Circadian Oscillation of Leukocyte Subpopulations and Inflammatory Cytokines over a 24-H Period in Horses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
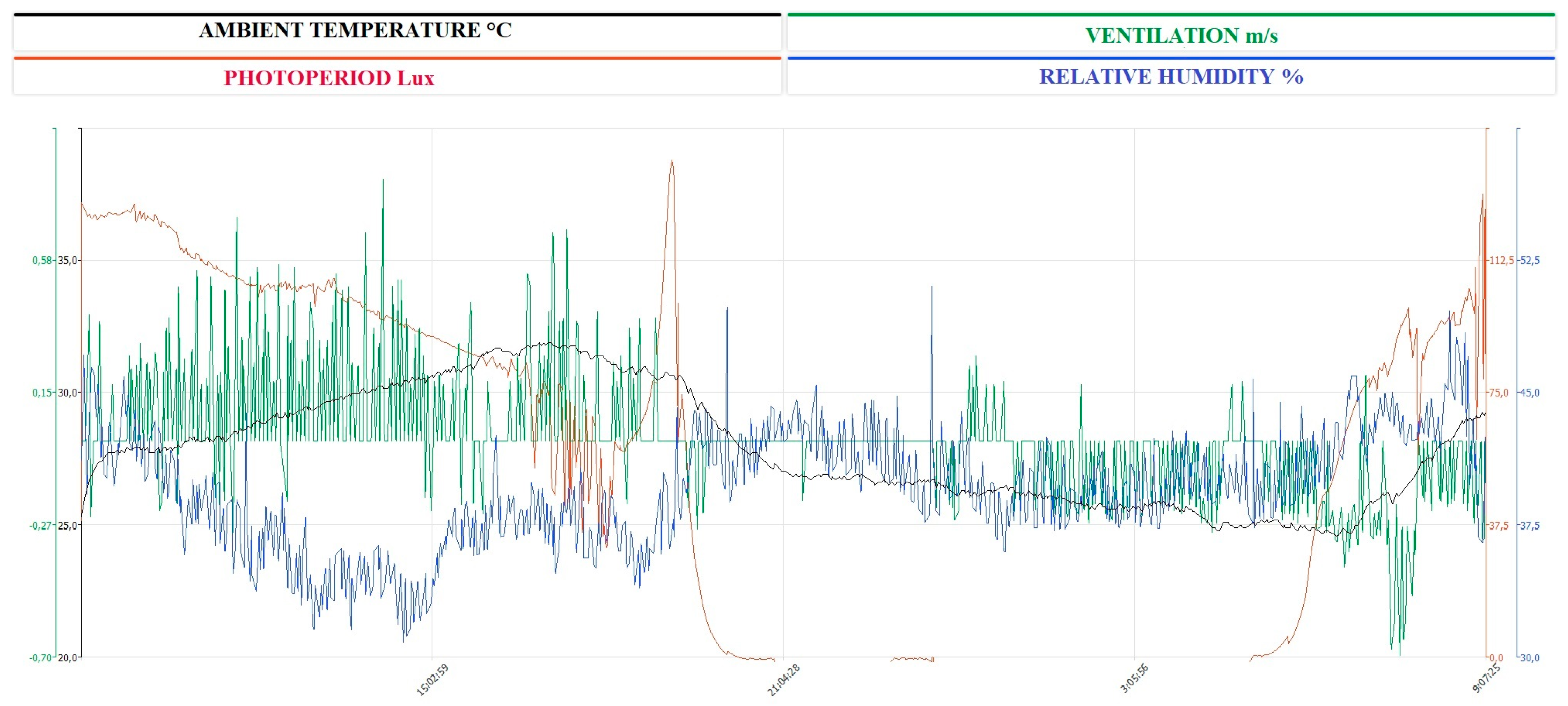
2.1. Sampling
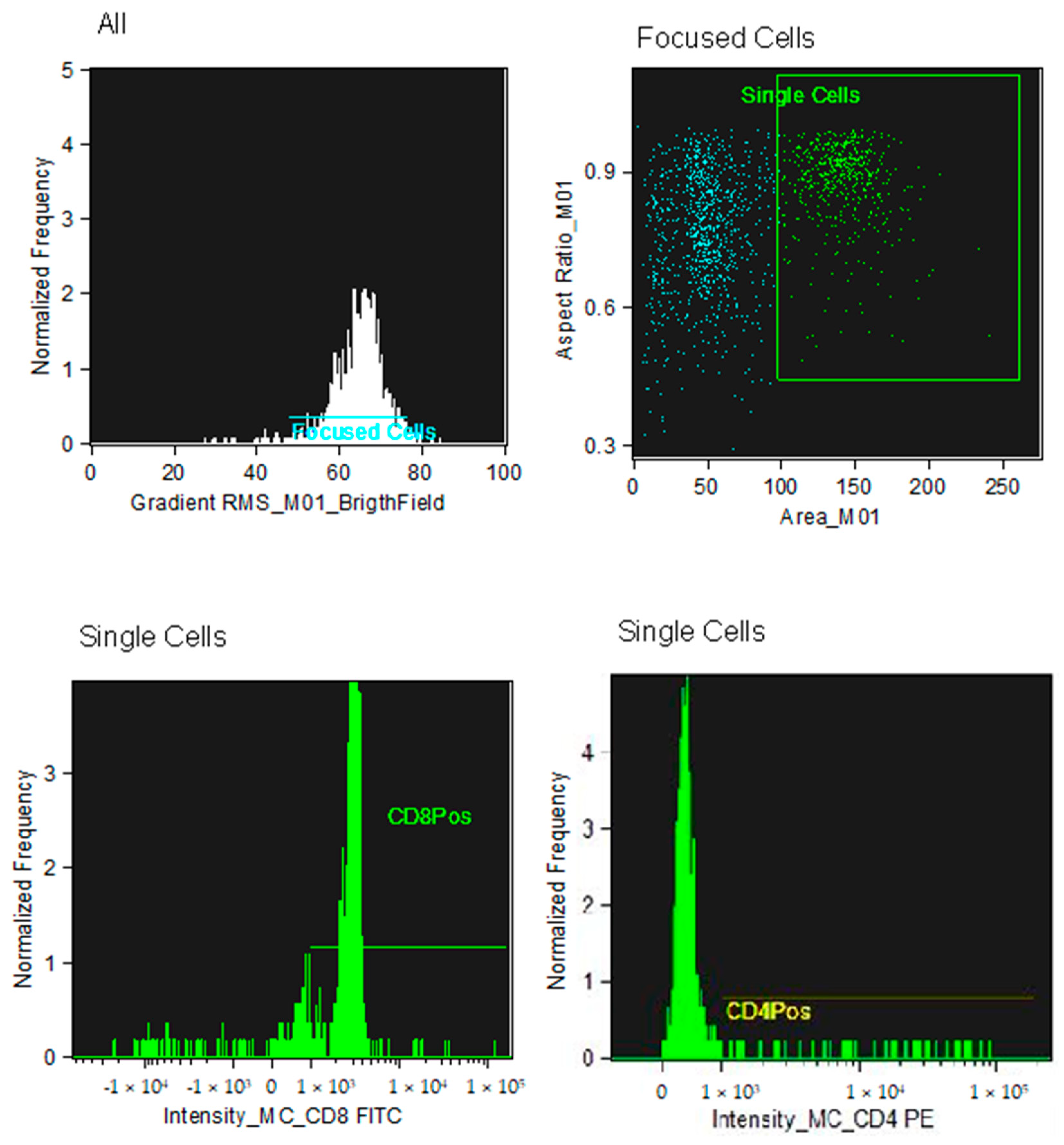
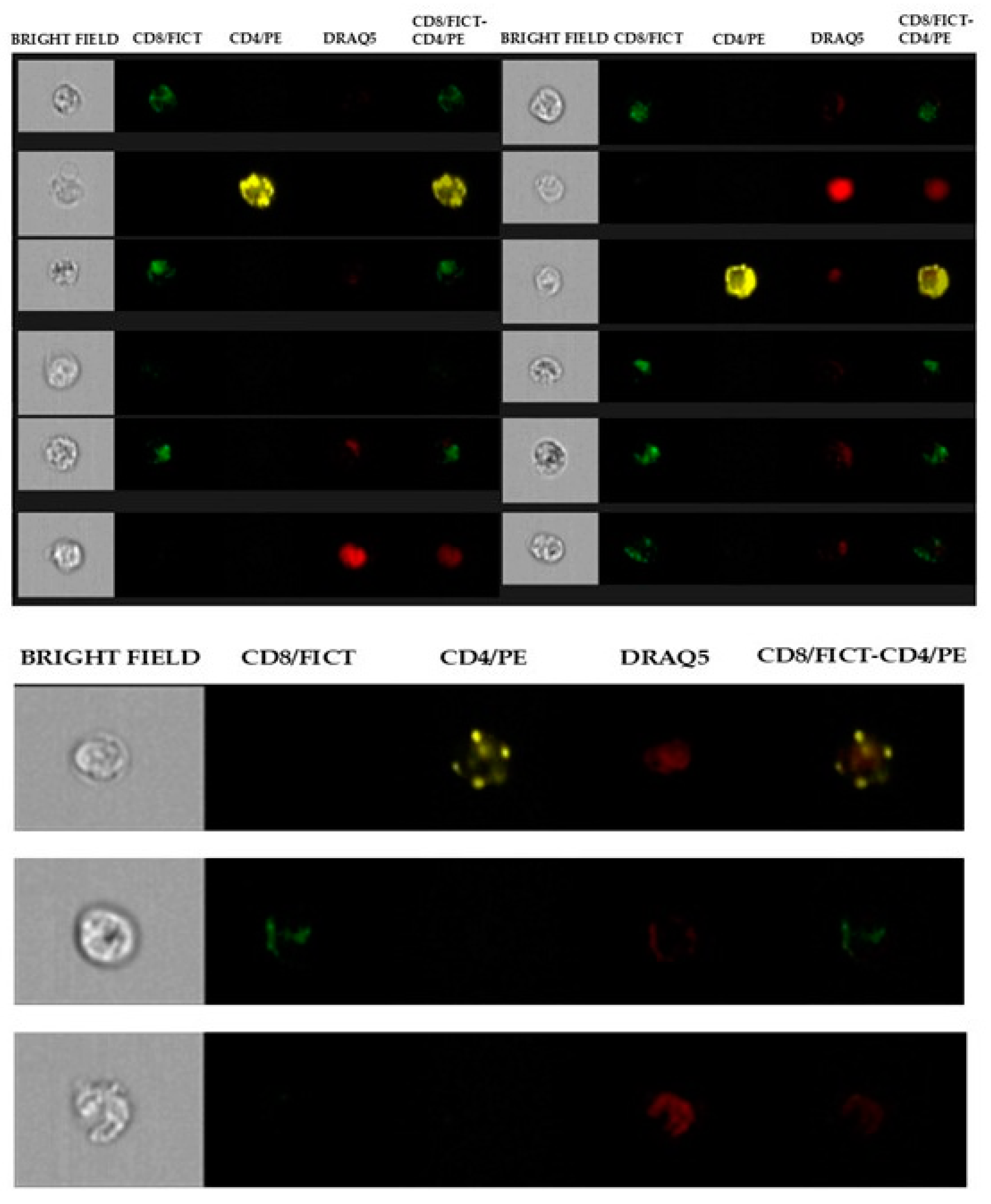
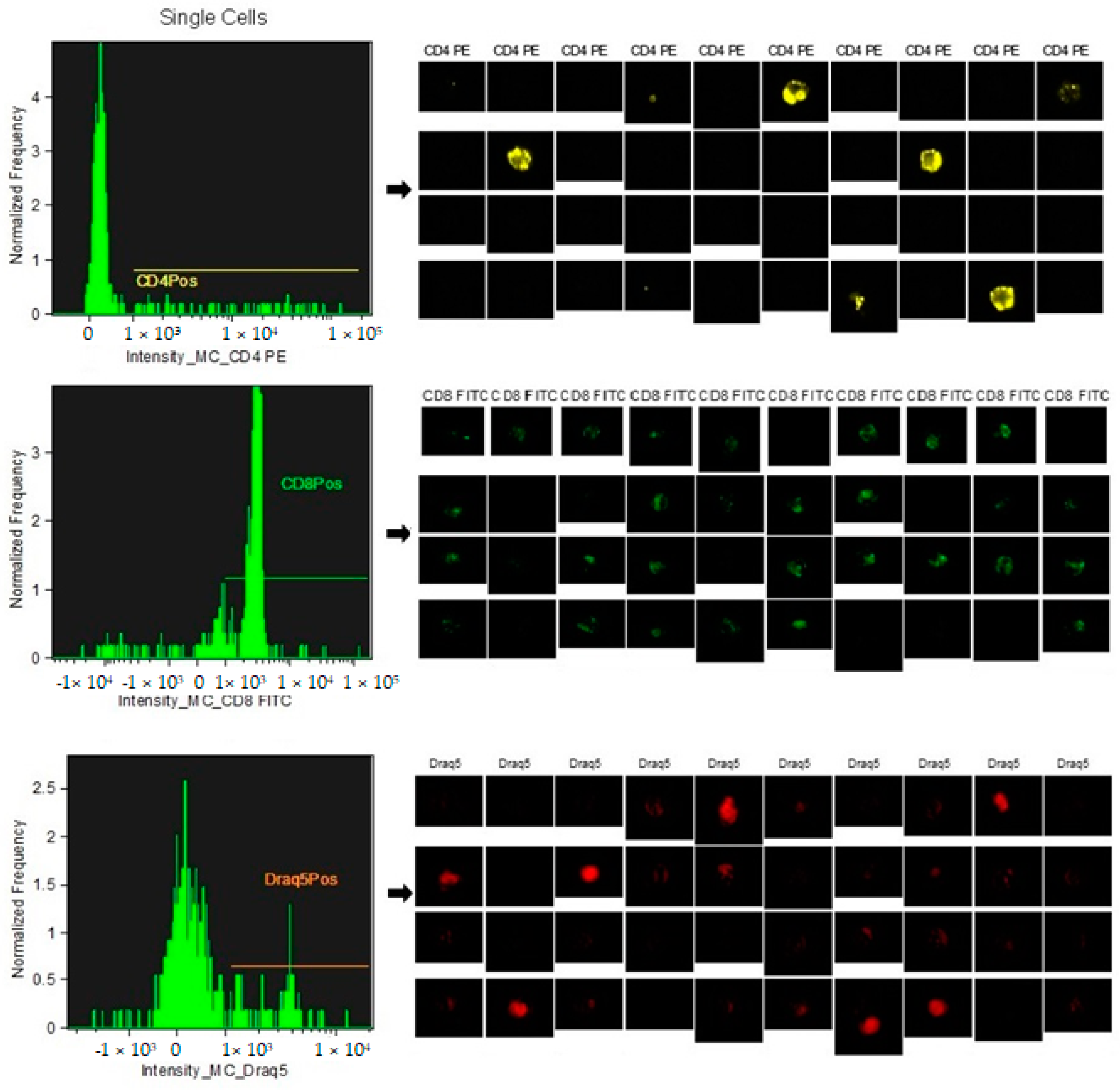
2.2. Flow Cytometry Analysis
2.3. Identification of CD4+ and CD8+ Subpopulations
2.4. Interleukine Analysis
2.5. Statistical Analysis
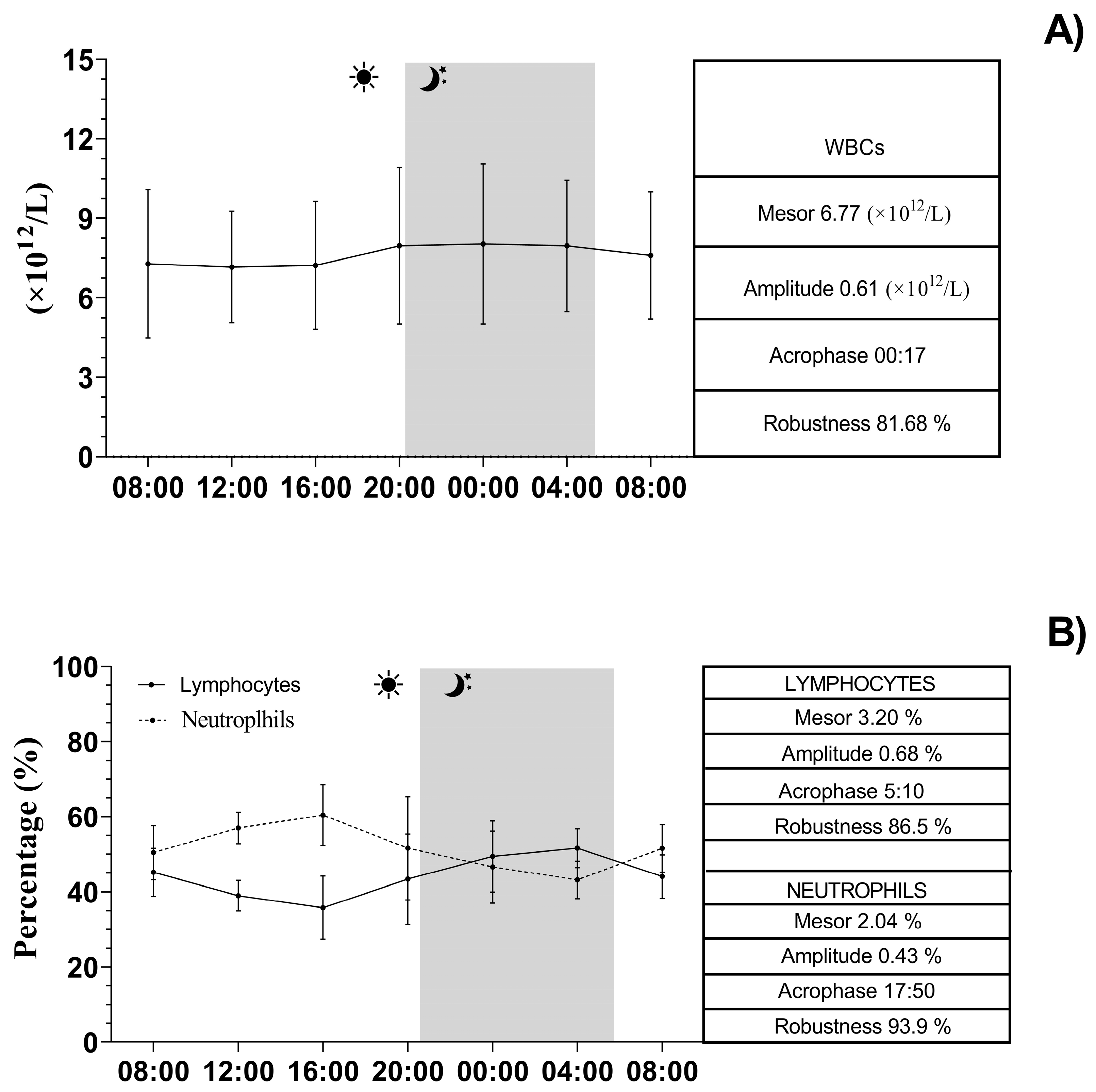
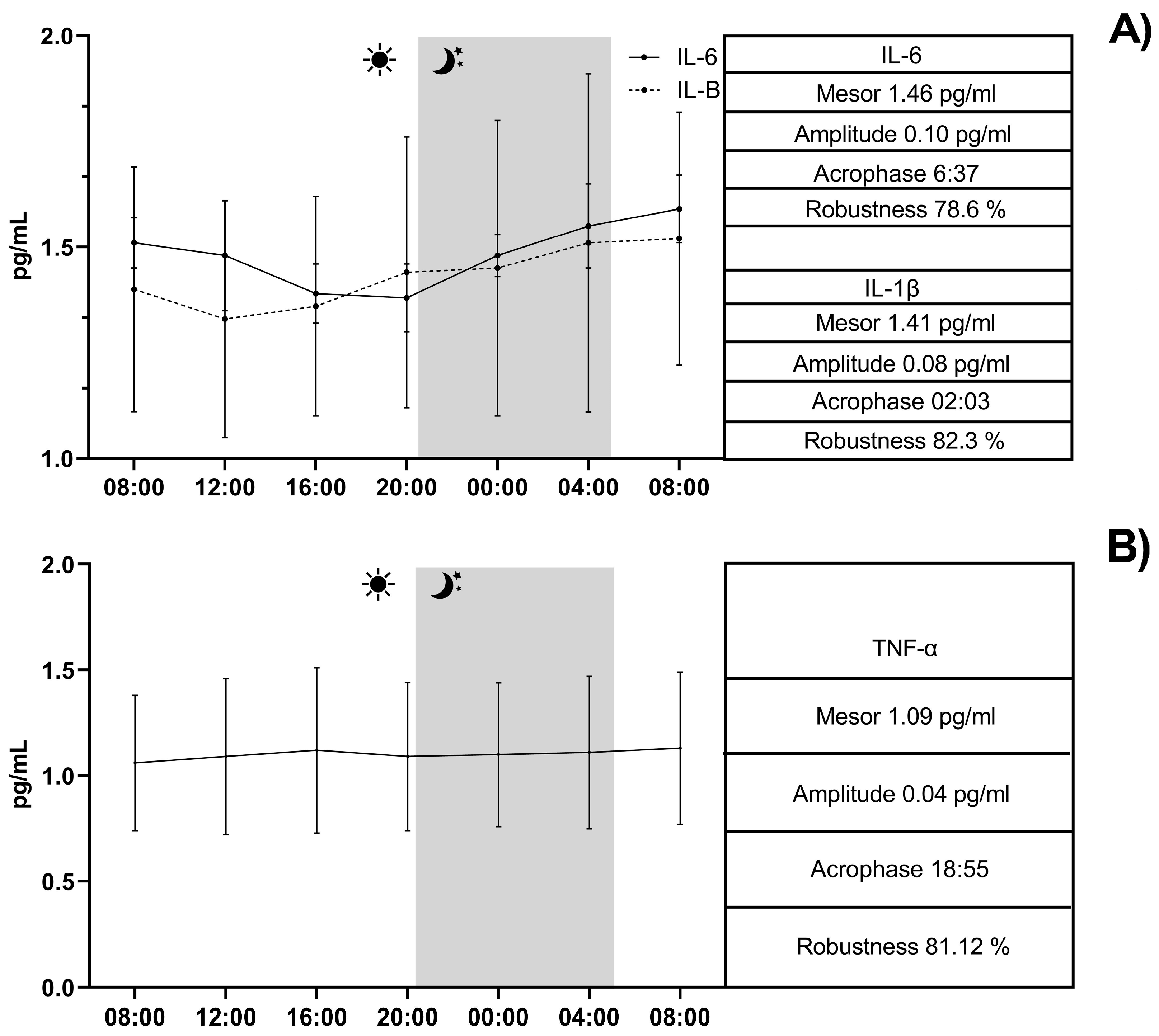
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aragona, F.; Arfuso, F.; Fazio, F.; De Caro, S.; Giudice, E.; Monteverde, V.; Piccione, G.; Giannetto, C. Circadian Variation of Peripheral Blood Cells in Horses Maintained in Different Environmental and Management Conditions. Animals 2023, 13, 1865. [Google Scholar] [CrossRef] [PubMed]
- Sauer, F.J.; Hermann, M.; Ramseyer, A.; Burger, D.; Riemer, S.; Gerber, V. Effects of Breed, Management and Personality on Cortisol Reactivity in Sport Horses. PLoS ONE 2019, 14, e0221794. [Google Scholar] [CrossRef] [PubMed]
- Padalino, B.; Raidal, S.L. Effects of Transport Conditions on Behavioural and Physiological Responses of Horses. Animals 2020, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Aragona, F.; Rizzo, M.; Arfuso, F.; Acri, G.; Fazio, F.; Piccione, G.; Giannetto, C. Eye Temperature Measured with Infrared Thermography to Assess Stress Responses to Road Transport in Horses. Animals 2024, 14, 1877. [Google Scholar] [CrossRef]
- Aragona, F.; Arfuso, F.; Rizzo, M.; Fazio, F.; Acri, G.; Piccione, G.; Giannetto, C. Using Infrared Thermography for the Evaluation of Road Transport Thermal Homeostasis in Athletic Horse. J. Equine Vet. Sci. 2024, 138, 105102. [Google Scholar] [CrossRef]
- Arrigo, F.; Aragona, F.; Faggio, C.; Giudice, E.; Giannetto, C.; Piccione, G.; Rizzo, M.; Arfuso, F. Monitoring the Physiological Inflammatory Alertness in Horse after Road Transport. Vet. Res. Commun. 2024, 48, 3331–3338. [Google Scholar] [CrossRef]
- Xu, H.; Huang, L.; Zhao, J.; Chen, S.; Liu, J.; Li, G. The Circadian Clock and Inflammation: A New Insight. Clin. Chim. Acta 2021, 512, 12–17. [Google Scholar] [CrossRef]
