Codonopsis pilosula Polysaccharides Exert Antiviral Effect Through Activating Immune Function in a Macrophage Model of Bovine Viral Diarrhea Virus Infection
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Virus, and Tested Compound
2.2. Cell Viability Assay
2.3. Antiviral Effect Assay
2.4. Real-Time Quantitative PCR (RT–qPCR)
2.5. Immunofluorescence Assay
2.6. Determination of Cell Phagocytic Function
2.7. Determination of the Cellular Antigen-Presenting Function
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Cell Apoptosis Assay
2.10. Statistical Analyses
3. Results
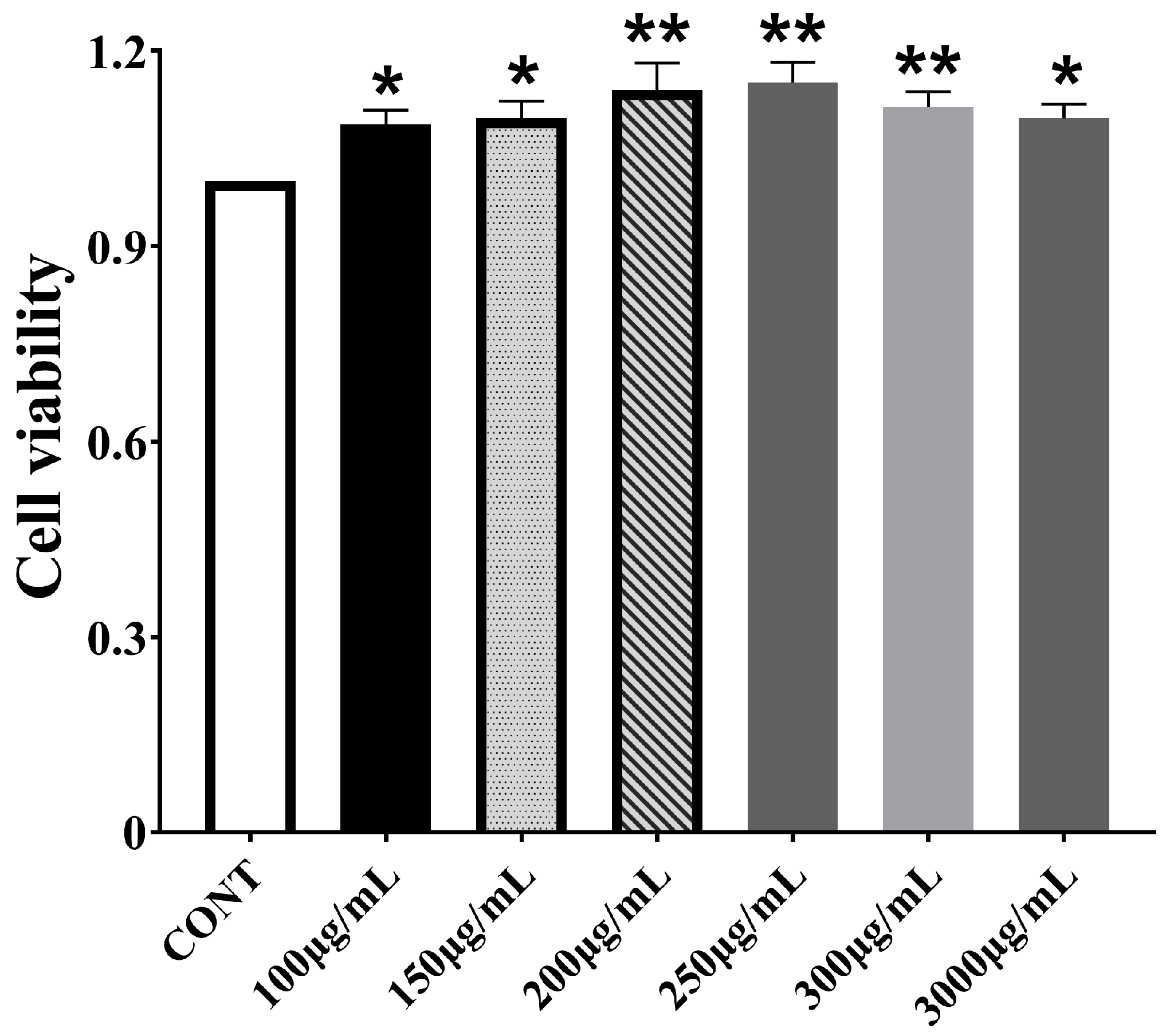
3.1. Effect of CPPs on Bomac Cells Viability
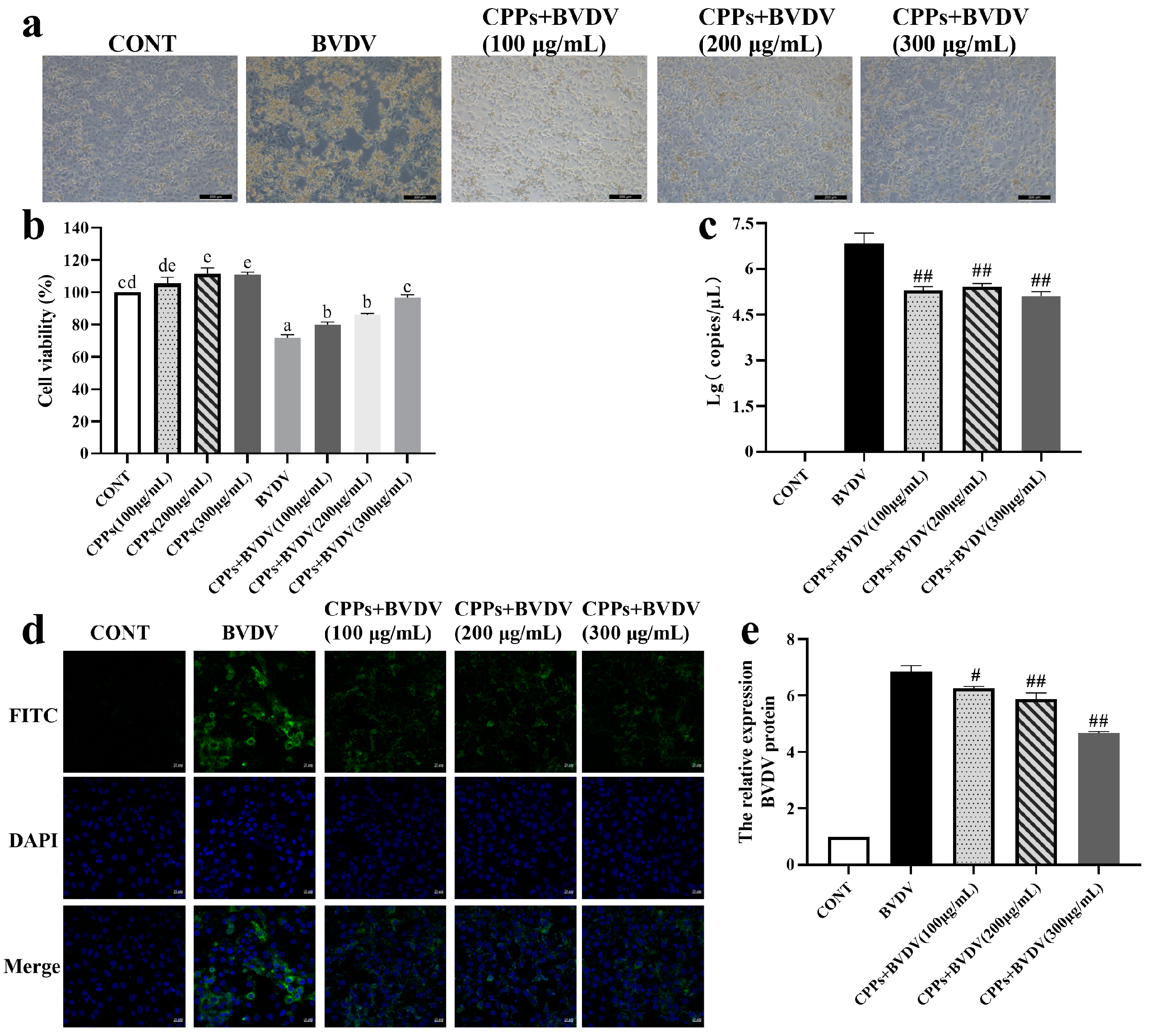
3.2. Blocking Effect of CPPs on BVDV Adsorption
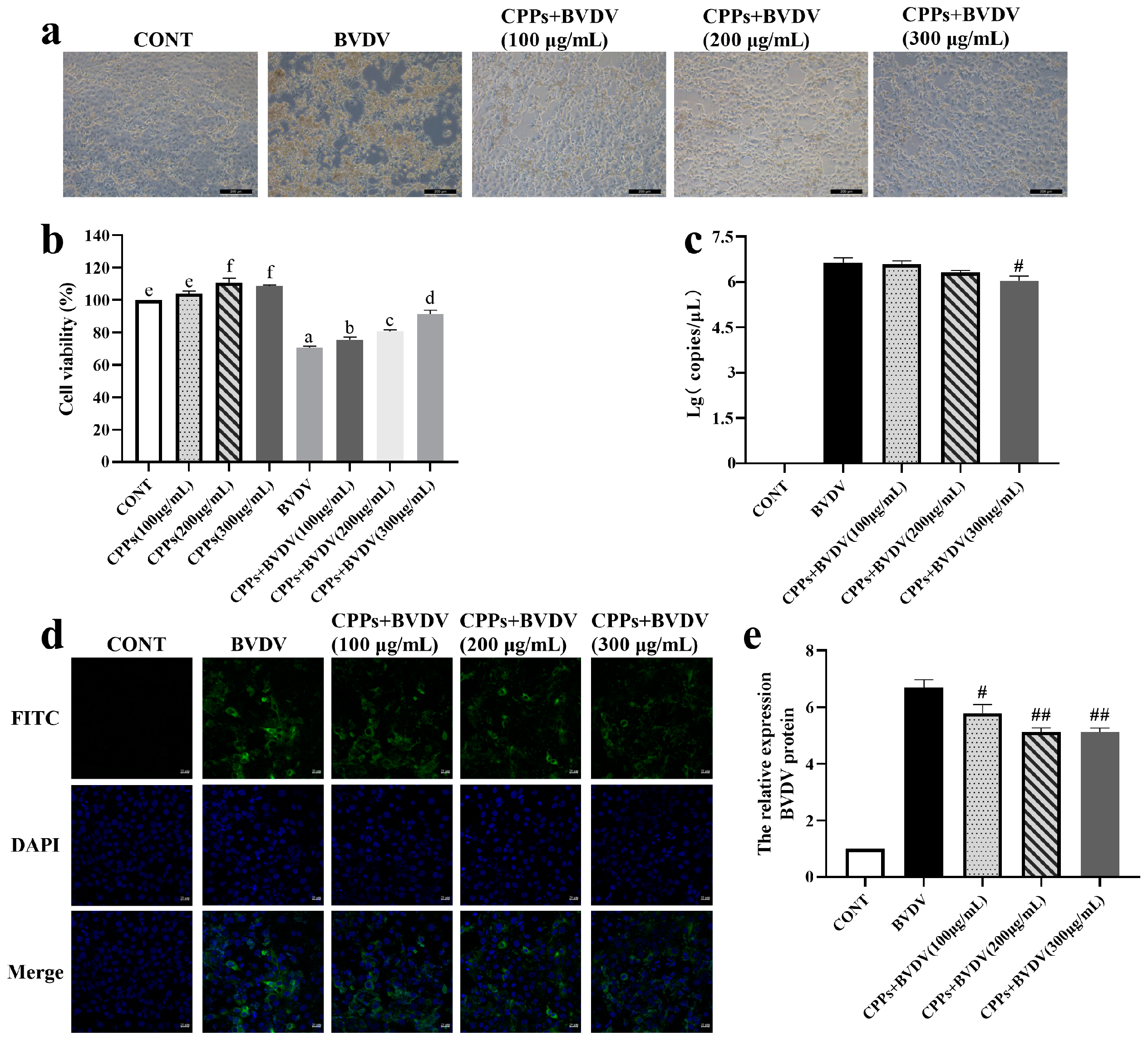
3.3. CPPs May Exert the Antiviral Effect by Directly Inactivating Virions
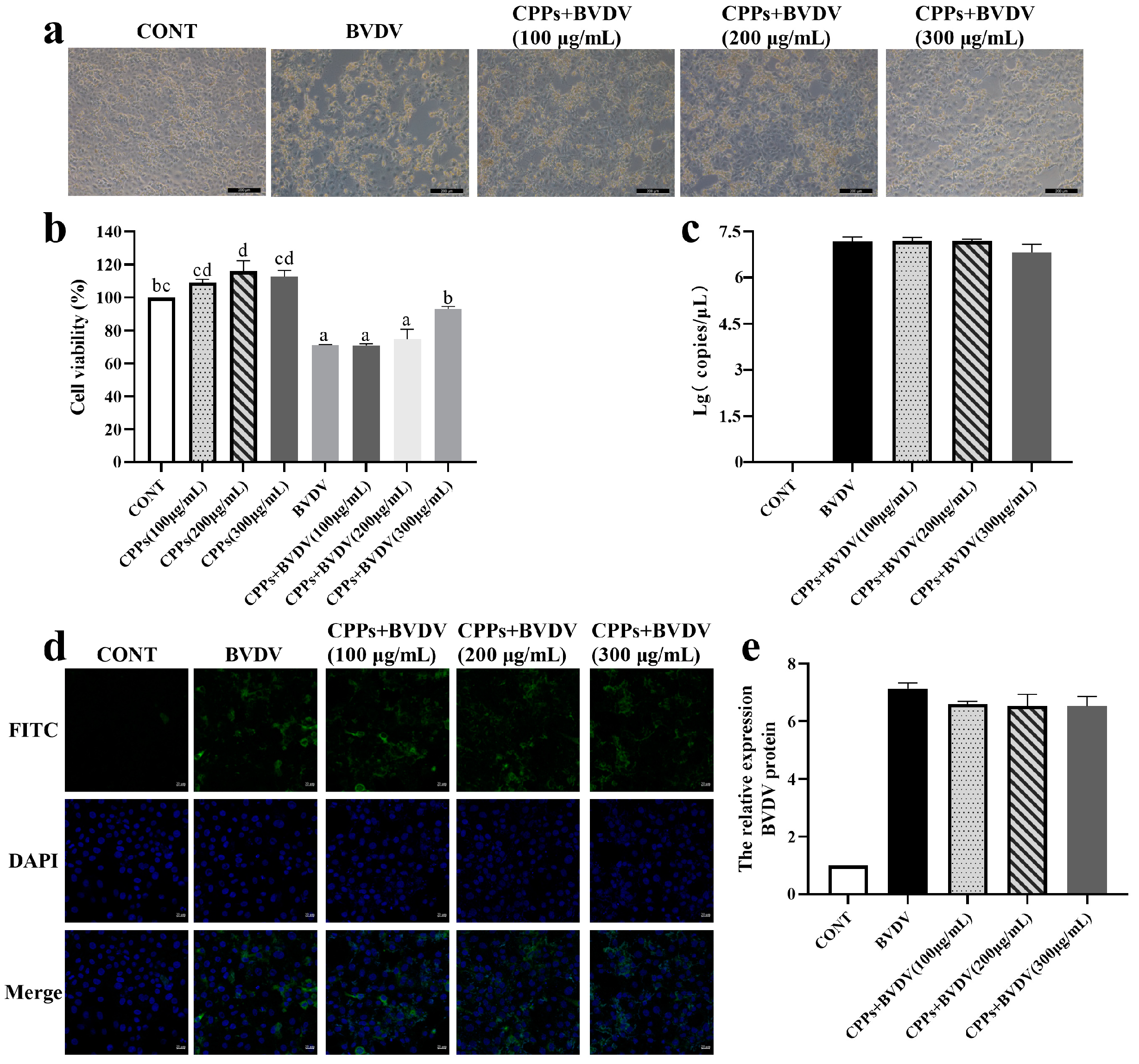
3.4. Blocking Effect of CPPs on BVDV Replication
3.5. Effects of CPPs on Cellular Phagocytosis Induced by BVDV in BoMac Cells
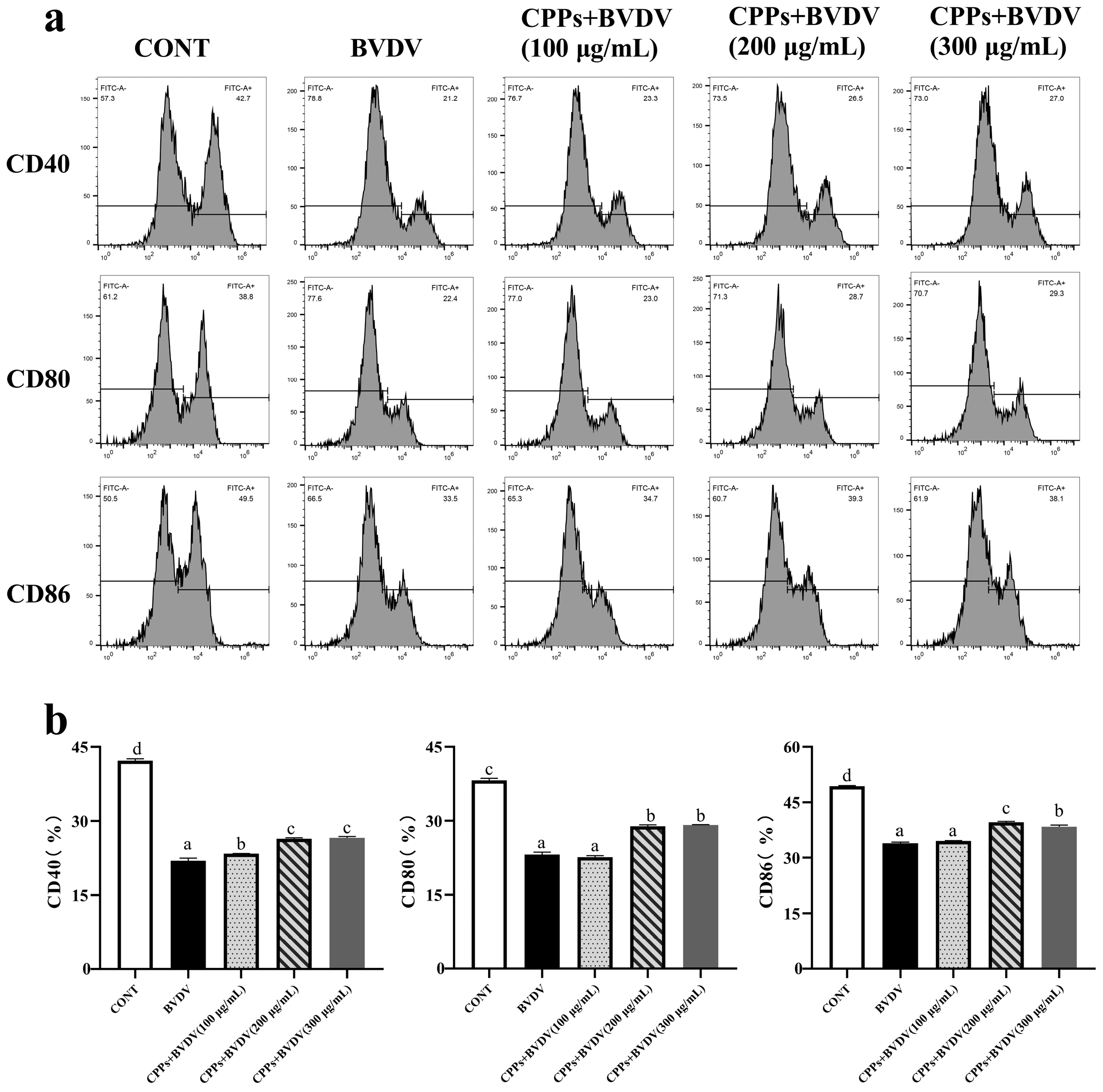
3.6. Effects of CPPs on Antigen-Presentating Function Induced by BVDV in BoMac Cells
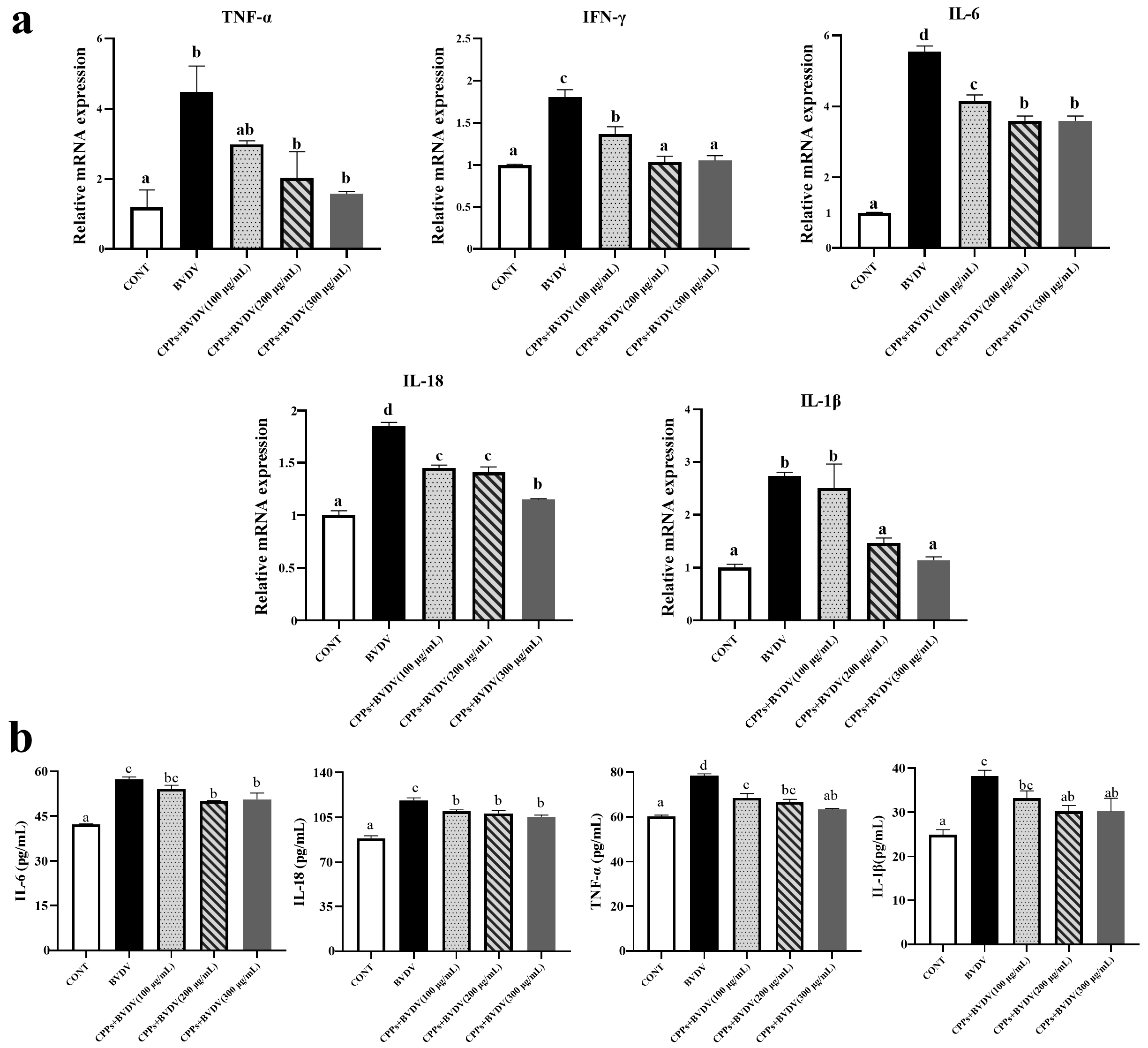
3.7. Effects of CPPs on the Expression of Inflammatory Factors Induced by BVDV in BoMac Cells
3.8. Effects of CPPs on Cell Apoptosis Induced by BVDV in BoMac Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BVD | Bovine viral diarrhea |
| BVDV | Bovine viral diarrhea virus |
| CPPs | Codonopsis pilosula polysaccharides |
| BoMac | Bovine macrophage |
| CP | Codonopsis pilosula |
| MDBK | Madin-Darby bovine kidney |
| FITC | Fluorescein isothiocyanate |
| MOI | Multiplicity of infection |
| OD | Optical density |
| RT-qPCR | Real-time quantitative PCR |
| ELISA | Enzyme-linked immunosorbent assay |
| E. coli | Escherichia coli |
| SEMs | Standard errors of means |
References
- Xie, M.Y.; Chen, K.J.; Liu, P.P.; Wang, X.D.; Chen, Y.X.; Shang, H.W.; Hao, Y.R.; Gao, P.Y.; He, H.L.; Xu, X.J. Seroprevalence of five diarrhea-related pathogens in bovine herds of scattered households in Inner Mongolia, China between 2019 and 2022. PeerJ 2023, 11, e16013. [Google Scholar] [CrossRef] [PubMed]
