmlui/handle/123456789/1279 (accessed on 8 February 2025).
- Aly, H.A.A.; Hassan, M.H. Potential Testicular Toxicity of Gentamicin in Adult Rats. Biochem. Biophys. Res. Commun. 2018, 497, 362–367. [Google Scholar] [CrossRef]
- Hfaiedh, M.; Brahmi, D.; Zourgui, L. Protective Role of Cactus Cladodes Extract on Sodium Dichromate-Induced Testicular Injury and Oxidative Stress in Rats. Biol. Trace Elem. Res. 2014, 159, 304–311. [Google Scholar] [CrossRef]
- Turner, T.T.; Lysiak, J.J. Oxidative Stress: A Common Factor in Testicular Dysfunction. J Androl 2008, 29, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, K.B.F.; Costa, N.M.B.; Alfenas, R.C.G.; Paula, S.O.; Bressan, J. Oxidative Stress: Concept, Implications and Modulating Factors. Rev. Nutr. 2010, 23, 629–643. [Google Scholar]
- Ding, X.; Yu, L.; Ge, C.; Ma, H. Protective Effect of DHEA on Hydrogen Peroxide-Induced Oxidative Damage and Apoptosis in Primary Rat Leydig Cells. Oncotarget 2017, 8, 16158–16169. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Grundy, S.M. Influence of Antioxidant Vitamins on LDL Oxidation. Ann. N. Y. Acad. Sci. 1992, 669, 237–247. [Google Scholar] [CrossRef]
- Luczaj, W. Skrzydlewska E Dna Damage Caused by Lipid Peroxidation Products. Cell. Mol. Biol. Lett. 2003, 8, 91–413. [Google Scholar]
- Shakeel, M.; Yoon, M. Heat Stress and Stallion Fertility. J. Anim. Sci. Technol. 2023, 65, 683–697. [Google Scholar] [CrossRef]
- Dovolou, E.; Giannoulis, T.; Nanas, I.; Amiridis, G.S. Heat Stress: A Serious Disruptor of the Reproductive Physiology of Dairy Cows. Animals 2023, 13, 1846. [Google Scholar] [CrossRef]
- Borges, J.O.; Silva, A.P.V.; Carvalho, R.A. Conforto Térmico de Ovinos Da Raça Santa Inês Confinados Com Dietas. Bol. Da Indústria Anim. Nova Odessa 2018, 75, 1–7. [Google Scholar]
- Celeghini, E.C.C.; Alves, M.B.R.; de Oliveira, B.M.M.; Batissaco, L.; Costa, S.C.; Pinto, V.H.G.G.; Gonzaga, V.H.G.; Nogueira, V.J.M.; Garcia-Oliveros, L.N.; dos Santos Almeida, F. Degeneração Testicular: Visão Científica. 2a Reunião Da Associação Brasileira de Andrologia Animal; Embrapa Pantanal: Corumba, Brazil, 2017. [Google Scholar]
- Boe-Hansen, G.B.; Rêgo, J.P.A.; Satake, N.; Venus, B.; Sadowski, P.; Nouwens, A.; Li, Y.; McGowan, M. Effects of Increased Scrotal Temperature on Semen Quality and Seminal Plasma Proteins in Brahman Bulls. Mol. Reprod. Dev. 2020, 87, 574–597. [Google Scholar] [CrossRef]
| Ingredients (g/kg) | ||||||
|---|---|---|---|---|---|---|
| Itens | Elephant C. Hay | Small Palm | IPA-Sertânia Palm | MEE Palm | Soybean Meal | Corn Meal |
| Dry matter | 931.10 | 118.00 | 144.30 | 117.30 | 899.90 | 885.50 |
| Organic matter | 888.60 | 890.10 | 893.60 | 889.30 | 913.50 | 985.70 |
| Mineral matter | 111.40 | 109.90 | 106.40 | 110.70 | 86.50 | 14.30 |
| Crude protein | 57.30 | 56.90 | 57.50 | 59.70 | 533.80 | 86.10 |
| Ether extract | 20.90 | 25.90 | 28.20 | 30.70 | 18.10 | 49.30 |
| Neutral detergent fiber | 767.60 | 289.10 | 291.90 | 285.80 | 232.50 | 175.20 |
| Neutral detergent fiber | 724.60 | 259.10 | 252.90 | 243.20 | 148.20 | 156.80 |
| Acid detergent fiber | 469.00 | 142.10 | 109.60 | 125.50 | 24.90 | 82.00 |