- Horohov, D.W. The Equine Immune Responses to Infectious and Allergic Disease: A Model for Humans? Mol. Immunol. 2015, 66, 89–96. [Google Scholar] [CrossRef]
- Labrecque, N.; Cermakian, N. Circadian Clocks in the Immune System. J. Biol. Rhythm. 2015, 30, 277–290. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Toll-like Receptors and Innate Immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625. [Google Scholar] [CrossRef]
- Nathan, P.; Gibbs, J.E.; Rainger, G.E.; Chimen, M. Changes in Circadian Rhythms Dysregulate Inflammation in Ageing: Focus on Leukocyte Trafficking. Front. Immunol. 2021, 12, 673405. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zheng, Y.; Sheng, J.; Han, Y.; Yang, Y.; Pan, H.; Yao, J. CD3+CD4-CD8- (Double-Negative) T Cells in Inflammation, Immune Disorders and Cancer. Front. Immunol. 2022, 13, 816005. [Google Scholar] [CrossRef]
- Kitchen, S.G.; Whitmire, J.K.; Jones, N.R.; Galic, Z.; Kitchen, C.M.R.; Ahmed, R.; Zack, J.A. The CD4 Molecule on CD8+ T Lymphocytes Directly Enhances the Immune Response to Viral and Cellular Antigens. Proc. Natl. Acad. Sci. USA 2005, 102, 3794–3799. [Google Scholar] [CrossRef] [PubMed]
- Topchyan, P.; Lin, S.; Cui, W. The Role of CD4 T Cell Help in CD8 T Cell Differentiation and Function During Chronic Infection and Cancer. Immune Netw. 2023, 23, e41. [Google Scholar] [CrossRef]
- Cavalli, G.; Colafrancesco, S.; Emmi, G.; Imazio, M.; Lopalco, G.; Maggio, M.C.; Sota, J.; Dinarello, C.A. Interleukin 1α: A Comprehensive Review on the Role of IL-1α in the Pathogenesis and Treatment of Autoimmune and Inflammatory Diseases. Autoimmun. Rev. 2021, 20, 102763. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Targeting Inflammatory Pathways in Cardiovascular Disease: The Inflammasome, Interleukin-1, Interleukin-6 and Beyond. Cells 2021, 10, 951. [Google Scholar] [CrossRef]
- Schnabel, C.L.; Steinig, P.; Schuberth, H.-J.; Koy, M.; Wagner, B.; Wittig, B.; Juhls, C.; Willenbrock, S.; Escobar, H.M.; Jaehnig, P.; et al. Influences of Age and Sex on Leukocytes of Healthy Horses and Their Ex Vivo Cytokine Release. Vet. Immunol. Immunopathol. 2015, 165, 64–74. [Google Scholar] [CrossRef]