- Chicoski, L.M.; Fritzen, J.T.T.; Lorenzetti, E.; da Costa, A.R.; Moro, E.; de Carvalho, E.R.; Alfieri, A.F.; Alfieri, A.A. Serological profile of respiratory viruses in unvaccinated steers upon their arrival at Brazilian feedlot facilities. Braz. J. Microbiol. 2023, 54, 3237–3244. [Google Scholar] [CrossRef]
- Yin, J.H.; Zhang, J.L.; Liu, Y.; Duan, C.; Wang, J.F. Bergamottin inhibits bovine viral diarrhea virus replication by suppressing ROS-mediated endoplasmic reticulum stress and apoptosis. Viruses 2024, 16, 1287. [Google Scholar] [CrossRef]
- Li, Z.J.; Zhang, Y.; Zhao, B.; Xue, Q.H.; Wang, C.J.; Wan, S.Y.; Wang, J.Y.; Chen, X.W.; Qi, X.F. Non-cytopathic bovine viral diarrhea virus (BVDV) inhibits innate immune responses via induction of mitophagy. Vet. Res. 2024, 55, 27. [Google Scholar] [CrossRef] [PubMed]
- Fernández, G.A.; Castro, E.F.; Rosas, R.A.; Fidalgo, D.M.; Adler, N.S.; Battini, L.; España de Marco, M.J.; Fabiani, M.; Bruno, A.M.; Bollini, M.; et al. Design and optimization of quinazoline derivatives: New non-nucleoside inhibitors of bovine viral diarrhea virus. Front. Chem. 2020, 8, 590235. [Google Scholar] [CrossRef]
- Pang, F.; Long, Q.Q.; Wei, M. Immune evasion strategies of bovine viral diarrhea virus. Front. Cell Infect. Microbiol. 2023, 13, 1282526. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, K.; Kaushik, R.S.; Chase, C. The involvement of neutrophil in the immune dysfunction associated with BVDV infection. Pathogens 2023, 12, 737. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, C.H.; Chen, N.N.; Li, Y.; Fan, C.L.; Zhao, S.Q.; Bai, T.T.; Zhao, Z.B.; Chen, J.W.; Su, S.Y.; et al. PD-1 blockade restores the proliferation of peripheral blood lymphocyte and inhibits lymphocyte apoptosis in a BALB/c mouse model of CP BVDV acute infection. Front. Immunol. 2021, 12, 727254. [Google Scholar] [CrossRef]
- Castillo, J.A.; Urcuqui-Inchima, S. Mechanisms of monocyte cell death triggered by dengue virus infection. Apoptosis 2018, 23, 576–586. [Google Scholar] [CrossRef]
