Yellow Fever in Non-Human Primates: A Veterinary Guide from a One Health Perspective
Simple Summary
Abstract
1. Introduction
2. Epidemiology of Yellow Fever in Non-Human Primates
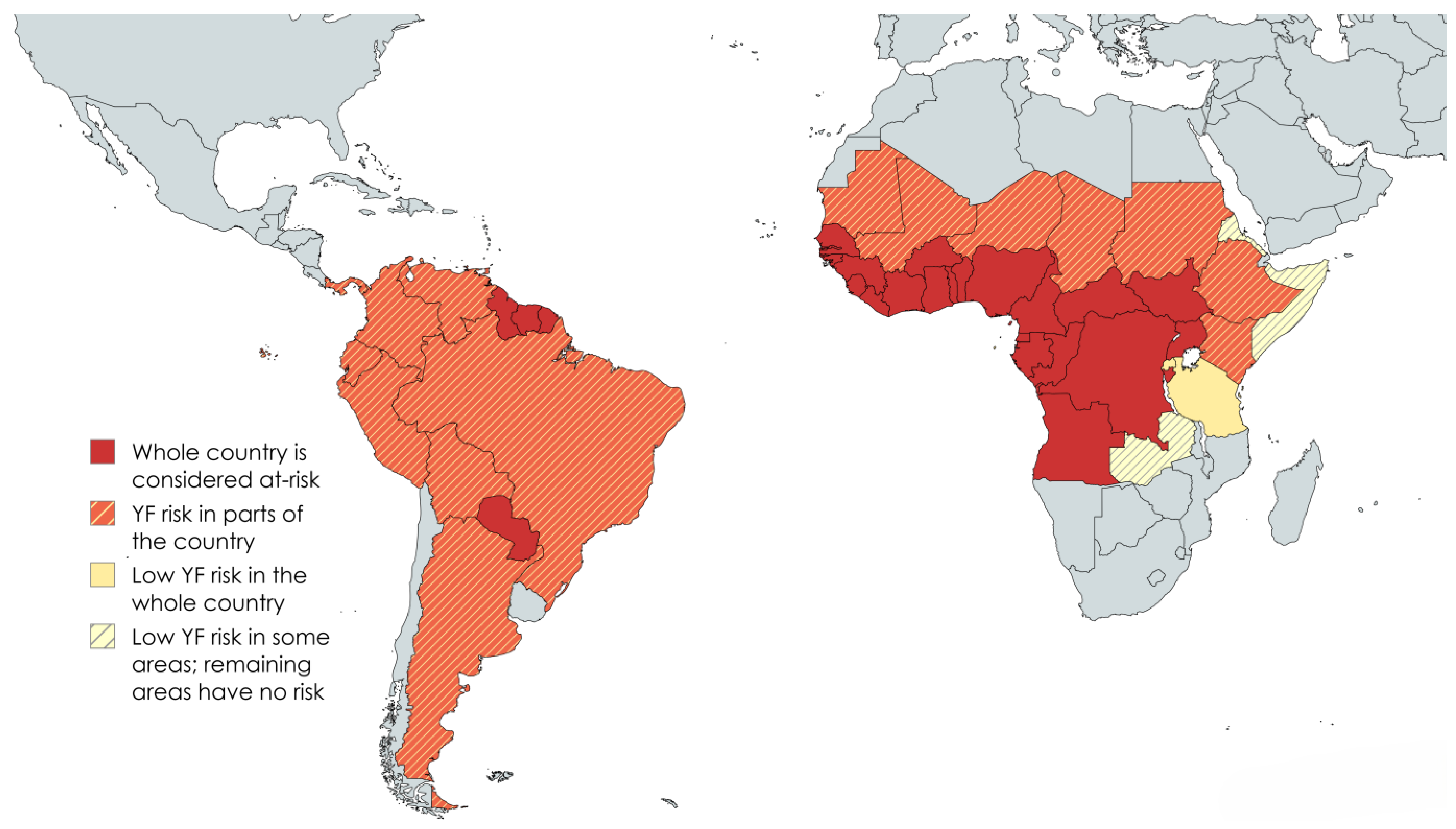
2.1. Geographic Distribution and Prevalence in NHP Populations
2.2. Recent Yellow Fever Outbreaks
3. Pathogenesis and Clinical Manifestations
3.1. Differences in Susceptibility and Clinical Outcomes Among NHP Species
3.2. Comparison of Clinical Signs in NHPs and Humans
3.3. Pathophysiology of Yellow Fever in NHPs
3.4. Pathological Changes Reported in NHPs
4. Vector Ecology and Disease Transmission
4.1. The Roles of Mosquitoes in the Transmission of Yellow Fever Virus
4.2. Transmission Cycles in Sylvatic, Urban, and Transitional Zones
4.3. The Impact of Landscape Modifications in Yellow Fever Epidemiology
4.4. The Role of Climate Change in Yellow Fever Virus Epidemiology
4.5. The Role of the Encroachment of Civilization on Natural Habitats
5. Zoonotic and One Health Perspectives
5.1. Role of NHPs as Sentinel Species for Yellow Fever Outbreaks
5.2. One Health in Surveillance and Control Strategies
6. Surveillance and Monitoring
7. Prevention and Control
7.1. Treatment of Infected NHPs
7.2. Vaccination Strategies for Humans and NHPs in Yellow Fever-Endemic Areas
7.3. Vector Control
7.3.1. Chemical Vector Control
7.3.2. Biological Vector Control
7.3.3. Physical Vector Control
8. Policy and Public Health Implications
9. Research Gaps and Future Directions
9.1. Yellow Fever Surveillance Systems
9.2. International Collaborations for Disease Control and Vaccination Programs
9.3. Long-Term Monitoring of Yellow Fever Dynamics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, M.R.; Dhar, P.S.; Rahman, M.M. The emergence of yellow fever: Outbreak, symptoms, transmission, prevention, treatment, and possible consequences. Int. J. Surg. 2023, 109, 3213–3214. [Google Scholar] [CrossRef] [PubMed]
- Oyono, M.G.; Kenmoe, S.; Abanda, N.N.; Takuissu, G.R.; Ebogo-Belobo, J.T.; Kenfack-Momo, R.; Kengne-Nde, C.; Mbaga, D.S.; Tchatchouang, S.; Kenfack-Zanguim, J.; et al. Epidemiology of yellow fever virus in humans, arthropods, and non-human primates in sub-Saharan Africa: A systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2022, 16, e0010610. [Google Scholar]
- Carrington, C.V.; Auguste, A.J. Evolutionary and ecological factors underlying the tempo and distribution of yellow fever virus activity. Infect. Genet. Evol. 2013, 13, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.D.; Monath, T.P. Epidemiology and ecology of yellow fever virus. Adv. Virus Res. 2003, 61, 291–315. [Google Scholar] [CrossRef]
- Huang, Y.J.; Higgs, S.; Horne, K.M.; Vanlandingham, D.L. Flavivirus-mosquito interactions. Viruses 2014, 6, 4703–4730. [Google Scholar] [CrossRef]
- Possas, C.; Lourenço-de-Oliveira, R.; Tauil, P.L.; Pinheiro, F.D.P.; Pissinatti, A.; Cunha, R.V.D.; Freire, M.; Martins, R.M.; Homma, A. Yellow fever outbreak in Brazil: The puzzle of rapid viral spread and challenges for immunisation. Mem. Inst. Oswaldo Cruz 2018, 113, e180278. [Google Scholar]
- Srivastava, S.; Dhoundiyal, S.; Kumar, S.; Kaur, A.; Khatib, M.N.; Gaidhane, S.; Zahiruddin, Q.S.; Mohanty, A.; Henao-Martinez, A.F.; Krsak, M.; et al. Yellow Fever: Global impact, epidemiology, pathogenesis, and integrated prevention approaches. Infez. Med. 2024, 32, 434–450. [Google Scholar] [CrossRef]
- Sadeghieh, T.; Sargeant, J.M.; Greer, A.L.; Berke, O.; Dueymes, G.; Gachon, P.; Ogden, N.H.; Ng, V. Yellow fever virus outbreak in Brazil under current and future climate. Infect. Dis. Model. 2021, 6, 664–677. [Google Scholar] [CrossRef]
- Strier, K.B.; Tabacow, F.P.; de Possamai, C.B.; Ferreira, A.I.G.; Nery, M.S.; de Melo, F.R.; Mendes, S.L. Status of the northern muriqui (Brachyteles hypoxanthus) in the time of yellow fever. Primates 2019, 60, 21–28. [Google Scholar] [CrossRef]
- Moreno, E.S.; Agostini, I.; Holzmann, I.; Di Bitetti, M.S.; Oklander, L.I.; Kowalewski, M.M.; Beldomenico, P.M.; Goenaga, S.; Martínez, M.; Lestani, E.; et al. Yellow fever impact on brown howler monkeys (Alouatta guariba clamitans) in Argentina: A metamodelling approach based on population viability analysis and epidemiological dynamics. Mem. Inst. Oswaldo Cruz 2015, 110, 865–876. [Google Scholar] [CrossRef]
- Berthet, M.; Mesbahi, G.; Duvot, G.; Zuberbühler, K.; Cäsar, C.; Bicca-Marques, J.C. Dramatic decline in a titi monkey population after the 2016-2018 sylvatic yellow fever outbreak in Brazil. Am. J. Primatol. 2021, 83, e23335. [Google Scholar] [CrossRef] [PubMed]
- Dietz, J.M.; Hankerson, S.J.; Alexandre, B.R.; Henry, M.D.; Martins, A.F.; Ferraz, L.P.; Ruiz-Miranda, C.R. Yellow fever in Brazil threatens successful recovery of endangered golden lion tamarins. Sci. Rep. 2019, 9, 12926. [Google Scholar] [CrossRef] [PubMed]
- Goenaga, S.; Fabbri, C.; Dueñas, J.C.; Gardenal, C.N.; Rossi, G.C.; Calderon, G.; Morales, M.A.; Garcia, J.B.; Enria, D.A.; Levis, S. Isolation of yellow fever virus from mosquitoes in Misiones province, Argentina. Vector Borne Zoonotic Dis. 2012, 12, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.I.O.; Sacchetto, L.; de Rezende, I.M.; Trindade, G.S.; LaBeaud, A.D.; de Thoisy, B.; Drumond, B.P. Recent sylvatic yellow fever virus transmission in Brazil: The news from an old disease. Virol. J. 2020, 17, 9. [Google Scholar] [CrossRef]
- Bicca-Marques, J.C.; de Freitas, D.S. The role of monkeys, mosquitoes, and humans in the occurrence of a Yellow Fever outbreak in a fragmented landscape in South Brazil: Protecting howler monkeys is a matter of public health. Trop. Conserv. Sci. 2010, 3, 78–89. [Google Scholar] [CrossRef]
- Bicca-Marques, J.C.; Rabelo, R.M.; de Almeida, M.A.B.; Sales, L.P. The risks of yellow fever to Asian Primates. Int. J. Primatol. 2022, 43, 74–91. [Google Scholar] [CrossRef]
- Bicca-Marques, J.C.; Calegaro-Marques, C.; Rylands, A.B.; Strier, K.B.; Mittermeier, R.A.; De Almeida, M.A.B.; De Castro, P.H.G.; Chaves, Ó.M.; Ferraz, L.P.; Fortes, V.B.; et al. Yellow Fever Threatens Atlantic Forest Primates; American Association for the Advancement of Science: Washington, DC, USA, 2017; Volume 3. [Google Scholar]
- Holzmann, I.; Agostini, I.; Areta, J.I.; Ferreyra, H.; Beldomenico, P.; Di Bitetti, M.S. Impact of yellow fever outbreaks on two howler monkey species (Alouatta guariba clamitans and A. caraya) in Misiones, Argentina. Am. J. Primatol. 2010, 72, 475–480. [Google Scholar] [CrossRef]
- Pigott, D.C. Hemorrhagic fever viruses. Crit. Care Clin. 2005, 21, 765–783. [Google Scholar] [CrossRef]
- Domingo, C.; Charrel, R.N.; Schmidt-Chanasit, J.; Zeller, H.; Reusken, C. Yellow fever in the diagnostics laboratory. Emerg. Microbes Infect. 2018, 7, 129. [Google Scholar] [CrossRef]
- Löwy, I. Leaking Containers: Success and Failure in Controlling the Mosquito Aedes aegypti in Brazil. Am. J. Public Health 2017, 107, 517–524. [Google Scholar] [CrossRef]
- Kleinert, R.D.V.; Montoya-Diaz, E.; Khera, T.; Welsch, K.; Tegtmeyer, B.; Hoehl, S.; Ciesek, S.; Brown, R.J.P. Yellow fever: Integrating current knowledge with technological innovations to identify strategies for controlling a re-emerging virus. Viruses 2019, 11, 960. [Google Scholar] [CrossRef] [PubMed]