- Coelho, C.S.; Sodre, T.D.R.P.; Sousa, L.N.; Siqueira, R.F.; Manso Filho, H.C.; Aragona, F.; Fazio, F. How Much Energy Vaquejada Horses Spend in a Field Simulation Test? Animals 2021, 11, 3421. [Google Scholar] [CrossRef]
- Fazio, F.; Aragona, F.; Piccione, G.; Pino, C.; Giannetto, C. Cardiac Biomarker Responses to Acute Exercise in Show Jumping Horses. J. Equine Vet. Sci. 2023, 128, 104882. [Google Scholar] [CrossRef]
- Deniz, Ö.; Ekinci, G.; Onmaz, A.C.; Derelli, F.M.; Fazio, F.; Aragona, F.; van den Hoven, R. Monitoring of Inflammatory Blood Biomarkers in Foals with Rhodococcus Equi Pneumonia during Antimicrobial Treatment. J. Equine Vet. Sci. 2024, 138, 105103. [Google Scholar] [CrossRef]
- Piccione, G.; Arfuso, F.; Giudice, E.; Aragona, F.; Pugliatti, P.; Panzera, M.F.; Zumbo, A.; Monteverde, V.; Bartolo, V.; Barbera, A.; et al. Dynamic Adaptation of Hematological Parameters, Albumin, and Non-Esterified Fatty Acids in Saddlebred and Standardbred Horses During Exercise. Animals 2025, 15, 300. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A. Temporal Regulation of Cytokines by the Circadian Clock. J. Immunol. Res. 2014, 2014, 614529. [Google Scholar] [CrossRef]
- Murphy, B.A. Circadian and Circannual Regulation in the Horse: Internal Timing in an Elite Athlete. J. Equine Vet. Sci. 2019, 76, 14–24. [Google Scholar] [CrossRef]
- Cox, S.L.; O’Siorain, J.R.; Fagan, L.E.; Curtis, A.M.; Carroll, R.G. Intertwining Roles of Circadian and Metabolic Regulation of the Innate Immune Response. Semin. Immunopathol. 2022, 44, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.J.; Gibbs, J.E. Adaptive Immunity, Chronic Inflammation and the Clock. Semin. Immunopathol. 2022, 44, 209–224. [Google Scholar] [CrossRef]
- Kouri, V.-P.; Olkkonen, J.; Kaivosoja, E.; Ainola, M.; Juhila, J.; Hovatta, I.; Konttinen, Y.T.; Mandelin, J. Circadian Timekeeping Is Disturbed in Rheumatoid Arthritis at Molecular Level. PLoS ONE 2013, 8, e54049. [Google Scholar] [CrossRef]
- Wang, C.; Lutes, L.K.; Barnoud, C.; Scheiermann, C. The Circadian Immune System. Sci. Immunol. 2022, 7, eabm2465. [Google Scholar] [CrossRef] [PubMed]
- Ella, K.; Mócsai, A.; Káldi, K. Circadian Regulation of Neutrophils: Control by a Cell-Autonomous Clock or Systemic Factors? Eur. J. Clin. Investig. 2018, 48, e12965. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian Control of the Immune System. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef]
