Prevalence and Genotyping of Mycobacterium avium subsp. paratuberculosis in Sheep from Inner Mongolia, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
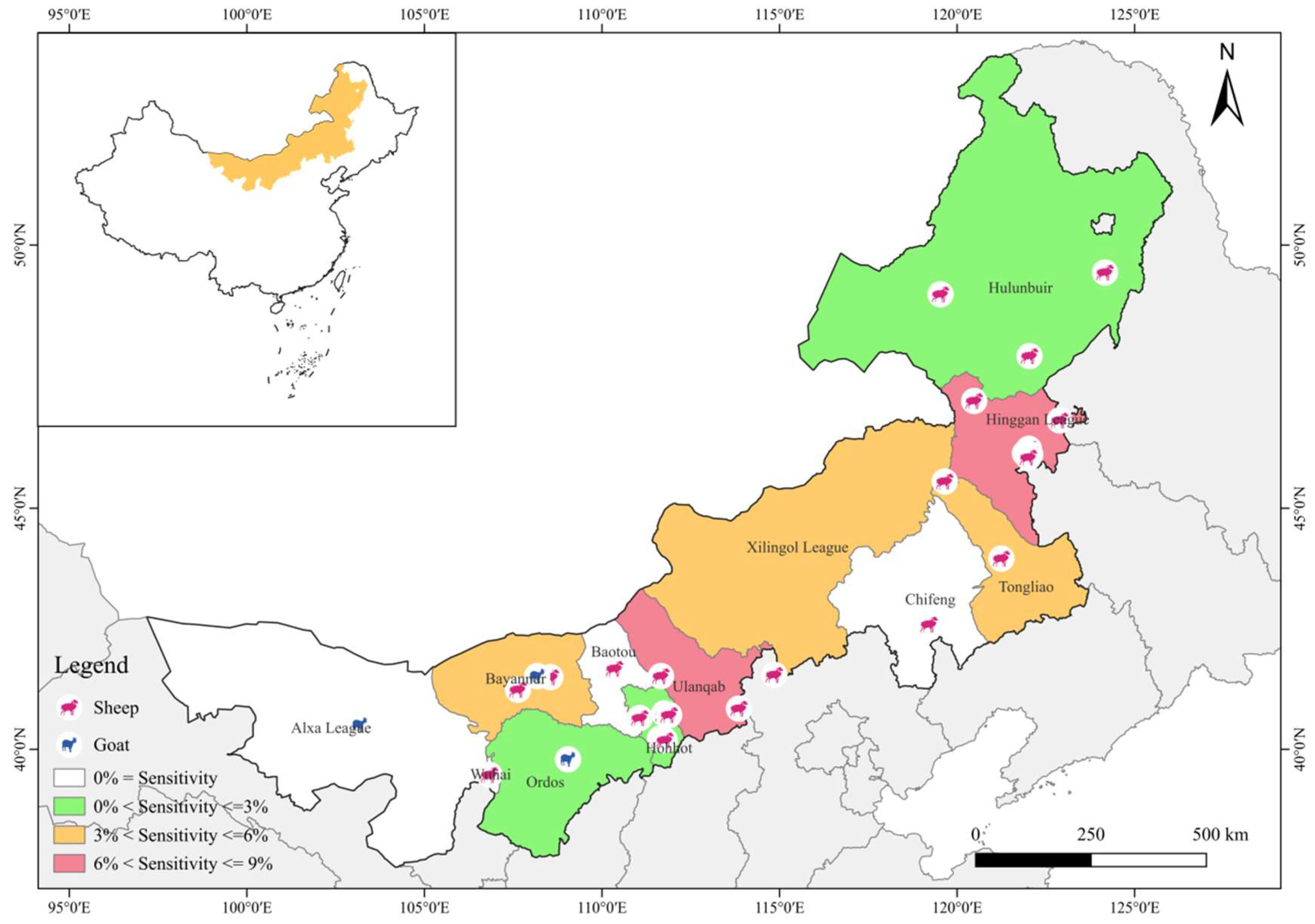
2.1. Study Areas and Sample Collection
2.2. DNA Extraction
2.3. qPCR
2.4. Statistical Analysis
3. Results
3.1. Infection Status of MAP
3.2. Genotype Identification MAP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Udainiya, S.; Tiwari, A. Johne’s Disease (Paratuberculosis). In The Handbook of Zoonotic Diseases of Goats, 1st ed.; Rana, T., Biswas, S., Eds.; CAB International: Wallingford, UK, 2024; Volume 18, pp. 220–231. [Google Scholar]
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Arrigoni, N.; Juste, R.; Sáez, J.L.; Dhand, N.; et al. Control of paratuberculosis: Who, why and how. A review of 48 countries. BMC Vet. Res. 2019, 15, 198. [Google Scholar]
- Kuenstner, L.; Kuenstner, J.T. Mycobacterium avium ssp. paratuberculosis in the food supply: A public health issue. Front. Public Health 2021, 9, 647448. [Google Scholar]
- Bryant, J.M.; Thibault, V.C.; Smith, D.G.; McLuckie, J.; Heron, I.; Sevilla, I.A.; Biet, F.; Harris, S.R.; Maskell, D.J.; Bentley, S.D.; et al. Phylogenomic exploration of the relationships between strains of Mycobacterium avium subspecies paratuberculosis. BMC Genom. 2016, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.; Zschöck, M.; Ewers, C.; Eisenberg, T. Genotyping methods and molecular epidemiology of Mycobacterium avium subsp. paratuberculosis (MAP). Int. J. Vet. Sci. Med. 2018, 6, 258–264. [Google Scholar] [CrossRef]
- Windsor, P.A. Paratuberculosis in sheep and goats. Vet. Microbiol. 2015, 181, 161–169. [Google Scholar]
- Liapi, M.; Leontidesb, L.; Kostoulasb, P.; Botsarisc, G.; Iacovoua, Y.; Reesc, C.; Georgioua, K.; Smithd, G.C.; Nasebye, D.C. Bayesian estimation of the true prevalence of Mycobacterium avium subsp. paratuberculosis infection in Cypriot dairy sheep and goat flocks. Small Rumin. Res. 2011, 95, 174–178. [Google Scholar]
- Manning, E.J.; Steinberg, H.; Krebs, V.; Collins, M.T. Diagnostic testing patterns of natural Mycobacterium paratuberculosis infection in pygmy goats. Can. J. Vet. Res. 2003, 67, 213–218. [Google Scholar]
- Salgado, M.; Kruze, J.; Collins, M.T. Diagnosis of paratuberculosis by fecal culture and ELISA on milk and serum samples in two types of Chilean dairy goat herds. J. Vet. Diagn. Investig. 2007, 19, 99–102. [Google Scholar]