- Cheng, Z.R.; Brown, L.E.; Wathes, D.C. Bovine viral diarrhoea virus infection disrupts uterine interferon stimulated gene regulatory pathways during pregnancy recognition in cows. Viruses 2019, 12, 1. [Google Scholar] [CrossRef]
- Ma, K.; Yi, X.; Yang, S.T.; Zhu, H.; Liu, T.Y.; Jia, S.S.; Fan, J.H.; Hu, D.J.; LV, G.P.; Huang, H. Isolation, purification, and structural characterization of polysaccharides from Codonopsis pilosula and its therapeutic effects on non-alcoholic fatty liver disease in vitro and in vivo. Int. J. Biol. Macromol. 2024, 265, 130988. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; An, J.Y.; Jiang, N.; Yang, K.; Guan, C.H.; Zhao, N.; Cheng, J.G.; Fu, S.B.; Ma, C.X.; Ma, X.N.; et al. Codonopsis pilosula polysaccharides promote osteogenic differentiation and inhibit lipogenic differentiation of rat bone marrow stem cells by activating β-catenin. Chem. Biol. Interact. 2023, 385, 110721. [Google Scholar] [CrossRef]
- Li, Y.T.; Guo, X.; Tian, Y.H.; Zhang, T.L.; Luo, Z.W.; Liu, X.J.; Qian, C.; Dai, J.X.; Duan, Y.X. Methylation in combination with temperature programming enables rapid identification of polysaccharides by ambient micro-fabrication glow discharge plasma (MFGDP) desorption ionization mass spectrometry. Talanta 2020, 218, 121156. [Google Scholar] [CrossRef]
- Liu, M.Q.; Zhang, G.Q.; Zhou, K.X.; Wen, J.L.; Zheng, F.X.; Sun, L.L.; Ren, X.L. Structural characterization, antioxidant activity, and the effects of Codonopsis pilosula polysaccharides on the solubility and stability of flavonoids. J. Pharmaceut. Biomed. 2023, 229, 115368. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.G.; Zhang, S.Y.; Gu, B.C.; Cao, J.S.; Mao, W.; Yao, Y.; Zhao, J.M.; Ren, P.P.; Zhang, K.; Liu, B. Codonopsis pilosula polysaccharides attenuate Escherichia coli-induced acute lung injury in mice. Food Funct. 2022, 13, 7999–8011. [Google Scholar] [CrossRef]
