Simple Summary
European hedgehogs can pose health risks to humans, particularly in areas where they live in close contact. A study of 134 hedgehogs (2020–2023) examined potential zoonotic fungi. While dermatophytes were rare (with only one case of Paraphyton mirabile observed), yeasts were more common, detected in 25.6% of the sampled hedgehogs. The most frequent yeast was Candida albicans, followed by Yarrowia lipolytica, Rhodotorula mucilaginosa, and Meyerozyma guilliermondii. Candida albicans isolates showed high susceptibility to antifungal treatments. This study highlights the importance of monitoring fungal species in wildlife and raising public awareness to protect human health.
Abstract
European hedgehogs are an important reservoir for many pathogens of health interest. Since hedgehogs live in close contact with humans, potential zoonotic fungi raise significant public health concerns, especially in areas with a high hedgehog density. From 2020 to 2023, 134 hedgehogs were surveyed for potential zoonotic fungi. Non-invasive methods were used, such as brushing live animals with a sterile toothbrush and taking oral and rectal swabs from deceased ones (86 animals). Dermatophytes were cultured on Dermasel agar and identified using molecular tools, while yeasts were isolated on Sabouraud agar with chloramphenicol and determined using Candida Chromogenic agar (MicroBiolDiagnostici®, Cagliari, Italy) and MALDI-TOF (Microflex LT Smart Biotyper with FlexControlBiotyper 3.4 software, Bruker Daltonics, Bremen, Germany). Minimum inhibitory concentrations (MICs) were determined for Candida albicans isolates. Dermatophytes were found in just one hedgehog (0.8%, 95% C.I.: 0–0.04), identified as Paraphyton mirabile. Yeasts were detected in 22 of 86 hedgehogs (25.6%, 95% C.I.: 16.4–34.8), with 25 isolates obtained, including 21 Candida albicans, 2 Yarrowia lipolytica, 1 Rhodotorula mucilaginosa, and 1 Meyerozyma guilliermondii. All C. albicans isolates showed a high susceptibility to the antimycotic panel tested. Monitoring zoonotic fungi harbored by European hedgehogs, as well as raising public awareness on the topic, is of great importance for public health.
1. Introduction
The European hedgehog (Erinaceus europaeus Linnaeus, 1758) is a small mammal widely distributed across Western and Central Europe [1]. Many studies have shown a substantial decline in the European hedgehog population in Northern Europe (the UK, Belgium, the Netherlands, Sweden, and Germany), likely caused by habitat loss and fragmentation, molluscicides and rodenticide poisoning, intensive agricultural practices, road traffic, and predation from European badgers (Meles meles Linnaeus, 1758) in some areas [2]. According to two consecutive assessments conducted by International Union for the Conservation of Nature (IUCN 2013, 2022), there is no evidence of a decline in the Italian population of European hedgehogs, which remains abundant and is not particularly affected by human factors such as traffic [3].