- Valentine, M.J.; Murdock, C.C.; Kelly, P.J. Sylvatic cycles of arboviruses in non-human primates. Parasites Vectors 2019, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- de Thoisy, B.; Dussart, P.; Kazanji, M. Wild terrestrial rainforest mammals as potential reservoirs for flaviviruses (yellow fever, dengue 2 and St Louis encephalitis viruses) in French Guiana. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Findlay, G.M.; Cockburn, T.A. Possible role of birds in the maintenance of yellow fever in West Africa. Nature 1943, 152, 245. [Google Scholar] [CrossRef]
- Câmara, F.P.; Gomes, A.L.; Carvalho, L.M.; Castello, L.G. Dynamic behavior of sylvatic yellow fever in Brazil (1954–2008). Rev. Soc. Bras. Med. Trop. 2011, 44, 297–299. [Google Scholar] [CrossRef]
- Medeiros-Sousa, A.R.; Laporta, G.Z.; Mucci, L.F.; Marrelli, M.T. Epizootic dynamics of yellow fever in forest fragments: An agent-based model to explore the influence of vector and host parameters. Ecol. Model. 2022, 466, 109884. [Google Scholar] [CrossRef]
- Rodhain, F. Yellow fever: A brief history of a tropical virosis. Presse Med. 2022, 51, 104132. [Google Scholar] [CrossRef]
- Bauer, J.H.; Mahaffy, A.F. The susceptibility of African monkeys to Yellow Fever. Am. J. Epidemiol. 1930, 12, 155–174. [Google Scholar] [CrossRef]
- Moreno, E.S.; Spinola, R.; Tengan, C.H.; Brasil, R.A.; Siciliano, M.M.; Coimbra, T.L.; Silveira, V.R.; Rocco, I.M.; Bisordi, I.; Souza, R.P.; et al. Yellow fever epizootics in non-human primates, São Paulo state, Brazil, 2008–2009. Rev. Inst. Med. Trop. Sao Paulo 2013, 55, 45–50. [Google Scholar] [CrossRef]
- Moreno, E.S.; Rocco, I.M.; Bergo, E.S.; Brasil, R.A.; Siciliano, M.M.; Suzuki, A.; Silveira, V.R.; Bisordi, I.; Souza, R.P. Reemergence of yellow fever: Detection of transmission in the State of São Paulo, Brazil, 2008. Rev. Soc. Bras Med. Trop. 2011, 44, 290–296. [Google Scholar] [CrossRef]
- Goes de Jesus, J.; Gräf, T.; Giovanetti, M.; Mares-Guia, M.A.; Xavier, J.; Lima Maia, M.; Fonseca, V.; Fabri, A.; Dos Santos, R.F.; Mota Pereira, F.; et al. Yellow fever transmission in non-human primates, Bahia, Northeastern Brazil. PLoS Neglected Trop. Dis. 2020, 14, e0008405. [Google Scholar] [CrossRef]
- Fernandes, N.C.C.A.; Cunha, M.S.; Guerra, J.M.; Réssio, R.A.; Cirqueira, C.D.S.; Iglezias, S.D.; de Carvalho, J.; Araujo, E.L.L.; Catão-Dias, J.L.; Díaz-Delgado, J. Outbreak of yellow fever among non-human primates, Espirito Santo, Brazil, 2017. Emerg. Infect. Dis. 2017, 23, 2038–2041. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.C.C.A.; Cunha, M.S.; Guerra, J.M.; Diaz-Delgado, J.; Ressio, R.A.; Cirqueira, C.S.; Kanamura, C.T.; Fuentes-Castillo, D.; Catão-Dias, J.L. Yellow fever as cause of death of Titi Monkeys (Callicebus spp.). Vet. Pathol. 2021, 58, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Mares-Guia, M.A.M.M.; Horta, M.A.; Romano, A.; Rodrigues, C.D.S.; Mendonça, M.C.L.; Dos Santos, C.C.; Torres, M.C.; Araujo, E.S.M.; Fabri, A.; de Souza, E.R.; et al. Yellow fever epizootics in non-human primates, Southeast and Northeast Brazil (2017 and 2018). Parasites Vectors 2020, 13, 90. [Google Scholar] [CrossRef]
- Saad, L.D.C.; Chiaravalloti-Neto, F. Reemergence of yellow fever in the state of São Paulo: The structuring role of surveillance of epizootics in non-human primates in a one health approach. Rev. Bras. Epidemiol. 2024, 27, e240064. [Google Scholar] [CrossRef]
- Leal, S.G.; Romano, A.P.; Monteiro, R.V.; Melo, C.B.; Vasconcelos, P.F.; Castro, M.B. Frequency of histopathological changes in Howler monkeys (Alouatta sp.) naturally infected with yellow fever virus in Brazil. Rev. Soc. Bras. Med. Trop. 2016, 49, 29–33. [Google Scholar] [CrossRef]
- Santos, D.O.D.; de Oliveira, A.R.; de Lucena, F.P.; de Mattos, S.A.; de Carvalho, T.P.; Costa, F.B.; Moreira, L.G.A.; Paixão, T.A.D.; Santos, R.L. Histopathologic patterns and susceptibility of neotropical primates naturally infected with yellow fever virus. Vet. Pathol. 2020, 57, 681–686. [Google Scholar] [CrossRef]
- Monath, T.P. Yellow fever: An update. Lancet Infect. Dis. 2001, 1, 11–20. [Google Scholar]
- Monath, T.P.; Barrett, A.D. Pathogenesis and pathophysiology of yellow fever. Adv. Virus Res. 2003, 60, 343–395. [Google Scholar] [CrossRef]
- Xavier, A.R.; Freitas, G.S.; Santos, C.F.; Januzzi, W.A.; Lacerda, G.S.; Carvalho, E.R.M.; Kanaan, S. Yellow fever: Laboratorial diagnosis and clinical manifestations. J. Bras. Patol. Med. Lab. 2018, 54, 296–305. [Google Scholar] [CrossRef]
- Patterson, J.L.; Lanford, R.E. Chapter 28. Experimental Infections of the Common Marmoset (Callithrix jacchus). In The Common Marmoset in Captivity and Biomedical Research; Marini, R., Wachtman, L., Tardif, S., Mansfield, K., Fox, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 515–523. [Google Scholar] [CrossRef]
- de Almeida, M.A.; Dos Santos, E.; da Cruz Cardoso, J.; da Fonseca, D.F.; Noll, C.A.; Silveira, V.R.; Maeda, A.Y.; de Souza, R.P.; Kanamura, C.; Brasil, R.A. Yellow fever outbreak affecting Alouatta populations in southern Brazil (Rio Grande do Sul State), 2008–2009. Am. J. Primatol. 2012, 74, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.L.; Kang, L.-I.; Zanella, L.G.F.d.A.B.D.; Silveira, C.G.T.; Ho, Y.-L.; Foquet, L.; Bial, G.; McCune, B.T.; Duarte-Neto, A.N.; Thomas, A.; et al. Consumptive coagulopathy of severe yellow fever occurs independently of hepatocellular tropism and massive hepatic injury. Proc. Natl. Acad. Sci. USA 2020, 117, 32648–32656. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.C. The susceptibility of marmosets to yellow fever virus. J. Exp. Med. 1930, 52, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.C. The transmission of yellow fever: Experiments with the “woolly monkey” (Lagothrix Lago-Tricha Humboldt), the “spider monkey” (Ateles Ater F. Cuvier), and the “squirrel monkey” (Saimiri Scireus Linnaeus). J. Exp. Med. 1930, 51, 703–720. [Google Scholar] [CrossRef]
- Davis, N.C.; Shannon, R.C. Studies on South American yellow fever: III. Transmission of the virus to Brazilian monkeys preliminary observations. J. Exp. Med. 1929, 50, 81–85. [Google Scholar] [CrossRef]
- Engelmann, F.; Josset, L.; Girke, T.; Park, B.; Barron, A.; Dewane, J.; Hammarlund, E.; Lewis, A.; Axthelm, M.K.; Slifka, M.K.; et al. Pathophysiologic and transcriptomic analyses of viscerotropic yellow fever in a rhesus macaque model. PLoS Neglected Trop. Dis. 2014, 8, e3295. [Google Scholar] [CrossRef]
- Sellards, A.W. The Behavior of the virus of yellow fever in monkeys and mice. Proc. Natl. Acad. Sci. USA 1931, 17, 339–343. [Google Scholar] [CrossRef]
- Groot, H. Serological reactions in Rhesus monkeys inoculated with the 17D strain of yellow fever virus. Bull. World Health Organ. 1962, 27, 709–715. [Google Scholar]
- Monath, T.P.; Brinker, K.R.; Chandler, F.W.; Kemp, G.E.; Cropp, C.B. Pathophysiologic correlations in a rhesus monkey model of yellow fever with special observations on the acute necrosis of B cell areas of lymphoid tissues. Am. J. Trop. Med. Hyg. 1981, 30, 431–443. [Google Scholar]