- Liu, F.; Geng, C.; Qu, Y.K.; Cheng, B.X.; Zhang, Y.; Wang, A.M.; Zahng, J.H.; Liu, B.; Tian, H.Y.; Yang, W.P.; et al. The feeding of dietary Codonopsis pilosula polysaccharide enhances the immune responses, the expression of immune-related genes and the growth performance of red swamp crayfish (Procambarus clarkii). Fish Shellfish. Immunol. 2020, 103, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Ming, K.; He, M.; Su, L.L.; Du, H.X.; Wang, D.Y.; Wu, Y.; Liu, J.G. The inhibitory effect of phosphorylated Codonopsis pilosula polysaccharide on autophagosomes formation contributes to the inhibition of duck hepatitis A virus replication. Poult. Sci. 2020, 99, 2146–2156. [Google Scholar] [CrossRef]
- Wang, C.L.; Zhang, Y.C.; Xue, H.Y.; Yang, M.J.; Leng, F.F.; Wang, Y.G. Extraction kinetic model of polysaccharide from Codonopsis pilosula and the application of polysaccharide in wound healing. Biomed. Mater. 2022, 17, 025012. [Google Scholar] [CrossRef]
- Ming, K.; Chen, Y.; Yao, F.K.; Shi, J.T.; Yang, J.J.; Du, H.X.; Wang, X.Y.; Wang, Y.X.; Liu, J.G. Phosphorylated Codonopsis pilosula polysaccharide could inhibit the virulence of duck hepatitis A virus compared with Codonopsis pilosula polysaccharide. Int. J. Biol. Macromol. 2017, 94, 28–35. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Li, E.; Fan, Q.; Wang, D.Y.; Zhang, C.S.; Li, P.; Li, X.P.; Chen, X.Y.; Qiu, Z.Z.; et al. Solomonseal polysaccharide and sulfated Codonopsis pilosula polysaccharide synergistically resist Newcastle disease virus. PLoS ONE 2015, 10, e0117916. [Google Scholar] [CrossRef]
- Li, N.; Xiong, Y.X.; Ye, F.; Jin, B.; Wu, J.J.; Han, M.M.; Liu, T.; Fan, Y.K.; Li, C.Y.; Liu, J.S.; et al. Isolation, purification, and structural characterization of polysaccharides from Codonopsis pilosula and their anti-tumor bioactivity by immunomodulation. Pharmaceuticals 2023, 16, 895. [Google Scholar] [CrossRef]
- Fu, Y.P.; Feng, B.; Zhu, Z.K.; Feng, X.; Chen, S.F.; Li, L.X.; Yin, Z.Q.; Huang, C.; Chen, X.F.; Zhang, B.Z.; et al. The polysaccharides from Codonopsis pilosula modulates the immunity and intestinal microbiota of cyclophosphamide-treated immunosuppressed mice. Molecules 2018, 23, 1801. [Google Scholar] [CrossRef] [PubMed]