- Dimitrov, S.; Benedict, C.; Heutling, D.; Westermann, J.; Born, J.; Lange, T. Cortisol and Epinephrine Control Opposing Circadian Rhythms in T Cell Subsets. Blood 2009, 113, 5134–5143. [Google Scholar] [CrossRef]
- Aragona, F.; Fazio, F.; Piccione, G.; Giannetto, C. Chronophysiology of Domestic Animals. Chronobiol. Int. 2024, 41, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Guo, Z.; Wu, M.; Chen, F.; Chen, L. Circadian Rhythm Regulates the Function of Immune Cells and Participates in the Development of Tumors. Cell Death Discov. 2024, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Egawa, G.; Chow, Z.; Asahina, R.; Otsuka, M.; Nakajima, S.; Nomura, T.; Shibuya, R.; Ishida, Y.; Nakamizo, S.; et al. Circadian Rhythm Affects the Magnitude of Contact Hypersensitivity Response in Mice. Allergy 2022, 77, 2748–2759. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.S.; Stangherlin, A.; Nagy, A.D.; Nicoll, M.P.; Efstathiou, S.; O’Neill, J.S.; Reddy, A.B. Cell Autonomous Regulation of Herpes and Influenza Virus Infection by the Circadian Clock. Proc. Natl. Acad. Sci. USA 2016, 113, 10085–10090. [Google Scholar] [CrossRef]
- Baxter, M.; Ray, D.W. Circadian Rhythms in Innate Immunity and Stress Responses. Immunology 2020, 161, 261–267. [Google Scholar] [CrossRef]
- Cossío, I.; Lucas, D.; Hidalgo, A. Neutrophils as Regulators of the Hematopoietic Niche. Blood 2019, 133, 2140–2148. [Google Scholar] [CrossRef]
- Pick, R.; He, W.; Chen, C.-S.; Scheiermann, C. Time-of-Day-Dependent Trafficking and Function of Leukocyte Subsets. Trends Immunol. 2019, 40, 524–537. [Google Scholar] [CrossRef]
- Ding, J.; Chen, P.; Qi, C. Circadian Rhythm Regulation in the Immune System. Immunology 2024, 171, 525–533. [Google Scholar] [CrossRef]
- Shen, Y.; Endale, M.; Wang, W.; Morris, A.R.; Francey, L.J.; Harold, R.L.; Hammers, D.W.; Huo, Z.; Partch, C.L.; Hogenesch, J.B.; et al. NF-κB Modifies the Mammalian Circadian Clock through Interaction with the Core Clock Protein BMAL1. PLoS Genet. 2021, 17, e1009933. [Google Scholar] [CrossRef]
- Morena da Silva, F.; Esser, K.A.; Murach, K.A.; Greene, N.P. Inflammation o’clock: Interactions of Circadian Rhythms with Inflammation-Induced Skeletal Muscle Atrophy. J. Physiol. 2024, 602, 6587–6607. [Google Scholar] [CrossRef]