- Ferreira, M.S.; Júnior, P.S.B.; Cerqueira, V.D.; Rivero, G.R.C.; Júnior, C.A.O.; Castro, P.H.G.; Silva, G.A.D.; Silva, W.B.D.; Imbeloni, A.A.; Sousa, J.R.; et al. Experimental yellow fever virus infection in the squirrel monkey (Saimiri spp.) I: Gross anatomical and histopathological findings in organs at necropsy. Mem. Inst. Oswaldo Cruz 2020, 115, e190501. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Martins, L.C.; de Melo, K.F.L.; da Silva, W.B.; Imbeloni, A.A.; Muniz, J.A.P.C.; de Oliveira, C.F.; Freitas, M.N.O.; Dos Santos, É.B.; Chagas, L.L.; et al. Experimental yellow fever in the Squirrel Monkey (Saimiri spp.): Hematological, Biochemical, and Immunological Findings. Viruses 2023, 15, 613. [Google Scholar] [CrossRef] [PubMed]
- Shinde, D.P.; Walker, J.; Reyna, R.A.; Scharton, D.; Mitchell, B.; Dulaney, E.; Bonam, S.R.; Hu, H.; Plante, J.A.; Plante, K.S.; et al. Mechanisms of Flavivirus Cross-Protection against Yellow Fever in a Mouse Model. Viruses 2024, 16, 836. [Google Scholar] [CrossRef] [PubMed]
- Beeuwkes, H. Clinical manifestations of yellow fever in the west African native as observed during four extensive epidemics of the disease in the Gold Coast and Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1936, 30, 61–86. [Google Scholar] [CrossRef]
- Harrison, M.F. The misunderstood coagulopathy of liver disease: A review for the acute setting. West. J. Emerg. Med. 2018, 19, 863–871. [Google Scholar] [CrossRef]
- Kopec, A.K.; Luyendyk, J.P. Coagulation in liver toxicity and disease: Role of hepatocyte tissue factor. Thromb. Res. 2014, 133 (Suppl. S1), S57–S59. [Google Scholar] [CrossRef]
- Kujovich, J.L. Coagulopathy in liver disease: A balancing act. Hematology Am. Soc. Hematol. Educ. Program. 2015, 2015, 243–249. [Google Scholar] [CrossRef]
- Monath, T.P.; Vasconcelos, P.F.C. Yellow Fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef]
- Guimarães, A.; Oliveira, M.C.; Kierulff, M.C.M.; Mendonça-Furtado, O.; Baptista, M.N.M.; Mendes, S.L.; Almada, G.L. Epidemiologic profile and histopathological findings in neotropical primates during and after the yellow fever outbreak in Espírito Santo, Brazil. An. Acad. Bras. Cienc. 2022, 94, e20211229. [Google Scholar] [CrossRef]
- Passos, P.H.O.; Ramos, D.G.; Romano, A.P.; Cavalcante, K.R.L.J.; Miranda, L.H.M.; Coelho, J.M.C.O.; Barros, R.C.; Martins Filho, A.J.; Quaresma, J.A.S.; Macêdo, I.L.; et al. Hepato-pathological hallmarks for the surveillance of yellow fever in South American non-human primates. Acta Trop. 2022, 231, 106468. [Google Scholar] [CrossRef]
- de Azevedo Fernandes, N.C.C.; Guerra, J.M.; Díaz-Delgado, J.; Cunha, M.S.; Saad, L.D.; Iglezias, S.D.; Ressio, R.A.; Dos Santos Cirqueira, C.; Kanamura, C.T.; Jesus, I.P.; et al. Differential Yellow Fever susceptibility in New World non-human primates, comparison with humans, and implications for surveillance. Emerg. Infect. Dis. 2021, 27, 47–56. [Google Scholar] [CrossRef]
- Shinde, D.P.; Plante, J.A.; Plante, K.S.; Weaver, S.C. Yellow Fever: Roles of Animal Models and Arthropod Vector Studies in Understanding Epidemic Emergence. Microorganisms 2022, 10, 1578. [Google Scholar] [CrossRef] [PubMed]
- Hanley, K.A.; Monath, T.P.; Weaver, S.C.; Rossi, S.L.; Richman, R.L.; Vasilakis, N. Fever versus fever: The role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect. Genet. Evol. 2013, 19, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Klitting, R.; Fischer, C.; Drexler, J.F.; Gould, E.A.; Roiz, D.; Paupy, C.; De Lamballerie, X. What does the future hold for yellow fever virus? (II). Genes 2018, 9, 425. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, F.V.S.; Ribeiro, I.P.; Ferreira-de-Brito, A.; dos Santos, A.A.C.; de Miranda, R.M.; Bonelly, I.D.S.; Neves, M.S.A.S.; Bersot, M.I.; dos Santos, T.P.; Gomes, M.Q.; et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019, 8, 218–231. [Google Scholar] [CrossRef]
- Simon, S.; Amaku, M.; Massad, E. Effects of migration rates and vaccination on the spread of yellow fever in Latin American communities. Rev. Panam. Salud Publica. 2023, 47, e86. [Google Scholar] [CrossRef]
- Cristóbal-Azkarate, J.; Arroyo-Rodríguez, V. Diet and activity pattern of howler monkeys (Alouatta palliata) in Los Tuxtlas, Mexico: Effects of habitat fragmentation and implications for conservation. Am. J. Primatol. 2007, 69, 1013–1029. [Google Scholar]
- Abreu, F.V.S.; de Andreazzi, C.S.; Neves, M.S.A.S.; Meneguete, P.S.; Ribeiro, M.S.; Dias, C.M.G.; de Albuquerque Motta, M.; Barcellos, C.; Romão, A.R.; Magalhães, M.A.F.M.; et al. Ecological and environmental factors affecting transmission of sylvatic yellow fever in the 2017-2019 outbreak in the Atlantic Forest, Brazil. Parasites Vectors 2022, 15, 23. [Google Scholar] [CrossRef]
- Kuno, G. Mechanisms of Yellow Fever transmission: Gleaning the overlooked records of importance and identifying problems, puzzles, serious issues, surprises and research questions. Viruses 2024, 16, 84. [Google Scholar] [CrossRef]
- Lataillade, L.G.; Vazeille, M.; Obadia, T.; Madec, Y.; Mousson, L.; Kamgang, B.; Chen, C.H.; Failloux, A.B.; Yen, P.S. Risk of yellow fever virus transmission in the Asia-Pacific region. Nat. Commun. 2020, 11, 5801. [Google Scholar] [CrossRef]
- dos Santos, C.R.; Santos, C.G.M.d.; Couto-Lima, D.; Souza, B.S.; Rahman, R.U.; Dornelas Ribeiro, M.; Lima, J.B.P.; Martins, A.J. Evaluation of yellow fever virus infection in Aedes aegypti mosquitoes from pakistan with distinct knockdown resistance genotypes. Insects 2025, 16, 33. [Google Scholar] [CrossRef]
- Gould, E.A.; de Lamballerie, X.; Zanotto, P.M.; Holmes, E.C. Origins, evolution, and vector/host coadaptations within the genus Flavivirus. Adv. Virus Res. 2003, 59, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, S.; Tambyah, P.A.; Lim, P.L. Yellow fever cases in Asia: Primed for an epidemic. Int. J. Infect. Dis. 2016, 48, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Cianchi, V.; Torelli, A.; Montomoli, E. Yellow Fever: Origin, epidemiology, preventive strategies and future prospects. Vaccines 2022, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Salomón, O.D.; Arias, A.R. The second coming of urban yellow fever in the Americas: Looking the past to see the future. An. Acad. Bras. Cienc. 2022, 94, e20201252. [Google Scholar] [CrossRef]
- Saad, L.D.C.; Barata, R.B. Yellow fever outbreaks in São Paulo State, Brazil, 2000–2010. Epidemiol. Serv. Saúde 2016, 25, 555–564. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Kulmann-Leal, B.; Kaminski, V.L.; Valverde-Villegas, J.M.; Da Veiga, A.B.G.; Spilki, F.R.; Fearnside, P.M.; Caesar, L.; Giatti, L.L.; Wallau, G.L.; et al. Beyond diversity loss and climate change: Impacts of Amazon deforestation on infectious diseases and public health. An. Acad. Bras. Ciênc. 2020, 92, 01375. [Google Scholar] [CrossRef]
- Ilacqua, R.C.; Medeiros-Sousa, A.R.; Ramos, D.G.; Obara, M.T.; Ceretti-Junior, W.; Mucci, L.F.; Marrelli, M.T.; Laporta, G.Z. Reemergence of Yellow Fever in Brazil: The Role of Distinct Landscape Fragmentation Thresholds. J. Environ. Public. Health 2021, 2021, 8230789. [Google Scholar] [CrossRef]
- Wilk-da-Silva, R.; Prist, P.R.; Medeiros-Sousa, A.R.; Laporta, G.Z.; Mucci, L.F.; Marrelli, M.T. The role of forest fragmentation in yellow fever virus dispersal. Acta Tropica. 2023, 245, 106983. [Google Scholar] [CrossRef]
- Wilk-da-Silva, R.; Medeiros-Sousa, A.R.; Laporta, G.Z.; Mucci, L.F.; Prist, P.R.; Marrelli, M.T. The influence of landscape structure on the dispersal pattern of yellow fever virus in the state of São Paulo. Acta Tropica. 2022, 228, 106333. [Google Scholar] [CrossRef]