- Kadiroğlu, B.; Yeşilbağ, K. Optimum processing conditions for a trivalent-inactivated bovine viral diarrhea virus (BVDV) vaccine using field strains and immunogenicity of candidate formulations with different adjuvants. Vet. Res. Commun. 2024, 49, 37. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.Q.; Shu, Q.L. Modulating butyric acid-producing bacterial community abundance and structure in the intestine of immunocompromised mice with neutral polysaccharides extracted from Codonopsis pilosula. Int. J. Biol. Macromol. 2024, 278, 134959. [Google Scholar] [CrossRef]
- Feng, H.P.; Zhang, K.; Guo, Z.T.; Liu, Q.; Wang, L.; Wang, X.Z.; Qiu, Z.Y.; Wang, G.B.; Zhang, J.Y.; Li, J.X. Antiviral activity and underlying mechanisms of baicalin against avian infectious bronchitis virus in vitro. Avian Pathol. 2022, 51, 574–589. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.C.; Xu, Y.; Zhang, W.; Pan, H.C.; Zhou, Z.Y.; Yao, J.T.; Guo, T.T.; Ni, B.; Gao, S. Hippophae rhamnoides polysaccharides dampen pseudorabies virus infection through downregulating adsorption, entry and oxidative stress. Int. J. Biol. Macromol. 2022, 207, 454–463. [Google Scholar] [CrossRef]
- Huan, C.C.; Yao, J.T.; Wang, X.T.; Zhang, H.Y.; Wang, X.B.; Jiang, L.Y.; Gao, S. Rehmmannia glutinosa polysaccharide exerts antiviral activity against pseudorabies virus and antioxidant activity. Int. J. Biol. Macromol. 2024, 274, 133455. [Google Scholar] [CrossRef]
- He, X.R.; Fang, J.C.; Guo, Q.; Wang, M.; Li, Y.S.; Meng, Y.B.; Huang, L.H. Advances in antiviral polysaccharides derived from edible and medicinal plants and mushrooms. Carbohydr. Polym. 2020, 229, 115548. [Google Scholar] [CrossRef]
- Casas-Rodríguez, A.; Cebadero-Dominguez, Ó.; Puerto, M.; Cameán, A.M.; Jos, A. Immunomodulatory effects of cylindrospermopsin in human T cells and monocytes. Toxins 2023, 15, 301. [Google Scholar] [CrossRef]
- Harker, J.A.; Greene, T.T.; Barnett, B.E.; Bao, P.; Dolgoter, A.; Zuniga, E.I. IL-6 and IL-27 play both distinct and redundant roles in regulating CD4 T-cell responses during chronic viral infection. Front. Immunol. 2023, 14, 1221562. [Google Scholar] [CrossRef]
- Joffe, A.M.; Bakalar, M.H.; Fletcher, D.A. Macrophage phagocytosis assay with reconstituted target particles. Nat. Protoc. 2020, 15, 2230–2246. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, J.L. Macrophages: Their Untold Story in T Cell Activation and Function. Int. Rev. Cell Mol. Biol. 2019, 342, 73–93. [Google Scholar] [CrossRef]