- Mercier, P.; Baudry, C.; Beaudeau, F.; Seegers, H.; Malher, X. Estimated prevalence of Mycobacterium avium subspecies paratuberculosis infection in herds of dairy goats in France. Vet. Rec. 2010, 167, 412–415. [Google Scholar]
- Singh, K.; Chandel, B.S.; Dadawala, A.I.; Singh, S.V.; Chauhan, H.C.; Singh, B.; Agrawal, N.D.; Gupta, S.; Chaubey, K.K. Incidence of Mycobacterium avium subspecies paratuberculosis in Mehsana Breed of Goats from North Gujarat using Multiple Tests. Adv. Anim. Vet. Sci. 2013, 1, 28–31. [Google Scholar]
- Fiorentino, M.A.; Gioffréb, A.; Cironea, K.; Morsellaa, C.; Alonsoc, B.; Delgadob, F.; Paolicchia, F. First isolation of Mycobacterium avium subsp. paratuberculosis in a dairy goat in Argentina: Pathology and molecular characterization. Small Rumin. Res. 2012, 108, 133–136. [Google Scholar]
- Magalhães, N.M.D.A.; Limeira, C.H.; Costa, R.D.L.; Portela, R.D.A.; Azevedo, S.S.D.; Alves, F.S.F.; Alves, C.J. Global prevalence of Mycobacterium avium subsp. paratuberculosis in sheep: Systematic review and meta-analysis. Small Rumin. Res. 2025, 243, 107430. [Google Scholar]
- Yu, Y.D.; Zhang, S.H.; Xu, G.Y.; Xu, D.F.; Zheng, H.; Li, B.; Shen, K.F.; Fu, L.Z. Identification of Mycobacterium avium subspecies paratuberculosis in sheep farms in Bayannaoer, Inner Mongolia, China (short communication). BMC Vet. Res. 2022, 18, 281. [Google Scholar]
- Zhao, L.; Wang, Y.; Wang, J.L.; Zhao, W.H.; Cheng, H.X.; Ma, Y.M.; Chai, H.L.; Zhang, Z.S.; Wang, L.F.; Miao, Z.Q.; et al. Serological investigation and genotyping of Mycobacterium avium subsp. paratuberculosis in sheep and goats in Inner Mongolia, China. PLoS ONE 2021, 16, e0256628. [Google Scholar]
- Wong, W.; Farr, R.; Joglekar, M.; Januszewski, A.; Hardikar, A. Probe-based Real-time PCR Approaches for Quantitative Measurement of microRNAs. Jove-J. Vis. Exp. 2015, 98, 52586. [Google Scholar]
- Hodgeman, R.; Liu, Y.; Rochfort, S.; Rodoni, B. Development and evaluation of genomics informed real-time PCR assays for the detection and strain typing of Mycobacterium avium subsp. paratuberculosis. J. Appl. Microbiol. 2024, 135, lxae107. [Google Scholar]
- Johne, H.A.; Frothingham, L. Ein eigenthuemlicher fall von tuberculose beim Rind [A peculiar case of tuberculose in a cow]. Dtsch. Z. Für Tiermed. Und Vgl. Pathol. 1895, 21, 438–455. [Google Scholar]
- Dziedzinska, R.; Slana, I. Mycobacterium avium subsp. paratuberculosis–An Overview of the Publications from 2011 to 2016. Curr. Clin. Microbiol. Rep. 2017, 4, 19–28. [Google Scholar]