| Hemicellulose | 265.60 | 117.00 | 143.40 | 117.80 | 66.20 | 131.90 |
| Total carbohydrates | 810.50 | 807.20 | 807.90 | 798.90 | 361.60 | 850.40 |
| Non-fibrous carbohydrates | 75.90 | 548.20 | 555.00 | 555.70 | 213.40 | 693.50 |
| Hydrocyanic acid | - | - | 0.05762 | 0.05347 | - | - |
| Total oxalates | 0.9671 | 1.7752 | 2.0774 | 2.5706 | 1.4009 | 0.9152 |
| Groups | ||||
|---|---|---|---|---|
| Ingredients (g/kg) | Control | IPA | SMALL | MEE |
| Elephant hay | 70.99 | 25.03 | 26.31 | 23.21 |
| IPA-Sertânea Palm | 0.00 | 54.00 | 0.00 | 0.00 |
| Palm IPA-Sertania | 0.00 | 0.00 | 51.65 | 0.00 |
| Mexican Elephant Ear Palm | 0.00 | 0.00 | 0.00 | 57.33 |
| Soybean meal | 14.50 | 19.08 | 20.05 | 17.7 |
| Corn | 12.85 | 0.00 | 0.00 | 0.00 |
| Urea | 0.62 | 0.56 | 0.44 | 0.00 |
| Sheep mineral salt 1 | 0.86 | 0.95 | 1.00 | 0.88 |
| Dicalcium phosphate | 0.14 | 0.33 | 0.50 | 0.79 |
| Ammonium sulphate | 0.05 | 0.05 | 0.05 | 0.09 |
| Total | 100.01 | 100 | 100 | 100 |
| Control | MEE | SMALL | IPA | P | |
|---|---|---|---|---|---|
| Body weight | 24.59 ± 3.20 a | 34.89 ± 3.36 b | 33.33 ± 1.58 b | 34.64 ± 4.20 b | 0.001 |
| Gonad weight | 0.076 ± 0.045 a | 0.19 ± 0.06 b | 0.180 ± 0.101 b | 0.148 ± 0.079 a | 0.04 |
| Gonadosomatic index | 0.304 ± 0.183 a | 0.53 ± 0.14 a | 0.535 ± 0.293 a | 0.416 ± 0.176 a | 0.1 |
| Tubular Diameter (µm) | 189.0 ± 2.6 a | 199.9 ± 4.5 b | 197.7 ± 3.9 b | 176.0 ± 3.8 c | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, G.W.C.; Dias, F.C.R.; do Carmo Cupertino, M.; do Nascimento Silva, A.A.; Batista, Â.M.V.; de Oliveira Filho, E.F.; de Carvalho, F.F.R.; Figueiredo Porto, A.L.; Júnior, V.A.d.S. Activity of Different Types of Cactus Forage on Testicular Function and Morphology of Sheep Subjected to Environmental Heat Stress. Vet. Sci. 2025, 12, 492. https://doi.org/10.3390/vetsci12050492
da Silva GWC, Dias FCR, do Carmo Cupertino M, do Nascimento Silva AA, Batista ÂMV, de Oliveira Filho EF, de Carvalho FFR, Figueiredo Porto AL, Júnior VAdS. Activity of Different Types of Cactus Forage on Testicular Function and Morphology of Sheep Subjected to Environmental Heat Stress. Veterinary Sciences. 2025; 12(5):492. https://doi.org/10.3390/vetsci12050492
Chicago/Turabian Styleda Silva, Giselle Woolley Cardoso, Fernanda Carolina Ribeiro Dias, Marli do Carmo Cupertino, Alluanan Adelson do Nascimento Silva, Ângela Maria Vieira Batista, Emanuel Felipe de Oliveira Filho, Francisco Fernando Ramos de Carvalho, Ana Lúcia Figueiredo Porto, and Valdemiro Amaro da Silva Júnior. 2025. "Activity of Different Types of Cactus Forage on Testicular Function and Morphology of Sheep Subjected to Environmental Heat Stress" Veterinary Sciences 12, no. 5: 492. https://doi.org/10.3390/vetsci12050492
APA Styleda Silva, G. W. C., Dias, F. C. R., do Carmo Cupertino, M., do Nascimento Silva, A. A., Batista, Â. M. V., de Oliveira Filho, E. F., de Carvalho, F. F. R., Figueiredo Porto, A. L., & Júnior, V. A. d. S. (2025). Activity of Different Types of Cactus Forage on Testicular Function and Morphology of Sheep Subjected to Environmental Heat Stress. Veterinary Sciences, 12(5), 492. https://doi.org/10.3390/vetsci12050492