- Salas-Rojas, M.; de Oliveira-Filho, E.F.; Almazán-Marín, C.; Rodas-Martínez, A.Z.; Aguilar-Setién, Á.; Drexler, J.F. Serological evidence for potential yellow fever virus infection in non-human primates, southeastern Mexico. One Health Outlook 2023, 5, 14. [Google Scholar] [CrossRef]
- Reiter, P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001, 109 (Suppl. S1), 141–161. [Google Scholar] [CrossRef] [PubMed]
- Aliaga-Samanez, A.; Romero, D.; Murray, K.; Segura, M.; Real, R.; Olivero, J. Potential climate change effects on the distribution of urban and sylvatic dengue and yellow fever vectors. Pathog. Glob. Health 2024, 118, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Colón-González, F.J.; Sewe, M.O.; Tompkins, A.M.; Sjödin, H.; Casallas, A.; Rocklöv, J.; Caminade, C.; Lowe, R. Projecting the risk of mosquito-borne diseases in a warmer and more populated world: A multi-model, multi-scenario intercomparison modelling study. Lancet Planet Health 2021, 5, e404–e414, Erratum in Lancet Planet Health 2021, 5, e504. https://doi.org/10.1016/S2542-5196(21)00207-2. [Google Scholar] [CrossRef] [PubMed]
- Reiter, P. Climate change and mosquito-borne disease: Knowing the horse before hitching the cart. Rev. Sci. Tech. 2008, 27, 383–398. [Google Scholar] [CrossRef]
- Huxley, P.J.; Murray, K.A.; Pawar, S.; Cator, L.J. The effect of resource limitation on the temperature dependence of mosquito population fitness. Proc. Biol. Sci. 2021, 288, 20203217. [Google Scholar] [CrossRef]
- Christiansen-Jucht, C.; Parham, P.E.; Saddler, A.; Koella, J.C.; Basáñez, M.G. Temperature during larval development and adult maintenance influences the survival of Anopheles gambiae s.s. Parasites Vectors 2014, 7, 489. [Google Scholar] [CrossRef]
- Nik Abdull Halim, N.M.H.; Che Dom, N.; Dapari, R.; Salim, H.; Precha, N. A systematic review and meta-analysis of the effects of temperature on the development and survival of the Aedes mosquito. Front. Public Health 2022, 10, 1074028. [Google Scholar] [CrossRef]
- Gaythorpe, K.A.; Hamlet, A.; Cibrelus, L.; Garske, T.; Ferguson, N.M. The effect of climate change on yellow fever disease burden in Africa. eLife 2020, 9, e55619. [Google Scholar] [CrossRef]
- Leta, S.; Beyene, T.J.; De Clercq, E.M.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef]
- Schrama, M.; Hunting, E.R.; Beechler, B.R.; Guarido, M.M.; Govender, D.; Nijland, W.; van ‘t Zelfde, M.; Venter, M.; van Bodegom, P.M.; Gorsich, E.E. Human practices promote presence and abundance of disease-transmitting mosquito species. Sci. Rep. 2020, 10, 13543. [Google Scholar] [CrossRef]
- Duval, P.; Antonelli, P.; Aschan-Leygonie, C.; Valiente Moro, C. Impact of human activities on disease-spreading mosquitoes in urban areas. J. Urban Health 2023, 100, 591–611. [Google Scholar] [CrossRef] [PubMed]
- Wilke, A.B.B.; Chase, C.; Vasquez, C.; Carvajal, A.; Medina, J.; Petrie, W.D.; Beier, J.C. Urbanization creates diverse aquatic habitats for immature mosquitoes in urban areas. Sci. Rep. 2019, 9, 15335. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, K.R.L.J.; Tauil, P.L. Epidemiological characteristics of yellow fever in Brazil, 2000-2012. Epidemiol. Serv. Saúde 2016, 25, 11–20. [Google Scholar] [CrossRef]
- Araújo, F.A.A.; Ramos, D.G.; Santos, A.L.; Passos, P.H.O.; Elkhoury, A.N.S.M.; Costa, Z.G.A.; Leal, S.G.; Romano, A.P.M. Epizootics in non-human primates during reemergence of yellow fever virus in Brazil, 2007 to 2009. Epidemiol. Serv. Saude 2011, 20, 527–536. [Google Scholar] [CrossRef]
- Almeida, M.A.; Cardoso Jda, C.; Dos Santos, E.; da Fonseca, D.F.; Cruz, L.L.; Faraco, F.J.; Bercini, M.A.; Vettorello, K.C.; Porto, M.A.; Mohrdieck, R.; et al. Surveillance for Yellow Fever virus in non-human primates in southern Brazil, 2001-2011: A tool for prioritizing human populations for vaccination. PLoS Neglected Trop. Dis. 2014, 8, e2741. [Google Scholar] [CrossRef]
- Sawyer, W.A.; Lloyd, W.D.; Kitchen, S.F. The preservation of yellow fever virus. J. Exp. Med. 1929, 50, 1–13. [Google Scholar] [CrossRef]
- Domingo, C.; Patel, P.; Yillah, J.; Weidmann, M.; Méndez, J.A.; Nakouné, E.R.; Niedrig, M. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J. Clin. Microbiol. 2012, 50, 4054–4060. [Google Scholar] [CrossRef]
- De Brito, T.; Siqueira, S.A.; Santos, R.T.; Nassar, E.S.; Coimbra, T.L.; Alves, V.A. Human fatal yellow fever. Immunohistochemical detection of viral antigens in the liver, kidney and heart. Pathol. Res. Pract. 1992, 188, 177–181. [Google Scholar] [CrossRef]
- Fischer, C.; Torres, M.C.; Patel, P.; Moreira-Soto, A.; Gould, E.A.; Charrel, R.N.; de Lamballerie, X.; Nogueira, R.M.R.; Sequeira, P.C.; Rodrigues, C.D.S.; et al. Lineage-specific real-time RT-PCR for yellow fever virus outbreak surveillance, Brazil. Emerg. Infect. Dis. 2017, 23, 1867–1871. [Google Scholar] [CrossRef]
- Cardoso, S.F.; Yoshikawa, A.A.G.; Pinheiro, I.C.; Granella, L.W.; Couto-Lima, D.; Neves, M.S.A.S.; Mansur, D.S.; Pitaluga, A.N.; Rona, L.D.P. Development and validation of RT-LAMP for detecting yellow fever virus in non-human primates samples from Brazil. Sci. Rep. 2024, 14, 22520. [Google Scholar] [CrossRef]
- Escadafal, C.; Faye, O.; Sall, A.A.; Faye, O.; Weidmann, M.; Strohmeier, O.; von Stetten, F.; Drexler, J.; Eberhard, M.; Niedrig, M.; et al. Rapid molecular assays for the detection of yellow fever virus in low-resource settings. PLoS Neglected Trop. Dis. 2014, 8, e2730. [Google Scholar] [CrossRef] [PubMed]
- Kumm, H.W.; Laemmerrt, H.W., Jr. The geographical distribution of immunity to yellow fever among the primates of Brazil. Am. J. Trop. Med. Hyg. 1950, 30, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Souza, N.C.S.E.; Félix, A.C.; de Paula, A.V.; Levi, J.E.; Pannuti, C.S.; Romano, C.M. Evaluation of serological cross-reactivity between yellow fever and other flaviviruses. Int. J. Infect. Dis. 2019, 81, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.C. On the use of immune serum at various intervals after the inoculation of yellow fever virus into Rhesus Monkeys. J. Immunol. 1934, 26, 361–390. [Google Scholar] [CrossRef]
- Lloyd, W.; Mahaffy, A.F. The serum antibody titer of macacus rhesus following repeated inoculations of yellow fever virus. J. Immunol. 1934, 26, 313–320. [Google Scholar] [CrossRef]
- Sawyer, W.A.; Kitchen, S.F.; Lloyd, W. Vaccination against yellow fever with immune serum and virus fixed for mice. J. Exp. Med. 1932, 55, 945–969. [Google Scholar] [CrossRef][Green Version]
- Monath, T.P. Treatment of yellow fever. Antiviral Res. 2008, 78, 116–124. [Google Scholar] [CrossRef]
- Ricciardi, M.J.; Rust, L.N.; Pedreño-Lopez, N.; Yusova, S.; Biswas, S.; Webb, G.M.; Gonzalez-Nieto, L.; Voigt, T.B.; Louw, J.J.; Laurino, F.D.; et al. Therapeutic neutralizing monoclonal antibody administration protects against lethal yellow fever virus infection. Sci. Transl. Med. 2023, 15, eade5795. [Google Scholar] [CrossRef]
- Frierson, J.G. The yellow fever vaccine: A history. Yale J. Biol. Med. 2010, 83, 77–85. [Google Scholar]
- Gotuzzo, E.; Yactayo, S.; Córdova, E. Efficacy and duration of immunity after yellow fever vaccination: Systematic review on the need for a booster every 10 years. Am. J. Trop. Med. Hyg. 2013, 89, 434–444. [Google Scholar] [CrossRef]
- Schlesinger, J.J.; Brandriss, M.W.; Cropp, C.B.; Monath, T.P. Protection against yellow fever in monkeys by immunization with yellow fever virus nonstructural protein NS1. J. Virol. 1986, 60, 1153–1155. [Google Scholar] [CrossRef] [PubMed]