Hedgehogs have nocturnal habits and an omnivorous diet, mostly feeding on arthropods and small invertebrates, such as earthworms and gastropods, that are dug up from the soil or actively hunted in the leaf litter [1,4]. Many of these invertebrates act as intermediate or paratenic hosts for several parasites such as bronchopulmonary nematodes Crenosoma striatum and Eucoleus aerophilus, and the fluke Brachylaemus erinacei, which can cause severe and sometimes fatal infestations in hedgehogs [4,5,6]. Furthermore, many of these parasites have a zoonotic potential, including Cryptosporidium spp. [7] and Giardia duodenalis [8], or can be transmitted to both humans and pets, such as E. aerophilus [5,6]. Moreover, hedgehogs can also harbor several harmful and potentially zoonotic bacteria from the genera Borrelia, Coxiella, Leptospira, Klebsiella, and Salmonella [9], with a high percentage of antimicrobial resistance [10]. Lastly, European hedgehogs constitute an important reservoir for various health-related fungi that inhabit their integument [11,12], such as Trichophyton erinacei, a zoophilic dermatophyte that was first isolated in hedgehogs from New Zealand, raising relevant public health concerns [13,14,15]. Hedgehogs are usually asymptomatic carriers, lacking detectable clinical lesions, whereas direct and indirect transmission can cause dermatophytosis on both humans [16,17,18] and dogs [19,20]. Mite infestations, sustained by Caparinia tripilis, which can cause scabbing and furfural shedding [9], have been shown to facilitate the transmission of zoonotic dermatophytes, such as Microsporum canis and Microsporum gypseum [21], or Trichophyton erinacei [22]. Moreover, hedgehogs can serve as reservoirs for geophilic fungi that are of low zoonotic concern [11]. Among the mycotic pathogens harbored by hedgehogs, infections caused by the commensal yeast Candida albicans have also been reported [9]. There is, in fact, evidence of both oral and intestinal candidiasis in European hedgehogs (E. europaeus) and African pygmy hedgehogs (Atelerix albiventris Wagner, 1841) [23,24].
Considering the numerous pathogens harbored by hedgehogs, it is important not to overlook the health of urban populations, as this may pose a threat to both humans and domestic animals. Since hedgehogs tend to live in close proximity to humans by foraging or hibernating in gardens and public parks, improper handling without the use of adequate protections, or practices that attract hedgehogs to human spaces—such as providing food and water sources shared with domestic animals—can facilitate the transmission of zoonotic pathogens from the wild to the domestic environment.
A mycological survey on pathogenic fungi was carried out to investigate dermatophytes and yeasts on European hedgehogs rescued from Central Italy. Furthermore, minimum inhibitory concentrations (MICs) were tested to interpret the susceptibility and resistance levels of the detected mycotic pathogens to the available antifungal drugs.
2. Materials and Methods
A total of 134 wild European hedgehogs rescued by WildUmbria Wildlife Rescue Center (Central Italy) between 2020 and 2023 were admitted to the University of Perugia Veterinary Teaching Hospital for emergency care and enrolled in the survey. Of these, 48 of the 134 animals were alive at the time of sampling, while 86 out of 134 were deceased due to causes independent of this study (i.e., road traffic or predation victims) and were sampled during necroscopic procedures. Sample collection for dermatophyte investigations was carried out via the toothbrush technique [25]. Samples from live animals were taken on admission by gently brushing the integument of the exposed parts without forcing the animal to uncurl to avoid stressful procedures; samples from deceased animals were taken by brushing all the available surfaces. The samples were inoculated on Dermasel agar, incubated at 25 ± 1 °C, and observed daily for 14 days. Oral and rectal swabs were also collected from deceased animals during the necroscopic inspections to detect yeasts. These samples were sown on Sabouraud Dextrose agar (SDA) supplemented with chloramphenicol and Candida Chromogenic agar (MicroBiolDiagnostici®, Cagliari, Italy) and incubated at 37 °C for 24–48 h. The identification of fungal growths attributable to dermatophytes and yeasts was based on visual inspection of macroscopic and microscopic features with the support of the mycology identification keys provided by The University of Adelaide (https://www.adelaide.edu.au/mycology/ accessed on 12 December 2024); accurate species identification was achieved through molecular and mass spectrometry investigations.