- McGlynn, O.F.; Browne, J.A.; Blake, C.M.; Murphy, B.A. Time of Day Influences Cytokine and Clock Gene Response to Immune Stimulation in Equine Whole Blood. Anim. Genet. 2010, 41, 202–204. [Google Scholar] [CrossRef]
- Mazzoccoli, G.; Sothern, R.B.; De Cata, A.; Giuliani, F.; Fontana, A.; Copetti, M.; Pellegrini, F.; Tarquini, R. A Timetable of 24-Hour Patterns for Human Lymphocyte Subpopulations. J. Biol. Regul. Homeost. Agents 2011, 25, 387–395. [Google Scholar]
- Davis, E.G.; Wilkerson, M.J.; Rush, B.R. Flow Cytometry: Clinical Applications in Equine Medicine. J. Vet. Intern. Med. 2002, 16, 404–410. [Google Scholar] [CrossRef]
- Kublicka, A.; Lorek, D.; Mikołajczyk-Martinez, A.; Chodaczek, G.; Chwirot, A.; Bażanów, B.; Matczuk, A.K. Imaging Flow Cytometry Reveals the Mechanism of Equine Arteritis Virus Entry and Internalization. Sci. Rep. 2025, 15, 3246. [Google Scholar] [CrossRef]
- Bulkeley, E.; Santistevan, A.C.; Varner, D.; Meyers, S. Imaging Flow Cytometry to Characterize the Relationship between Abnormal Sperm Morphologies and Reactive Oxygen Species in Stallion Sperm. Reprod. Domest. Anim. 2023, 58, 10–19. [Google Scholar] [CrossRef]
- Refinetti, R. Non-Stationary Time Series and the Robustness of Circadian Rhythms. J. Theor. Biol. 2004, 227, 571–581. [Google Scholar] [CrossRef]
- Giannetto, C.; Aragona, F.; Arfuso, F.; Piccione, G.; De Caro, S.; Fazio, F. Diurnal Variation in Rectal and Cutaneous Temperatures of Horses Housed under Different Management Conditions. Int. J. Biometeorol. 2022, 66, 1601–1611. [Google Scholar] [CrossRef]
- Aragona, F.; Di Pietro, S.; Arfuso, F.; Fazio, F.; Piccione, G.; Giudice, E.; Giannetto, C. Correlation between Ocular and Rectal Temperature with Intra Ocular Pressure in Horse during Exercise. Animals 2022, 12, 1850. [Google Scholar] [CrossRef]
- Deniz, Ö.; Aragona, F.; Murphy, B.A.; Tümer, K.Ç.; Bozacı, S.; Fazio, F. Climate Change Impact on Blood Haemogram in the Horse: A Three-Year Preliminary Study. Front. Vet. Sci. 2024, 11, 1482268. [Google Scholar] [CrossRef]