- Amanna, I.J.; Thomas, A.; Engelmann, F.; Hammarlund, E.; Raué, H.P.; Bailey, A.L.; Poore, E.A.; Quintel, B.K.; Lewis, A.D.; Axthelm, M.K.; et al. Development of a hydrogen peroxide-inactivated vaccine that protects against viscerotropic yellow fever in a non-human primate model. Cell Rep. Med. 2024, 5, 101655. [Google Scholar] [CrossRef] [PubMed]
- Piras-Douce, F.; Broudic, K.; Chautard, E.; Raynal, F.; Courtois, V.; Gautheron, S.; Mantel, N. Evaluation of safety and immuno-efficacy of a next generation live-attenuated yellow fever vaccine in cynomolgus macaques. Vaccine 2023, 41, 1457–1470. [Google Scholar] [CrossRef] [PubMed]
- Theiler, M.; Smith, H.H. The use of yellow fever virus modified by in vitro cultivation for human immunization. J. Exp. Med. 1937, 65, 787–800. [Google Scholar] [CrossRef]
- Medina-Magües, L.G.; Mühe, J.; Jasny, E.; Medina-Magües, E.S.; Roth, N.; Lopera-Madrid, J.; Salas-Quinchucua, C.; Knuese, C.; Petsch, B.; Osorio, J.E. Immunogenicity and protective activity of mRNA vaccine candidates against yellow fever virus in animal models. NPJ Vaccines 2023, 8, 31. [Google Scholar] [CrossRef]
- Mantel, N.; Piras-Douce, F.; Chautard, E.; Marcos-Lopez, E.; Bodinham, C.L.; Cosma, A.; Courtois, V.; Dhooge, N.; Gautheron, S.; Kaufmann, S.H.E.; et al. Cynomolgus macaques as a translational model of human immune responses to yellow fever 17D vaccination. J. Virol. 2024, 98, e0151623. [Google Scholar] [CrossRef]
- Nederlof, R.A.; Sainmaa, S.; Wissink-Argilaga, N.; Koo, B.-S.; Bakker, J. Preventative vaccination of nonhuman primates. J. Zool. Bot. Gard. 2025, 6, 8. [Google Scholar] [CrossRef]
- Mason, R.A.; Tauraso, N.M.; Spertzel, R.O.; Ginn, R.K. Yellow fever vaccine: Direct challenge of monkeys given graded doses of 17D vaccine. Appl. Microbiol. 1973, 25, 539–544. [Google Scholar] [CrossRef]
- Mason, R.A.; Tauraso, N.M.; Ginn, R.K.; O’Brien, T.C.; Trimmer, R.W. Yellow Fever Vaccine. V. Antibody Response in Monkeys Inoculated with Graded Doses of the 17D Vaccine. Appl. Microbiol. 1972, 235, 908–913. [Google Scholar] [CrossRef]
- de Miranda, R.M.; Fernandes, R.S.; da Silva-Fernandes, A.T.; Ferreira-de-Brito, A.; Moreira, S.B.; Pereira, R.C.; da Silva Mendes, Y.; de Lima, S.M.B.; Pissinatti, A.; Freire, M.D.S.; et al. Neotropical sylvatic mosquitoes and Aedes aegypti are not competent to transmit 17DD attenuated yellow fever virus from vaccinated viremic New World non-human primates. Viruses 2022, 14, 2231. [Google Scholar] [CrossRef]
- Tavares da Silva Fernandes, A.; Moreira, S.B.; Gaspar, L.P.; Simões, M.; Cajaraville, A.C.D.R.A.; Pereira, R.C.; Gomes, M.P.B.; Linhares, J.H.R.; Santos, V.O.; Santos, R.T.; et al. Safety and immunogenicity of 17DD attenuated yellow fever vaccine in howler monkeys (Alouatta spp.). J. Med. Primatol. 2021, 50, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Silva-Fernandes, A.T.; Moreira, S.B.; Gaspar, L.P.; Cajaraville, A.C.R.A.; Simões, M.; Pereira, R.C.; Gomes, M.P.B.; Santos, V.O.; Santos, R.T.; Vieira da Silva, A.M.; et al. Safety and immunogenicity of different 17DD yellow fever vaccines in golden-headed tamarins (Leontopithecus chrysomelas): Inhibition of viremia and RNAemia after homologous live-attenuated vaccination. Vaccine 2025, 48, 126721. [Google Scholar] [CrossRef] [PubMed]
- Massad, E.; Miguel, M.M.; Coutinho, F.A.B. Is vaccinating monkeys against yellow fever the ultimate solution for the Brazilian recurrent epizootics? Epidemiol. Infect. 2018, 146, 1622–1624. [Google Scholar] [CrossRef] [PubMed]
- Danet, L.; Beauclair, G.; Berthet, M.; Moratorio, G.; Gracias, S.; Tangy, F.; Choumet, V.; Jouvenet, N. Midgut barriers prevent the replication and dissemination of the yellow fever vaccine in Aedes aegypti. PLoS Neglected Trop. Dis. 2019, 13, e0007299. [Google Scholar] [CrossRef]
- Achee, N.L.; Grieco, J.P.; Vatandoost, H.; Seixas, G.; Pinto, J.; Ching-Ng, L.; Martins, A.J.; Juntarajumnong, W.; Corbel, V.; Gouagna, C.; et al. Alternative strategies for mosquito-borne arbovirus control. PLoS Neglected Trop. Dis. 2019, 13, e0006822, Erratum in PLoS Neglected Trop. Dis. 2019, 13, e0007275. https://doi.org/10.1371/journal.pntd.0007275. [Google Scholar] [CrossRef]
- Martínez Rodríguez, E.J.; Evans, P.; Kalsi, M.; Rosenblatt, N.; Stanley, M.; Piermarini, P.M. Larvicidal Activity of Carbon Black against the yellow fever mosquito Aedes aegypti. Insects 2022, 13, 307. [Google Scholar] [CrossRef]
- Hustedt, J.C.; Boyce, R.; Bradley, J.; Hii, J.; Alexander, N. Use of pyriproxyfen in control of Aedes mosquitoes: A systematic review. PLoS Neglected Trop. Dis. 2020, 14, e0008205. [Google Scholar] [CrossRef]
- Abad-Franch, F.; Carvajal-Cortés, J.J.; Rabelo, A.C.L.; Gusmão, E.V.V.; Soares, S.S.S.; Luz, S.L.B. Mosquito-disseminated pyriproxyfen for mosquito-borne disease control in Belo Horizonte, Brazil: A pragmatic, before-after control-intervention paired-series trial. Lancet Infect. Dis. 2024, 25, 176–187. [Google Scholar] [CrossRef]
- Abad-Franch, F.; Zamora-Perea, E.; Ferraz, G.; Padilla-Torres, S.D.; Luz, S.L. Mosquito-disseminated pyriproxyfen yields high breeding-site coverage and boosts juvenile mosquito mortality at the neighborhood scale. PLoS Neglected Trop. Dis. 2015, 9, e0003702. [Google Scholar] [CrossRef]
- Moura, L.; de Nadai, B.L.; Corbi, J.J. What does not kill it does not always make it stronger: High temperatures in pyriproxyfen treatments produce Aedes aegypti adults with reduced longevity and smaller females. J. Asia-Pac. Entomol. 2020, 23, 529–535. [Google Scholar] [CrossRef]
- Mmbaga, A.T.; Lwetoijera, D.W. Current and future opportunities of autodissemination of pyriproxyfen approach for malaria vector control in urban and rural Africa. Wellcome Open Res. 2023, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Seixas, G.; Paul, R.E.L.; Pires, B.; Alves, G.; de Jesus, A.; Silva, A.C.; Devine, G.J.; Sousa, C.A. An evaluation of efficacy of the auto-dissemination technique as a tool for Aedes aegypti control in Madeira, Portugal. Parasit. Vectors. 2019, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.A.; Bauzer, L.G.S.D.R.; Borges, D.T.; Lima, J.B.P. Assessing the efficacy of two new formulations of larvicide pyriproxyfen for the control of Aedes aegypti using dissemination stations in two sites of Rio de Janeiro city. Mem. Inst. Oswaldo Cruz 2020, 115, e200271. [Google Scholar] [CrossRef] [PubMed]
- Unlu, I.; Rochlin, I.; Suman, D.S.; Wang, Y.; Chandel, K.; Gaugler, R. Large-scale operational pyriproxyfen autodissemination deployment to suppress the immature Asian Tiger Mosquito (Diptera: Culicidae) Populations. J. Med. Entomol. 2020, 57, 1120–1130. [Google Scholar] [CrossRef]
- Kitchen, L.W.; Lawrence, K.L.; Coleman, R.E. The role of the United States military in the development of vector control products, including insect repellents, insecticides, and bed nets. J. Vector Ecol. 2009, 34, 50–61. [Google Scholar] [CrossRef]
- Leal, W.S. The enigmatic reception of DEET—The gold standard of insect repellents. Curr. Opin. Insect Sci. 2014, 6, 93–98. [Google Scholar] [CrossRef]
- Chen-Hussey, V.; Behrens, R.; Logan, J.G. Assessment of methods used to determine the safety of the topical insect repellent N,N-diethyl-m-toluamide (DEET). Parasites Vectors 2014, 7, 173. [Google Scholar] [CrossRef]