- Cui, Z.L. Bacterial sexually transmitted diseases in dairy cows. In Diseases of Dairy Cattle, 1st ed.; Cui, Z.L., Li, Y.Q., Chen, H.T., Zhang, J.C., Eds.; China Agriculture: Beijing, China, 2007; Volume 5, pp. 220–223. [Google Scholar]
- Grant, I. Mycobacterium avium subsp. paratuberculosis in Animal-Derived Foods and the Environment. In Paratuberculosis Organism, Disease, Control, 2nd ed.; Behr, M., Stevenson, K., Kapur, V., Eds.; CAB International: Wallingford, UK, 2020; Volume 2, pp. 14–28. [Google Scholar]
- Xue, S.Y.; Ma, W.; Li, M.Y.; Meng, W.K.; Ding, Y.L.; Yang, B.; Lv, Y.R.; Chen, R.B.; Wu, Z.H.; Tunala, S.; et al. The Impact of Mycobacterium avium subsp. Paratuberculosis on Intestinal Microbial Community Composition and Diversity in Small-Tail Han Sheep. Pathogens 2024, 13, 1118. [Google Scholar] [CrossRef]
- Alonso-Hearn, M.; Ballesteros, A.; Navarro, A.; Badia-Bringué, G.; Casais, R. Lateral-flow assays for bovine paratuberculosis diagnosis. Front. Vet. Sci. 2023, 10, 1257488. [Google Scholar]
- Idris, S.M.; Eltom, K.H.; Okuni, J.B.; Ojok, L.; Elmagzoub, W.A.; El Wahed, A.A.; Eltayeb, E.; Gameel, A.A. Paratuberculosis: The Hidden Killer of Small Ruminants. Animals 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Hines, M.E., 2nd; Stabel, J.R.; Sweeney, R.W.; Griffin, F.; Talaat, A.M.; Bakker, D.; Benedictus, G.; Davis, W.C.; de Lisle, G.W.; Gardner, I.A.; et al. Experimental challenge models for Johne’s disease: A review and proposed international guidelines. Vet. Microbiol. 2007, 122, 197–222. [Google Scholar] [CrossRef] [PubMed]
- Köhler, H.; Soschinka, A.; Meyer, M.; Kather, A.; Reinhold, P.; Liebler-Tenorio, E. Characterization of a caprine model for the subclinical initial phase of Mycobacterium avium subsp. paratuberculosis infection. BMC Vet. Res. 2015, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Vaughan, J.A.; Stiles, P.L.; Noske, P.J.; Tizard, M.L.; Prowse, S.J.; Michalski, W.P.; Butler, K.L.; Jones, S.L. A long-term study in Merino sheep experimentally infected with Mycobacterium avium subsp. paratuberculosis: Clinical disease, faecal culture and immunological studies. Vet. Microbiol. 2004, 104, 165–178. [Google Scholar] [CrossRef]
- Kawaji, S.; Begg, D.J.; Plain, K.M.; Whittington, R.J. A longitudinal study to evaluate the diagnostic potential of a direct faecal quantitative PCR test for Johne’s disease in sheep. Vet. Microbiol. 2011, 148, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Marquetoux, N.; Mitchell, R.; Ridler, A.; Heuer, C.; Wilson, P. A synthesis of the patho–physiology of Mycobacterium avium subspecies paratuberculosis infection in sheep to inform mathematical modelling of ovine paratuberculosis. Vet. Res. 2018, 49, 27. [Google Scholar] [CrossRef] [PubMed]