- Deniz, Ö.; Aragona, F.; Pezzino, G.; Cancellieri, E.; Bozaci, S.; Tümer, K.Ç.; Fazio, F. Modeling Climate Change Effects on Some Biochemical Parameters in Horse. Res. Vet. Sci. 2025, 189, 105630. [Google Scholar] [CrossRef]
- Cermakian, N.; Stegeman, S.K.; Tekade, K.; Labrecque, N. Circadian Rhythms in Adaptive Immunity and Vaccination. Semin. Immunopathol. 2022, 44, 193–207. [Google Scholar] [CrossRef]
- Kilgallen, A.B.; van den Akker, F.; Feyen, D.A.M.; Crnko, S.; Snijders Blok, C.J.B.; Gremmels, H.; du Pré, B.C.; Reijers, R.; Doevendans, P.A.; de Jager, S.C.A.; et al. Circadian Dependence of the Acute Immune Response to Myocardial Infarction. Front. Pharmacol. 2022, 13, 869512. [Google Scholar] [CrossRef] [PubMed]
- Andriichuk, A.; Tkachenko, H. Seasonal Variations of Hematological Indices in Equines Involved in Recreational Horse Riding. Balt. Coast. Zone J. Ecol. Prot. Coastline 2015, 19, 11–22. [Google Scholar]
- Giannetto, C.; Fazio, F.; Alberghina, D.; Giudice, E.; Piccione, G. Clock Genes Expression in Peripheral Leukocytes and Plasma Melatonin Daily Rhythm in Horses. J. Equine Vet. Sci. 2020, 84, 102856. [Google Scholar] [CrossRef]
- Berger, J. A Two-Clock Model of Circadian Timing in the Immune System of Mammals. Pathol. Biol. 2008, 56, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Aroca-Crevillén, A.; Adrover, J.M.; Hidalgo, A. Circadian Features of Neutrophil Biology. Front. Immunol. 2020, 11, 576. [Google Scholar] [CrossRef]
- Gibbs, J.; Ince, L.; Matthews, L.; Mei, J.; Bell, T.; Yang, N.; Saer, B.; Begley, N.; Poolman, T.; Pariollaud, M.; et al. An Epithelial Circadian Clock Controls Pulmonary Inflammation and Glucocorticoid Action. Nat. Med. 2014, 20, 919–926. [Google Scholar] [CrossRef]
- Adrover, J.M.; Aroca-Crevillén, A.; Crainiciuc, G.; Ostos, F.; Rojas-Vega, Y.; Rubio-Ponce, A.; Cilloniz, C.; Bonzón-Kulichenko, E.; Calvo, E.; Rico, D.; et al. Programmed “disarming” of the Neutrophil Proteome Reduces the Magnitude of Inflammation. Nat. Immunol. 2020, 21, 135–144. [Google Scholar] [CrossRef]
- Hakamata, Y.; Hori, H.; Mizukami, S.; Izawa, S.; Yoshida, F.; Moriguchi, Y.; Hanakawa, T.; Inoue, Y.; Tagaya, H. Blunted Diurnal Interleukin-6 Rhythm Is Associated with Amygdala Emotional Hyporeactivity and Depression: A Modulating Role of Gene-Stressor Interactions. Front. Psychiatry 2023, 14, 1196235. [Google Scholar] [CrossRef]