- Weeks, J.A.; Guiney, P.D.; Nikiforov, A.I. Assessment of the environmental fate and ecotoxicity of N,N-diethyl-m-toluamide (DEET). Integr. Environ. Assess. Manag. 2012, 8, 120–134. [Google Scholar] [CrossRef]
- Aronson, D.; Weeks, J.; Meylan, B.; Guiney, P.D.; Howard, P.H. Environmental release, environmental concentrations, and ecological risk of N,N-Diethyl-m-toluamide (DEET). Integr. Environ. Assess. Manag. 2012, 8, 135–166. [Google Scholar] [CrossRef]
- Costanzo, S.D.; Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Sandstrom, M.W. Is there a risk associated with the insect repellent DEET (N,N-diethyl-m-toluamide) commonly found in aquatic environments? Sci. Total Environ. 2007, 384, 214–220. [Google Scholar] [CrossRef]
- Liu, L.; Qin, W.; Nie, L.; Wang, X.; Dong, X. Correlation between environmental DEET exposure and the mortality rate of cancer survivors: A large-sample cross-sectional investigation. BMC Cancer 2024, 24, 1410. [Google Scholar] [CrossRef] [PubMed]
- Achee, N.L.; Bangs, M.J.; Farlow, R.; Killeen, G.F.; Lindsay, S.; Logan, J.G.; Moore, S.J.; Rowland, M.; Sweeney, K.; Torr, S.J.; et al. Spatial repellents: From discovery and development to evidence-based validation. Malar. J. 2012, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi Niroumand, M.; Farzaei, M.H.; Karimpour Razkenari, E.; Amin, G.; Khanavi, M.; Akbarzadeh, T.; Shams-Ardekani, M.R. An evidence-based review on medicinal plants used as insecticide and Insect Repellent in Traditional Iranian Medicine. Iran. Red Crescent Med. J. 2016, 18, e22361. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, A.; Khoobdel, M.; Zahraei-Ramazani, A.; Azarmi, S.; Mosawi, S.H. Effectiveness of plant-based repellents against different Anopheles species: A systematic review. Malar. J. 2019, 18, 436. [Google Scholar] [CrossRef]
- Hazarika, H.; Krishnatreyya, H.; Tyagi, V.; Islam, J.; Gogoi, N.; Goyary, D.; Chattopadhyay, P.; Zaman, K. The fabrication and assessment of mosquito repellent cream for outdoor protection. Sci. Rep. 2022, 12, 2180. [Google Scholar] [CrossRef]
- Dube, F.F.; Tadesse, K.; Birgersson, G.; Seyoum, E.; Tekie, H.; Ignell, R.; Hill, S.R. Fresh, dried or smoked? Repellent properties of volatiles emitted from ethnomedicinal plant leaves against malaria and yellow fever vectors in Ethiopia. Malar. J. 2011, 10, 375. [Google Scholar] [CrossRef]
- Paluch, G.; Grodnitzky, J.; Bartholomay, L.; Coats, J. Quantitative structure-activity relationship of botanical sesquiterpenes: Spatial and contact repellency to the yellow fever mosquito, Aedes aegypti. J. Agric. Food Chem. 2009, 57, 7618–7625. [Google Scholar] [CrossRef]
- Paluch, G.; Bartholomay, L.; Coats, J. Mosquito repellents: A review of chemical structure diversity and olfaction. Pest Manag. Sci. 2010, 66, 925–935, Erratum in Pest Manag Sci. 2010, 66, 1155. [Google Scholar] [CrossRef]
- Peach, D.A.H.; Almond, M.; Gries, R.; Gries, G. Lemongrass and Cinnamon Bark: Plant Essential Oil Blend as a Spatial Repellent for Mosquitoes in a Field Setting. J. Med. Entomol. 2019, 56, 1346–1352, Erratum in J. Med. Entomol. 2019, 56, 1750. https://doi.org/10.1093/jme/tjz135. [Google Scholar] [CrossRef]
- Njoroge, T.M.; Hamid-Adiamoh, M.; Duman-Scheel, M. Maximizing the Potential of Attractive Targeted Sugar Baits (ATSBs) for Integrated Vector Management. Insects 2023, 14, 585. [Google Scholar] [CrossRef]
- Fiorenzano, J.M.; Koehler, P.G.; Xue, R.-D. Attractive Toxic Sugar Bait (ATSB) For Control of Mosquitoes and Its Impact on Non-Target Organisms: A Review. Int. J. Environ. Res. Public Health 2017, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; Xue, R.D.; et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar. J. 2020, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Zembere, K. The potential for attractive toxic sugar baits to complement core malaria interventions strategies: The need for more evidence. Malar. J. 2024, 23, 356. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Gupta, S.K.; Pasi, S. Attractive toxic sugar baits: A magic bullet for control of malaria and dengue in urban settings of India? J. Vector Borne Dis. 2023, 60, 340–341. [Google Scholar] [CrossRef]
- Junnila, A.; Revay, E.E.; Müller, G.C.; Kravchenko, V.; Qualls, W.A.; Xue, R.D.; Allen, S.A.; Beier, J.C.; Schlein, Y. Efficacy of attractive toxic sugar baits (ATSB) against Aedes albopictus with garlic oil encapsulated in beta-cyclodextrin as the active ingredient. Acta Trop. 2015, 152, 195–200. [Google Scholar] [CrossRef]
- Kyomuhangi, I.; Yukich, J.; Saili, K.; Orange, E.; Masuzyo, M.H.; Mwenya, M.; Mambo, P.; Hamainza, B.; Wagman, J.; Miller, J.; et al. Evaluating trends in damage to attractive targeted sugar baits (ATSBs) deployed during the second year of a two-year Phase III trial in Western Zambia. Malar. J. 2024, 23, 263. [Google Scholar] [CrossRef]
- Chiu, M.C.; Neoh, K.B.; Hwang, S.Y. The effect of attractive toxic sugar bait on the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae) in community farms in Northern Taiwan. Acta Trop. 2024, 250, 107102. [Google Scholar] [CrossRef]
- Owusu-Akyaw, M.; Owusu-Asenso, C.M.; Abdulai, A.; Mohammed, A.R.; Sraku, I.K.; Boadu, E.N.; Aduhene, E.; Attah, S.K.; Afrane, Y.A. Risk of arboviral transmission and insecticide resistance status of Aedes mosquitoes during a yellow fever outbreak in Ghana. BMC Infect. Dis. 2024, 24, 731. [Google Scholar] [CrossRef]
- Dusfour, I.; Vontas, J.; David, J.P.; Weetman, D.; Fonseca, D.M.; Corbel, V.; Raghavendra, K.; Coulibaly, M.B.; Martins, A.J.; Kasai, S.; et al. Management of insecticide resistance in the major Aedes vectors of arboviruses: Advances and challenges. PLoS Neglected Trop. Dis. 2019, 13, e0007615. [Google Scholar] [CrossRef]
- Kamgang, B.; Yougang, A.P.; Tchoupo, M.; Riveron, J.M.; Wondji, C. Temporal distribution and insecticide resistance profile of two major arbovirus vectors Aedes aegypti and Aedes albopictus in Yaoundé, the capital city of Cameroon. Parasites Vectors 2017, 10, 469. [Google Scholar] [CrossRef]
- Vontas, J.; Kioulos, E.; Pavlidi, N.; Morou, E.; della Torre, A.; Ranson, H. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pestic. Biochem. Physiol. 2012, 104, 126–131. [Google Scholar] [CrossRef]
- Jones, R.T.; Tytheridge, S.J.; Vegvari, C.; Meredith, H.R.; Pretorius, E.A.; Ant, T.H.; Logan, J.G. The effectiveness of putative wearable repellent technologies to protect against mosquito biting and Aedes-borne diseases, and their economic impact. PLoS Neglected Trop. Dis. 2024, 18, e0012621. [Google Scholar] [CrossRef]
- Londono-Renteria, B.; Patel, J.C.; Vaughn, M.; Funkhauser, S.; Ponnusamy, L.; Grippin, C.; Jameson, S.B.; Apperson, C.; Mores, C.N.; Wesson, D.M.; et al. Long-Lasting Permethrin-Impregnated Clothing Protects Against Mosquito Bites in Outdoor Workers. Am. J. Trop. Med. Hyg. 2015, 93, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Flores, H.A.; O’Neill, S.L. Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Benelli, G.; Jeffries, C.L.; Walker, T. Biological control of mosquito vectors: Past, present, and future. Insects 2016, 7, 52. [Google Scholar] [CrossRef]