- David, C.; Verney, C.; Si-Tahar, M.; Guillon, A. The deadly dance of alveolar macrophages and influenza virus. Eur. Respir. Rev. 2024, 33, 240132. [Google Scholar] [CrossRef] [PubMed]
- Luft, O.; Khattar, R.; Farrokhi, K.; Ferri, D.; Yavorska, N.; Zhang, J.; Sadozai, H.; Adeyi, O.; Chruscinski, A.; Levy, G.A.; et al. Inhibition of the Fibrinogen-Like Protein 2: FcγRIIB/RIII immunosuppressive pathway enhances antiviral T-cell and B-cell responses leading to clearance of lymphocytic choriomeningitis virus clone 13. Immunology 2018, 154, 476–489. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, W.M.; Fu, X.; Hou, J.; Wang, Y.; Shi, F.F.; Hu, S.H. Molecular mechanisms underlying macrophage immunomodulatory activity of Rubus chingii Hu polysaccharides. Int. J. Biol. Macromol. 2021, 185, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.L.; Wang, X.L.; Jiang, S.; Li, Y.; Yao, X.; Wang, M.; Zhao, J.H.; Sun, X.B.; Jiang, X.X.; Zhong, L.H.; et al. Identification of key proteins of cytopathic biotype bovine viral diarrhoea virus involved in activating NF-κB pathway in BVDV-induced inflammatory response. Virulence 2022, 13, 1884–1899. [Google Scholar] [CrossRef]
- Wu, J.M.; Qiao, Y.Y.; Jin, W.; Jia, F.Y.; Wang, Z.M.; Li, L.; Cheng, F.; Zhao, W.F.; Cheng, Y.F.; Zhao, L.; et al. Metabolomics and 16S rDNA sequencing of intestinal flora reveal the regulation of Sparassis latifolia polysaccharides on splenic immune function in lead-exposed mice. Int. J. Biol. Macromol. 2024, 280, 136084. [Google Scholar] [CrossRef]
- Da Silva, A.A.S.; de Oliveira, S.A.; Battistone, M.A.; Hinton, B.T.; Cerri, P.S.; Sasso-Cerri, E. hACE2 upregulation and participation of macrophages and clear cells in the immune response of epididymis to SARS-CoV-2 in K18-hACE2 mice. Andrology 2024. Advance online publication. [Google Scholar] [CrossRef]
- Yang, J.; Tan, H.L.; Gu, L.Y.; Song, M.L.; Wu, Y.Y.; Peng, J.B.; Lan, Z.B.; Wei, Y.Y.; Hu, T.J. Sophora subprosrate polysaccharide inhibited cytokine/chemokine secretion via suppression of histone acetylation modification and NF-κb activation in PCV2 infected swine alveolar macrophage. Int. J. Biol. Macromol. 2017, 104, 900–908. [Google Scholar] [CrossRef]