- Lombard, J.E.; Gardner, I.A.; Jafarzadeh, S.R.; Fossler, C.P.; Harris, B.; Capsel, R.T.; Wagner, B.A.; Johnson, W.O. Herd-level prevalence of Mycobacterium avium subsp. paratuberculosis infection in United States dairy herds in 2007. Prev. Vet. Med. 2013, 108, 234–238. [Google Scholar] [CrossRef]
- Corbett, C.S.; Naqvi, S.A.; Bauman, C.A.; De Buck, J.; Orsel, K.; Uehlinger, F.; Kelton, D.F.; Barkema, H.W. Prevalence of Mycobacterium avium ssp. paratuberculosis infections in Canadian dairy herds. J. Dairy Sci. 2018, 101, 11218–11228. [Google Scholar] [CrossRef]
- Han, M.; Zhang, L.; Wang, J.L.; Ding, J.B.; Liu, L.X.; Gu, Z.Y.; Ge, L.J.; Yang, H.J. Epidemiological Investigation of Large-scale Dairy Farm on Tuberculosis and Paratuberculosis in Shandong Province. Chin. Agric. Sci. Bull. 2014, 30, 10–13. [Google Scholar]
- Yue, R.; Liu, C.; Barrow, P.; Liu, F.; Cui, Y.; Yang, L.; Zhao, D.; Zhou, X. The isolation and molecular characterization of Mycobacterium avium subsp. paratuberculosis in Shandong province, China. Gut Pathog. 2016, 8, 9. [Google Scholar] [CrossRef]
- Jiménez-Martín, D.; García-Bocanegra, I.; Risalde, M.A.; Fernández-Molera, V.; Jiménez-Ruiz, S.; Isla, J.; Cano-Terriza, D. Epidemiology of paratuberculosis in sheep and goats in southern Spain. Prev. Vet. Med. 2022, 202, 105637. [Google Scholar] [CrossRef] [PubMed]
- De Silva, K.R.; Eda, S.; Lenhart, S. Modeling environmental transmission of MAP infection in dairy cows. Math. Biosci. Eng. 2017, 14, 1001–1017. [Google Scholar] [CrossRef] [PubMed]
- Imada, J.; Kelton, D.F.; Barkema, H.W. Epidemiology, Global Prevalence and Economics of Infection. In Paratuberculosis Organism, Disease, Control, 2nd ed.; Behr, M., Stevenson, K., Kapur, V., Eds.; CAB International: Wallingford, UK, 2020; Volume 1, pp. 1–13. [Google Scholar]
- Stevenson, K.; Ahlstrom, C. Comparative Genomics and Genomic Epidemiology of Mycobacterium avium subsp. paratuberculosis Strains. In Paratuberculosis Organism, Disease, Control, 2nd ed.; Behr, M., Stevenson, K., Kapur, V., Eds.; CAB International: Wallingford, UK, 2020; Volume 6, pp. 76–91. [Google Scholar]
- Sevilla, I.x.; Singh, S.V.; Garrido, J.M.; Aduriz, G.; Rodríguez, S.; Geijo, M.V.; Whittington, R.J.; Saunders, V.; Whitlock, R.H.; Juste, R.A. Molecular typing of Mycobacterium avium subspecies paratuberculosis strains from different hosts and regions. Rev. Sci. Et Tech. 2005, 24, 1061–1066. [Google Scholar] [CrossRef]