- Britch, S.C.; Nyberg, H.; Aldridge, R.L.; Swan, T.; Linthicum, K.J. Acoustic Control of Mosquito Larvae In Artificial Drinking Water Containers. J. Am. Mosq. Control Assoc. 2016, 32, 341–344. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Tseng, L.-C.; Murugan, K.; Panneerselvam, C.; Aziz, A.T.; Benelli, G.; Hwang, J.-S. Ultrasonic Technology Applied against Mosquito Larvae. Appl. Sci. 2020, 10, 3546. [Google Scholar] [CrossRef]
- Bitencourt, R.d.O.B.; Faria, F.d.S.; Marchesini, P.; dos Santos-Mallet, J.R.; Camargo, M.G.; Bittencourt, V.R.E.P.; Pontes, E.G.; Pereira, D.B.; Chaves, D.S.d.A.; Angelo, I.d.C. Entomopathogenic fungi and Schinus molle essential oil: The combination of two eco-friendly agents against Aedes aegypti larvae. J. Invertebr. Pathol. 2022, 194, 107827. [Google Scholar] [CrossRef]
- Bitencourt, R.d.O.B.; Mallet, J.R.d.S.; Mesquita, E.; Gôlo, P.S.; Fiorotti, J.; Bittencourt, V.R.E.P.; Pontes, E.G.; Angelo, I.d.C. Larvicidal activity, route of interaction and ultrastructural changes in Aedes aegypti exposed to entomopathogenic fungi. Acta Trop. 2021, 213, 105732. [Google Scholar] [CrossRef]
- Moreira, H.V.S.; Bitencourt, R.d.O.B.; da Rocha, I.U.; Ribeiro, M.L.; Bittencourt, V.R.E.P.; Gôlo, P.S.; Angelo, I.d.C. Entomopathogenic fungi against the dengue vector Aedes aegypti: Laboratory and semifield assessment. Contrib. A LAS Cienc. SOCIALES 2024, 17, e7559. [Google Scholar] [CrossRef]
- Kataki, A.S.; Baldini, F.; Naorem, A.S. Evaluation of synergistic effect of entomopathogenic fungi Beauveria bassiana and Lecanicillium lecacii on the mosquito Culex quinquefaciatus. PLoS ONE 2024, 19, e0308707. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Kanuka, H.; Rakotondrafara, A.; Tani, M.; Aiuchi, D. Pathogenicity and sub-lethal activity of orally administered entomopathogenic fungi against two adult mosquito species, Aedes aegypti (Diptera: Culicidae) and Anopheles stephensi (Diptera: Culicidae). J. Invertebr. Pathol. 2024, 207, 108233. [Google Scholar] [CrossRef] [PubMed]
- Renuka, S.; Vani, H.C.; Alex, E. Entomopathogenic Fungi as a Potential Management Tool for the Control of Urban Malaria Vector, Anopheles stephensi (Diptera: Culicidae). J. Fungi 2023, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Scholte, E.J.; Knols, B.G.; Samson, R.A.; Takken, W. Entomopathogenic fungi for mosquito control: A review. J. Insect Sci. 2004, 4, 19. [Google Scholar] [CrossRef]
- Evans, H.C.; Elliot, S.L.; Barreto, R.W. Entomopathogenic fungi and their potential for the management of Aedes aegypti (Diptera: Culicidae) in the Americas. Mem. Inst. Oswaldo Cruz 2018, 113, 206–214. [Google Scholar] [CrossRef]
- Dobson, S.L. When More is Less: Mosquito Population Suppression Using Sterile, Incompatible and Genetically Modified Male Mosquitoes. J. Med. Entomol. 2021, 58, 1980–1986. [Google Scholar] [CrossRef]
- Bouyer, J. Current status of the sterile insect technique for the suppression of mosquito populations on a global scale. Infect. Dis. Poverty 2024, 13, 68. [Google Scholar] [CrossRef]
- Gato, R.; Menéndez, Z.; Rodríguez, M.; Gutiérrez-Bugallo, G.; Del Carmen Marquetti, M. Advancing the art of mosquito control: The journey of the sterile insect technique against Aedes aegypti in Cuba. Infect. Dis. Poverty 2024, 13, 61. [Google Scholar] [CrossRef]
- de Castro Poncio, L.; Dos Anjos, F.A.; de Oliveira, D.A.; Rebechi, D.; de Oliveira, R.N.; Chitolina, R.F.; Fermino, M.L.; Bernardes, L.G.; Guimaeres, D.; Lemos, P.A.; et al. Novel Sterile Insect Technology Program Results in Suppression of a Field Mosquito Population and Subsequently to Reduced Incidence of Dengue. J. Infect. Dis. 2021, 224, 1005–1014. [Google Scholar] [CrossRef]
- Dumont, Y.; Yatat-Djeumen, I.V. Sterile insect technique with accidental releases of sterile females. Impact on mosquito-borne diseases control when viruses are circulating. Math. Biosci. 2022, 343, 108724. [Google Scholar] [CrossRef]
- Bansal, S.; Lim, J.T.; Chong, C.S.; Dickens, B.; Ng, Y.; Deng, L.; Lee, C.; Tan, L.Y.; Kakani, E.G.; Yoong, Y.; et al. Effectiveness of Wolbachia-mediated sterility coupled with sterile insect technique to suppress adult Aedes aegypti populations in Singapore: A synthetic control study. Lancet Planet. Health 2024, 8, e617–e628. [Google Scholar] [CrossRef] [PubMed]
- Iturbe-Ormaetxe, I.; Walker, T.; O’ Neill, S.L. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 2011, 12, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Ant, T.H.; Mancini, M.V.; McNamara, C.J.; Rainey, S.M.; Sinkins, S.P. Wolbachia-Virus interactions and arbovirus control through population replacement in mosquitoes. Pathog. Glob. Health 2023, 117, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.I.L.; Suzuki, Y.; Carvajal, T.; Muñoz, M.N.M.; Watanabe, K. Intracellular interactions between arboviruses and Wolbachia in Aedes aegypti. Front. Cell Infect. Microbiol. 2021, 11, 690087. [Google Scholar] [CrossRef]
- van den Hurk, A.F.; Hall-Mendelin, S.; Pyke, A.T.; Frentiu, F.D.; McElroy, K.; Day, A.; Higgs, S.; O’Neill, S.L. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Neglected Trop. Dis. 2012, 6, e1892. [Google Scholar] [CrossRef]
- Rocha, M.N.; Duarte, M.M.; Mansur, S.B.; Silva, B.D.M.E.; Pereira, T.N.; Adelino, T.É.R.; Giovanetti, M.; Alcantara, L.C.J.; Santos, F.M.; Costa, V.R.M.; et al. Pluripotency of Wolbachia against Arboviruses: The case of yellow fever. Gates Open Res. 2019, 3, 161. [Google Scholar] [CrossRef]
- Ritchie, S.A.; van den Hurk, A.F.; Smout, M.J.; Staunton, K.M.; Hoffmann, A.A. Mission Accomplished? We Need a Guide to the ‘Post Release’ World of Wolbachia for Aedes-borne Disease Control. Trends Parasitol. 2018, 34, 217–226. [Google Scholar] [CrossRef]
- Montenegro, D.; Cortés-Cortés, G.; Balbuena-Alonso, M.G.; Warner, C.; Camps, M. Wolbachia-based emerging strategies for control of vector-transmitted disease. Acta Trop. 2024, 260, 107410. [Google Scholar] [CrossRef]
- Alphey, L.; McKemey, A.; Nimmo, D.; Neira Oviedo, M.; Lacroix, R.; Matzen, K.; Beech, C. Genetic control of Aedes mosquitoes. Pathog. Glob. Health 2013, 107, 170–179. [Google Scholar] [CrossRef]
- Wang, G.H.; Gamez, S.; Raban, R.R.; Marshall, J.M.; Alphey, L.; Li, M.; Rasgon, J.L.; Akbari, O.S. Combating mosquito-borne diseases using genetic control technologies. Nat. Commun. 2021, 12, 4388. [Google Scholar] [CrossRef]
- Wang, G.H.; Du, J.; Chu, C.Y.; Madhav, M.; Hughes, G.L.; Champer, J. Symbionts and gene drive: Two strategies to combat vector-borne disease. Trends Genet. 2022, 38, 708–723. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Li, M.; McMeniman, C.J.; Akbari, O.S. CRISPR-Mediated Genome Engineering in Aedes aegypti. Methods Mol. Biol. 2022, 2509, 23–51. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.H.; Gaddelapati, S.C.; Jiao, Y.; Koo, J.; Palli, S.R. CRISPR-Cas9 Genome Editing Uncovers the Mode of Action of Methoprene in the yellow fever mosquito, Aedes aegypti. CRISPR J. 2022, 5, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, X.; Xie, X.; Li, C. Advances in CRISPR/Cas9-Based Gene Editing Technology in Mosquitoes. Zoonoses 2024, 4, 971. [Google Scholar] [CrossRef]
- Williams, A.E.; Franz, A.W.E.; Reid, W.R.; Olson, K.E. Antiviral Effectors and Gene Drive Strategies for Mosquito Population Suppression or Replacement to Mitigate Arbovirus Transmission by Aedes aegypti. Insects 2020, 11, 52. [Google Scholar] [CrossRef]