- Chen, Q.; Pan, X.H.; Wang, Q.H.; Bai, J.J.; Jiang, L.Q.; Li, Y.H.; Zhao, L.; Xie, X.D.; Qin, Y.; Hu, T.J. Sophora subprostrate polysaccharide targets LncRNA MSTRG.5823.1 to suppress PCV2-mediated immunosuppression via TNF/NF-κB signaling. Int. Immunopharmacol. 2024, 139, 112701. [Google Scholar] [CrossRef]
- Krüger, M. Remove, refine, reduce: Cell death in biological systems. Int. J. Mol. Sci. 2023, 24, 7028. [Google Scholar] [CrossRef]
- Lee, E.; Song, C.H.; Bae, S.J.; Ha, K.T.; Karki, R. Regulated cell death pathways and their roles in homeostasis, infection, inflammation, and tumorigenesis. Exp. Mol. Med. 2023, 55, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhou, L.; Wu, J.J.; Weng, W.L.; Wang, H.; Ye, M.M.; Qu, Y.J.; Hao, Y.X.; Zhang, Y.N.; Ge, X.N.; et al. Riding apoptotic bodies for cell-cell transmission by African swine fever virus. Proc. Natl. Acad. Sci. USA 2023, 120, e2309506120. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.Y.; Li, X.L.; Xu, G.R.; Chen, L.M.; Zhao, L.Y. Quercetin attenuates the symptoms of osteoarthritis in vitro and in vivo by suppressing ferroptosis via activation of AMPK/Nrf2/Gpx4 signaling. Mol. Med. Rep. 2025, 31, 60. [Google Scholar] [CrossRef] [PubMed]
- Kokoulin, K.S.; Kuzmich, A.S.; Filshtein, A.P.; Prassolov, V.S.; Romanenko, L.A. Capsular polysaccharide from the marine bacterium Cobetia marina induces apoptosis via both caspase-dependent and mitochondria-mediated pathways in HL-60 cells. Carbohydr. Polym. 2025, 347, 122791. [Google Scholar] [CrossRef]
- Yang, L.; Song, S.Y.; Li, X.L.; Wang, J.Q.; Bao, Y.N.; Wang, X.X.; Lian, L.W.; Liu, X.B.; Ma, W. Neuroprotective effect of Codonopsis pilosula polysaccharide on Aβ25-35-induced damage in PC12 cells via the p38MAPK signaling pathways. Pharmaceuticals 2024, 17, 1231. [Google Scholar] [CrossRef]


| Target Genes | Primer Sequence (5′-3′) | Product Length (bp) | Serial Number |
|---|---|---|---|
| TNF-α | F: AGGGGTCATGTGTGTGGAGAGC | 99 | NM_001166486.1 |