- Agorastos, A.; Hauger, R.L.; Barkauskas, D.A.; Moeller-Bertram, T.; Clopton, P.L.; Haji, U.; Lohr, J.B.; Geracioti, T.D.; Patel, P.M.; Chrousos, G.P.; et al. Circadian Rhythmicity, Variability and Correlation of Interleukin-6 Levels in Plasma and Cerebrospinal Fluid of Healthy Men. Psychoneuroendocrinology 2014, 44, 71–82. [Google Scholar] [CrossRef]
- Nilsonne, G.; Lekander, M.; Åkerstedt, T.; Axelsson, J.; Ingre, M. Diurnal Variation of Circulating Interleukin-6 in Humans: A Meta-Analysis. PLoS ONE 2016, 11, e0165799. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N.; McNair, P.; Harrison, L.C. Diurnal Rhythms of Pro-Inflammatory Cytokines: Regulation by Plasma Cortisol and Therapeutic Implications. Cytokine 1998, 10, 307–312. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 01550. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Bixler, E.O.; Lin, H.-M.; Prolo, P.; Trakada, G.; Chrousos, G.P. IL-6 and Its Circadian Secretion in Humans. Neuroimmunomodulation 2005, 12, 131–140. [Google Scholar] [CrossRef]
- Beynon, A.L.; Coogan, A.N. Diurnal, Age, and Immune Regulation of Interleukin-1β and Interleukin-1 Type 1 Receptor in the Mouse Suprachiasmatic Nucleus. Chronobiol. Int. 2010, 27, 1546–1563. [Google Scholar] [CrossRef]
- Ertosun, M.G.; Kocak, G.; Ozes, O.N. The Regulation of Circadian Clock by Tumor Necrosis Factor Alpha. Cytokine Growth Factor Rev. 2019, 46, 10–16. [Google Scholar] [CrossRef]
- Arfuso, F.; Giudice, E.; Panzera, M.; Rizzo, M.; Fazio, F.; Piccione, G.; Giannetto, C. Interleukin-1Ra (Il-1Ra) and Serum Cortisol Level Relationship in Horse as Dynamic Adaptive Response during Physical Exercise. Vet. Immunol. Immunopathol. 2022, 243, 110368. [Google Scholar] [CrossRef]
- Balakin, E.; Yurku, K.; Ivanov, M.; Izotov, A.; Nakhod, V.; Pustovoyt, V. Regulation of Stress-Induced Immunosuppression in the Context of Neuroendocrine, Cytokine, and Cellular Processes. Biology 2025, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Glovatchcka, V.; Ennes, H.; Mayer, E.A.; Bradesi, S. Chronic Stress-Induced Changes in pro-Inflammatory Cytokines and Spinal Glia Markers in the Rat: A Time Course Study. Neuroimmunomodulation 2012, 19, 367–376. [Google Scholar] [CrossRef]