- Stevenson, K.; Hughes, V.M.; de Juan, L.; Inglis, N.F.; Wright, F.; Sharp, J.M. Molecular characterization of pigmented and nonpigmented isolates of Mycobacterium avium subsp. paratuberculosis. J. Clin. Microbiol. 2002, 40, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, G.G.; Narnaware, S.D.; Tripathi, B.N. Molecular epidemiology of Mycobacterium avium subspecies paratuberculosis in ruminants in different parts of India. Int. J. Mycobacteriol. 2016, 5, 59–65. [Google Scholar] [CrossRef]
- O’Brien, R.; Mackintosh, C.G.; Bakker, D.; Kopecna, M.; Pavlik, I.; Griffin, J.F. Immunological and molecular characterization of susceptibility in relationship to bacterial strain differences in Mycobacterium avium subsp. paratuberculosis infection in the red deer (Cervus elaphus). Infect. Immun. 2006, 74, 3530–3537. [Google Scholar] [CrossRef]
- Djonne, B. Paratuberculosis in goats. In Paratuberculosis Organism, Disease, Control, 2nd ed.; Behr, M., Stevenson, K., Kapur, V., Eds.; CAB International: Wallingford, UK, 2020; Volume 13, pp. 174–187. [Google Scholar]
- Salgado, M.; Steuer, P.; Troncoso, E.; Collins, M.T. Evaluation of PMS-PCR technology for detection of Mycobacterium avium subsp. paratuberculosis directly from bovine fecal specimens. Vet. Microbiol. 2013, 167, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Roberto, J.P.L.; Limeira, C.H.; Araújo Júnior, J.P.; Malossi, C.D.; Ullmann, L.S.; Silva, M.L.C.R.; Dantas, A.F.M.; do Nascimento, M.J.R.; de Azevedo, S.S.; Alves, C.J. Clinical, histopathological, and molecular findings for Mycobacterium avium subspecies paratuberculosis (MAP) in dairy goats under semiarid conditions. Tuberculosis 2023, 139, 102319. [Google Scholar] [CrossRef]
- Bruczyńska, M.; Didkowska, A.; Brzezińska, S.; Nowak, M.; Filip-Hutsch, K.; Kalicki, M.; Augustynowicz-Kopeć, E.; Anusz, K. Mycobacterium avium Subspecies paratuberculosis in Asymptomatic Zoo Herbivores in Poland. Animals 2023, 13, 1022. [Google Scholar] [CrossRef] [PubMed]
- de Noronha Xavier, A.; de Sá, L.M.N.; de Nazaré Santos Ferreira, M.; de Oliveira, P.R.F.; de Moraes Peixoto, R.; Mota, R.A.; Junior, J.W.P. First serological diagnosis of Mycobacterium avium subsp. paratuberculosis infection in sheep in the state of Pernambuco, Brazil. Vet. Res. Commun. 2024, 48, 1293–1299. [Google Scholar] [CrossRef]