- Reid, W.R.; Olson, K.E.; Franz, A.W.E. Current effector and gene-drive developments to engineer arbovirus-resistant Aedes aegypti (Diptera: Culicidae) for a sustainable population replacement strategy in the field. J. Med. Entomol. 2021, 58, 1987–1996. [Google Scholar] [CrossRef]
- Noble, C.; Adlam, B.; Church, G.M.; Esvelt, K.M.; Nowak, M.A. Current CRISPR gene drive systems are likely to be highly invasive in wild populations. Elife 2018, 7, e33423. [Google Scholar] [CrossRef]
- Chae, K.; Dawson, C.; Valentin, C.; Contreras, B.; Zapletal, J.; Myles, K.M.; Adelman, Z.N. Engineering a self-eliminating transgene in the yellow fever mosquito, Aedes aegypti. PNAS Nexus 2022, 1, pgac037. [Google Scholar] [CrossRef]
- Pacca, C.C.; Severino, A.A.; Mondini, A.; Rahal, P.; D’avila, S.G.; Cordeiro, J.A.; Nogueira, M.C.; Bronzoni, R.V.; Nogueira, M.L. RNA interference inhibits yellow fever virus replication in vitro and in vivo. Virus Genes 2009, 38, 224–231. [Google Scholar] [CrossRef]
- Samuel, G.H.; Pohlenz, T.; Dong, Y.; Coskun, N.; Adelman, Z.N.; Dimopoulos, G.; Myles, K.M. RNA interference is essential to modulating the pathogenesis of mosquito-borne viruses in the yellow fever mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2023, 120, e2213701120. [Google Scholar] [CrossRef]
- Bargielowski, I.; Kaufmann, C.; Alphey, L.; Reiter, P.; Koella, J. Flight performance and teneral energy reserves of two genetically-modified and one wild-type strain of the yellow fever mosquito Aedes aegypti. Vector Borne Zoonotic Dis. 2012, 12, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Julander, J.G. Experimental therapies for yellow fever. Antiviral. Res. 2013, 97, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Qi, Z. Mosquito-Borne Flaviviruses and Current Therapeutic Advances. Viruses 2022, 14, 1226. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Dimopoulos, G. Antiviral compounds for blocking arboviral transmission in mosquitoes. Viruses 2021, 13, 108. [Google Scholar] [CrossRef]
- Ren, H.; Wang, J.; Tang, H.; Qian, X.; Xia, B.; Luo, Z.; Xu, Z.; Qi, Z.; Zhao, P. Tiratricol inhibits yellow fever virus replication through targeting viral RNA-dependent RNA polymerase of NS5. Antivir. Res. 2023, 219, 105737. [Google Scholar] [CrossRef]
- Qian, X.; Wu, B.; Tang, H.; Luo, Z.; Xu, Z.; Ouyang, S.; Li, X.; Xie, J.; Yi, Z.; Leng, Q.; et al. Rifapentine is an entry and replication inhibitor against yellow fever virus both in vitro and in vivo. Emerg. Microbes Infect. 2022, 11, 873–884. [Google Scholar] [CrossRef]
- Heinz, S.; Kolimenakis, A.; Horstick, O.; Yakob, L.; Michaelakis, A.; Lowery Wilson, M. Systematic review: Yellow fever control through environmental management mechanisms. Trop. Med. Int. Health 2021, 26, 1411–1418. [Google Scholar] [CrossRef]
- Banks, S.D.; Murray, N.; Wilder-Smith, A.; Logan, J.G. Insecticide-treated clothes for the control of vector-borne diseases: A review on effectiveness and safety. Med. Vet. Entomol. 2014, 28 (Suppl. S1), 14–25. [Google Scholar] [CrossRef]
- Barrera, R. New tools for Aedes control: Mass trapping. Curr. Opin. Insect Sci. 2022, 52, 100942. [Google Scholar] [CrossRef]
- Jaffal, A.; Fite, J.; Baldet, T.; Delaunay, P.; Jourdain, F.; Mora-Castillo, R.; Olive, M.M.; Roiz, D. Current evidences of the efficacy of mosquito mass-trapping interventions to reduce Aedes aegypti and Aedes albopictus populations and Aedes-borne virus transmission. PLoS Neglected Trop. Dis. 2023, 17, e0011153. [Google Scholar] [CrossRef] [PubMed]
- Barrera, R.; Harris, A.; Hemme, R.R.; Felix, G.; Nazario, N.; Muñoz-Jordan, J.L.; Rodriguez, D.; Miranda, J.; Soto, E.; Martinez, S.; et al. Citywide Control of Aedes aegypti (Diptera: Culicidae) during the 2016 Zika Epidemic by Integrating Community Awareness, Education, Source Reduction, Larvicides, and Mass Mosquito Trapping. J. Med. Entomol. 2019, 56, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Durán, J.A.; Hamer, G.L.; Reyes-Villanueva, F.; Fernández-Santos, N.A.; Uriegas-Camargo, S.; Rodríguez-Martínez, L.M.; Estrada-Franco, J.G.; Rodríguez-Pérez, M.A. Effectiveness of mass trapping interventions using autocidal gravid ovitraps (AGO) for the control of the dengue vector, Aedes (Stegomyia) aegypti, in Northern Mexico. Parasites Vectors 2024, 17, 344. [Google Scholar] [CrossRef] [PubMed]
- Debebe, Y.; Tekie, H.; Dugassa, S.; Hopkins, R.J.; Hill, S.R.; Ignell, R. Mosquito odour-baited mass trapping reduced malaria transmission intensity: A result from a controlled before-and-after intervention study. BMC Med. 2024, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Rapley, L.P.; Williams, C.; Johnson, P.H.; Larkman, M.; Silcock, R.M.; Long, S.A.; Russell, R.C. A lethal ovitrap-based mass trapping scheme for dengue control in Australia: I. Public acceptability and performance of lethal ovitraps. Med. Vet. Entomol. 2009, 23, 295–302. [Google Scholar] [CrossRef]
- Johnson, B.J.; Ritchie, S.A.; Fonseca, D.M. The State of the Art of Lethal Oviposition Trap-Based Mass Interventions for Arboviral Control. Insects 2017, 8, 5. [Google Scholar] [CrossRef]
- Mensah, E.A.; Gyasi, S.O.; Nsubuga, F.; Alali, W.Q. A proposed One Health approach to control yellow fever outbreaks in Uganda. One Health Outlook 2024, 6, 9. [Google Scholar] [CrossRef]
- Enitan, S.; Gbise, D.; Dogonyaro, B.; Eke, S.; Iduh, U.; Dada, M.; Adebola, O.; Osareniro, O.; John-Ugwuanya, G.; Itodo, G.; et al. One health approach in the fight against yellow fever in Nigeria. Microbes Infect. Dis. 2024, 5, 972–990. [Google Scholar] [CrossRef]
- Kelly, T.R.; Machalaba, C.; Karesh, W.B.; Crook, P.Z.; Gilardi, K.; Nziza, J.; Uhart, M.M.; Robles, E.A.; Saylors, K.; Joly, D.O.; et al. Implementing One Health approaches to confront emerging and re-emerging zoonotic disease threats: Lessons from PREDICT. One Health Outlook 2020, 2, 1. [Google Scholar] [CrossRef]
- Gaythorpe, K.A.; Hamlet, A.; Jean, K.; Garkauskas Ramos, D.; Cibrelus, L.; Garske, T.; Ferguson, N. The global burden of yellow fever. eLife 2021, 10, e64670. [Google Scholar] [CrossRef]
- Faburay, B. The case for a ‘one health’ approach to combating vector-borne diseases. Infect. Ecol. Epidemiol. 2015, 5, 28132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nederlof, R.A.; Virgilio, T.; Stemkens, H.J.J.; da Silva, L.C.C.P.; Montagna, D.R.; Abdussamad, A.M.; Chipangura, J.; Bakker, J. Yellow Fever in Non-Human Primates: A Veterinary Guide from a One Health Perspective. Vet. Sci. 2025, 12, 339. https://doi.org/10.3390/vetsci12040339
Nederlof RA, Virgilio T, Stemkens HJJ, da Silva LCCP, Montagna DR, Abdussamad AM, Chipangura J, Bakker J. Yellow Fever in Non-Human Primates: A Veterinary Guide from a One Health Perspective. Veterinary Sciences. 2025; 12(4):339. https://doi.org/10.3390/vetsci12040339
Chicago/Turabian StyleNederlof, Remco A., Tommaso Virgilio, Hendrickus J. J. Stemkens, Luiz C. C. Pereira da Silva, Daniela R. Montagna, Abdussamad M. Abdussamad, John Chipangura, and Jaco Bakker. 2025. "Yellow Fever in Non-Human Primates: A Veterinary Guide from a One Health Perspective" Veterinary Sciences 12, no. 4: 339. https://doi.org/10.3390/vetsci12040339
APA StyleNederlof, R. A., Virgilio, T., Stemkens, H. J. J., da Silva, L. C. C. P., Montagna, D. R., Abdussamad, A. M., Chipangura, J., & Bakker, J. (2025). Yellow Fever in Non-Human Primates: A Veterinary Guide from a One Health Perspective. Veterinary Sciences, 12(4), 339. https://doi.org/10.3390/vetsci12040339