| R: GTGTGCAGGTGCCGGTTCAG | |||
| IFN-γ | F: GAGCCTGTGAGCGTGCTTTT | 163 | NM_001077840.1 |
| R: TGGTGCTGAGGATGACATGG | |||
| IL-6 | F: GCCTTCACTCCATTCGCTGTC | 109 | NM_173923.2 |
| R: TGCCTGGGGTGGTGTCATT | |||
| IL-18 | F: TCATTAACCAGGGAAATCAACC | 84 | NM_174091.2 |
| R: TGATAAATATGGTCTGGGGTGC | |||
| IL-1β | F: CCTCGGTTCCATGGGAGATG | 119 | NM_174093.1 |
| R: AGGCACTGTTCCTCAGCTTC | |||
| Bim | F: AGCCCGGCACCCATGAGTTGT | 215 | XM_010809717.4 |
| R: GCCTGGTGACGCACGAAGACCCT | |||
| Bcl-xL | F: CGACGGGCATTCAGCGACCT | 122 | XM_005214498.5 |
| R: GCCACAATGCGACCCCAGTTCACC | |||
| Caspase-3 | F: CTGGACCCTCATCCATCCTT | 168 | NM_001077840.1 |
| R: GGCACCCTGGTTCTTTCATTT | |||
| β-actin | F: GCGGCATTCACGAAACTACCTT | 268 | NM_173979.3 |
| R: TCCTGCTTGCTGATCCACATCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Wang, L.; Zhang, J.; Feng, H.; Chen, X.; Wang, J.; Shi, M.; Zhang, K.; Li, J. Codonopsis pilosula Polysaccharides Exert Antiviral Effect Through Activating Immune Function in a Macrophage Model of Bovine Viral Diarrhea Virus Infection. Vet. Sci. 2025, 12, 415. https://doi.org/10.3390/vetsci12050415
Feng X, Wang L, Zhang J, Feng H, Chen X, Wang J, Shi M, Zhang K, Li J. Codonopsis pilosula Polysaccharides Exert Antiviral Effect Through Activating Immune Function in a Macrophage Model of Bovine Viral Diarrhea Virus Infection. Veterinary Sciences. 2025; 12(5):415. https://doi.org/10.3390/vetsci12050415
Chicago/Turabian StyleFeng, Xiaowei, Lei Wang, Jingyan Zhang, Haipeng Feng, Xiaoliang Chen, Junyan Wang, Mingxian Shi, Kang Zhang, and Jianxi Li. 2025. "Codonopsis pilosula Polysaccharides Exert Antiviral Effect Through Activating Immune Function in a Macrophage Model of Bovine Viral Diarrhea Virus Infection" Veterinary Sciences 12, no. 5: 415. https://doi.org/10.3390/vetsci12050415
APA StyleFeng, X., Wang, L., Zhang, J., Feng, H., Chen, X., Wang, J., Shi, M., Zhang, K., & Li, J. (2025). Codonopsis pilosula Polysaccharides Exert Antiviral Effect Through Activating Immune Function in a Macrophage Model of Bovine Viral Diarrhea Virus Infection. Veterinary Sciences, 12(5), 415. https://doi.org/10.3390/vetsci12050415