- Plain, K.M.; Marsh, I.B.; Waldron, A.M.; Galea, F.; Whittington, A.M.; Saunders, V.F.; Begg, D.J.; de Silva, K.; Purdie, A.C.; Whittington, R.J. High-throughput direct fecal PCR assay for detection of Mycobacterium avium subsp. paratuberculosis in sheep and cattle. J. Clin. Microbiol. 2014, 52, 745–757. [Google Scholar] [PubMed]
- Yang, J.; Kemps-Mols, B.; Spruyt-Gerritse, M.; Anholts, J.; Claas, F.; Eikmans, M. The source of SYBR green master mix determines outcome of nucleic acid amplification reactions. BMC Res. Notes 2016, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, E.; Aranaz, A.; Romero, B.; de Juan, L.; Alvarez, J.; Bezos, J.; Rodrı´guez, S.; Stevenson, K.; Mateos, A.; Domínguez, L. Polymorphisms in gyrA and gyrB Genes among Mycobacterium avium subsp. paratuberculosis Type I, II, and III Isolates. J. Clin. Microbiol. 2007, 45, 3439. [Google Scholar]
- Möbius, P.; Fritsch, I.; Luyven, G.; Hotzel, H.; Köhler, H. Unique genotypes of Mycobacterium avium subsp. paratuberculosis strains of Type III. Vet. Microbiol. 2009, 139, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Hodgeman, R.; Mann, R.; Savin, K.; Djitro, N.; Rochfort, S.; Rodoni, B. Molecular characterisation of Mycobacterium avium subsp. paratuberculosis in Australia. BMC Microbiol. 2021, 21, 101. [Google Scholar]
- Mizzi, R.; Plain, K.; Timms, V.; Marsh, I.; Whittington, R. Characterisation of IS1311 in Mycobacterium avium subspecies paratuberculosis genomes: Typing, continental clustering, microbial evolution and host adaptation. PLoS ONE 2024, 19, e0294570. [Google Scholar]
- Mizzi, R.; Timms, V.; Price-Carter, M.; Gautam, M.; Whittington, R.; Heuer, C.; Biggs, P.; Plain, K. Comparative Genomics of Mycobacterium avium Subspecies Paratuberculosis Sheep Strains. Front. Vet. Sci. 2021, 8, 637637. [Google Scholar]

| Factor | Sample Size | No. Positive (%) (95% CI) | p Value | OR (95% CI) | C Type | S Type | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Positive (%) (95% CI) | p Value | OR (95% CI) | No. Positive (%) (95% CI) | p Value | OR (95% CI) | |||||||||
| Breed | Sheep | 1346 | 51 (3.79%) (2.77–4.81) | 0.017 | 4.667 (1.128–l19.299) | 44 (3.27%) (2.32–4.22) | 0.036 | 4.005 (0.964–16.631) | 7 (0.52%) (0.14–0.90) | 0.603 | – | |||
| Goat | 239 | 2 (0.84%) (−0.33–2.00) | 2 (0.84%) (−0.33–2.00) | 0 | ||||||||||
| Feeding method | Intensive | 903 | 31 (3.56%) (2.24–4.62) | 0.888 | 0.938 (0.538–1.634) | 28 (3.10%) (1.97–4.24) | 0.879 | 1.084 (0.595–1.978) | 3 (0.33%) (0.04–0.71) | 0.472 | 0.565 (0.126–2.533) | |||