- Aragona, F.; Tabbì, M.; Gugliandolo, E.; Giannetto, C.; D’Angelo, F.; Fazio, F.; Interlandi, C. Role of Cannabidiolic Acid or the Combination of Cannabigerol/Cannabidiol in Pain Modulation and Welfare Improvement in Horses with Chronic Osteoarthritis. Front. Vet. Sci. 2024, 11, 1496473. [Google Scholar] [CrossRef]


| Acrophase | CD4 + | CD8 + | WBCs | Lymphocytes | Neutrophils | IL-6 | IL-1β | TNF-α |
|---|---|---|---|---|---|---|---|---|
| CD4+ | <0.0001 | <0.0001 | <0.001 | <0.0001 | <0.0001 | |||
| CD8+ | <0.0001 | <0.01 | <0.0001 | <0.0001 | ||||
| WBCs | <0.0001 | <0.01 | <0.0001 | <0.001 | <0.0001 | |||
| Lymphocytes | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| Neutrophils | <0.001 | <0.001 | <0.0001 | <0.01 | ||||
| IL-6 | <0.0001 | <0.0001 | <0.0001 | |||||
| IL-1β | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| TNF-α | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragona, F.; Rizzo, M.; Giudice, E.; Fazio, F.; Costa, A.; Di Bella, B.; De Caro, S.; Arfuso, F.; Briglia, M.; Piccione, G.; et al. Circadian Oscillation of Leukocyte Subpopulations and Inflammatory Cytokines over a 24-H Period in Horses. Vet. Sci. 2025, 12, 386. https://doi.org/10.3390/vetsci12040386
Aragona F, Rizzo M, Giudice E, Fazio F, Costa A, Di Bella B, De Caro S, Arfuso F, Briglia M, Piccione G, et al. Circadian Oscillation of Leukocyte Subpopulations and Inflammatory Cytokines over a 24-H Period in Horses. Veterinary Sciences. 2025; 12(4):386. https://doi.org/10.3390/vetsci12040386
Chicago/Turabian StyleAragona, Francesca, Maria Rizzo, Elisabetta Giudice, Francesco Fazio, Antonino Costa, Beatrice Di Bella, Salvatore De Caro, Francesca Arfuso, Marilena Briglia, Giuseppe Piccione, and et al. 2025. "Circadian Oscillation of Leukocyte Subpopulations and Inflammatory Cytokines over a 24-H Period in Horses" Veterinary Sciences 12, no. 4: 386. https://doi.org/10.3390/vetsci12040386
APA StyleAragona, F., Rizzo, M., Giudice, E., Fazio, F., Costa, A., Di Bella, B., De Caro, S., Arfuso, F., Briglia, M., Piccione, G., & Giannetto, C. (2025). Circadian Oscillation of Leukocyte Subpopulations and Inflammatory Cytokines over a 24-H Period in Horses. Veterinary Sciences, 12(4), 386. https://doi.org/10.3390/vetsci12040386