| Free range | 682 | 22 (3.23%) (1.90–4.56) | 18 (2.64%) (1.43–3.85) | 4 (0.59%) (0.01–1.16) | ||||||||||
| Regions | Eastern region | Hinggan League | 194 | 15 (7.73%) | 654 | 31 (4.74%) a (3.11–6.37) | 0.432 | 1.351 (0.709–2.571) | 29 (4.43%) a (2.85–6.02) | 0.087 | 1.985 (0.930–4.238) | 2 (0.31%) ab (−0.12–0.73) | 0.111 | 0.239 (0.046–1.236) |
| Hulun Buir | 160 | 4 (2.50%) | ||||||||||||
| Chifeng | 100 | 0 (0%) | ||||||||||||
| Tongliao | 200 | 12 (6.00%) | ||||||||||||
| Central region | Hohhot | 184 | 3 (1.63%) | 394 | 14 (3.55%) ab (1.72–5.39) | 0.002 | 3.290 (1.500–7.220) | 9 (1.08%) ab (0.80–3.77) | 0.004 | 3.068 (1.391–6.769) | 5 (1.30%) b (0.16–2.38) | 0.504 | – | |
| Xilingol League | 100 | 4 (4.00%) | ||||||||||||
| Ulanqab | 110 | 7 (6.36%) | ||||||||||||
| Western region | Alxa League | 98 | 0 (0%) | 537 | 8 (1.49%) b (0.46–2.52) | 0.049 | 2.436 (1.012–5.865) | 8 (1.49%) b (0.46–2.52) | 0.459 | 1.546 (0.591–4.043) | 0 a | 0.012 | – | |
| Ordos | 100 | 2 (2.00%) | ||||||||||||
| Baotou | 100 | 0 (0%) | ||||||||||||
| Wuhai | 84 | 1 (1.19%) | ||||||||||||
| Bayannur | 155 | 5 (3.22%) | ||||||||||||
| Total | 1585 | 3.34% (53/1585) | 2.90% (46/1585) | 0.44% (7/1585) | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Lv, Y.-R.; Yang, B.; Wang, H.; Jia, J.-T.; Wu, Z.-H.; Nie, M.; Sun, L.-Y.; Xue, S.-Y.; Ding, Y.-L.; et al. Prevalence and Genotyping of Mycobacterium avium subsp. paratuberculosis in Sheep from Inner Mongolia, China. Vet. Sci. 2025, 12, 326. https://doi.org/10.3390/vetsci12040326
Zhang R, Lv Y-R, Yang B, Wang H, Jia J-T, Wu Z-H, Nie M, Sun L-Y, Xue S-Y, Ding Y-L, et al. Prevalence and Genotyping of Mycobacterium avium subsp. paratuberculosis in Sheep from Inner Mongolia, China. Veterinary Sciences. 2025; 12(4):326. https://doi.org/10.3390/vetsci12040326
Chicago/Turabian StyleZhang, Rong, Yue-Rong Lv, Bo Yang, Hao Wang, Jun-Tao Jia, Zhi-Hong Wu, Ming Nie, Lian-Yang Sun, Shi-Yuan Xue, Yu-Lin Ding, and et al. 2025. "Prevalence and Genotyping of Mycobacterium avium subsp. paratuberculosis in Sheep from Inner Mongolia, China" Veterinary Sciences 12, no. 4: 326. https://doi.org/10.3390/vetsci12040326
APA StyleZhang, R., Lv, Y.-R., Yang, B., Wang, H., Jia, J.-T., Wu, Z.-H., Nie, M., Sun, L.-Y., Xue, S.-Y., Ding, Y.-L., Chen, R.-B., Tunala, S., Zhao, L., & Liu, Y.-H. (2025). Prevalence and Genotyping of Mycobacterium avium subsp. paratuberculosis in Sheep from Inner Mongolia, China. Veterinary Sciences, 12(4), 326. https://doi.org/10.3390/vetsci12040326