Dermatophyte colonies grown on Dermasel agar were subjected to DNA extraction using the QIAamp DNA mini kit (QIAGEN®, Hilden, Germany) following a modified Gram-positive protocol (Appendix C of the handbook: Protocols for Bacteria. https://www.qiagen.com/us/resources/resourcedetail?id=62a200d6-faf4-469b-b50f-2b59cf738962&lang=en, accessed on 12 December 2024). An end-point PCR was carried out to confirm dermatophyte isolation using universal fungal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) [26]. PCR amplification was carried out at a total volume of 50 µL. The reaction mixture was prepared as follows: 5X Colorless GoTaq® Flexi Buffer (Promega, Madison, WI, USA), 2.5 mM of MgCl2 (Promega, Madison, WI, USA); 0.2 mM of each dNTP (Global Life Sciences Solutions Operations, Little Chalfont, UK); 0.4 µM of each primer; 1.25 units of GoTaq® Hot Start Polymerase (Promega, Madison, WI, USA); 3 µL of template DNA and Nuclease-Free Water (Thermo Fisher Scientific, Austin, TX, USA). PCR amplification was performed in a Mastercycler Nexus X2 (Eppendorf AG, Hamburg, Germany) set to the following conditions: denaturation at 95 °C for 5 min; 35 cycles of 94 °C for 45 s, 56 °C for 45 s, and 72 °C for 1 min; and a final extension at 72 °C for 10 min. The PCR product was run in a 2% agarose gel containing Midori Green Advance (NIPPON Genetics®, Europe GmbH, Düren, Germany). Gel electrophoresis on a 2% agarose gel stained with Midori Green Advance DNA stain (NIPPON Genetics Europe GmbH®, Düren, Germany) allowed for the visualization of the PCR products. The amplicons were purified using a QIAquick PCR Purification Kit (QIAGEN®, Hilden, Germany) to perform a sequencing reaction using a BrilliantDyeTM Terminator v3.1 Cycle Sequencing Kit (NimaGen®, Nijmegen, The Netherlands). The obtained forward and reverse sequences were run in a 3500 Genetic Analyzer (Applied Biosystem, Foster City, CA, USA). Eventually, the consensus sequences were created by BioEdit Sequence Alignment Editor software v7.2.5 [27] and aligned in the Westerdijk Fungal BioDiversity Institute database.
Yeast colonies grown on SDA and Candida Chromogenic agar were identified through a Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight (MALDI-TOF) instrument (Microflex LT Smart Biotyper with FlexControlBiotyper 3.4 software, Bruker Daltonics, Bremen, Germany). MALDI-TOF MS analysis was performed directly from the subcultures obtained on agar plates. A small quantity of the yeast colony was smeared on a spot of a 96-spot stainless steel target plate (Bruker Daltonics, Bremen, Germany). Then, 1 μL of a 70% formic acid solution overlapped each spot. After that, 1 μL of HCCA matrix (a-cyano-4-hydroxycinnamic acid supplied with MALDI-TOF reagents by Bruker Daltonics, Bremen, Germany) was applied on each spot and air-dried before analysis with MALDI-TOFMS. The Bruker Biotyper 3.4 software and library were used for spectral analysis. Following the manufacturer’s instructions, scores ≥ 2.0 were interpreted as successful identifications at the genus and species levels. Furthermore, C. albicans strains were also tested for the sensitivity to the most common antifungals using the broth micro-dilution method utilizing SensititreTM YeastOne YO10® (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Colonies grown on SDA and Candida Chromogenic agar were suspended in sterile water, adjusted to the 2 McFarland standard set (Liofilchem Dianostici ®, Roseto degli Abruzzi, Italy), and diluted in the yeast broth; a 100 µL of the obtained suspension was dispensed in each well. The plates were incubated at 37 °C for 24–48 h. The concentration in the first blue-colored well was recorded as the minimum inhibitory concentration (MIC). The antifungal susceptibility of specific Candida species was interpreted through clinical breakpoints (CBPs) based on the CLSI M60 [28,29]. The antifungal molecules tested included anidulafungin, micafungin, caspofungin, 5-fluorocytosine, posaconazole, voriconazole, itraconazole, fluconazole, and amphotericin B.
3. Results
Clinical examinations did not reveal skin lesions such as spike loss, crusty skin, or erythema, which could suggest dermatophytosis; similarly, deceased animals sampling during the necroscopic inspections to detect yeasts did not exhibit mucosal lesions suggestive of mycosis.
Dermatophytes were identified in just 1 of the 134 hedgehogs (0.8%, 95% C.I.: 0–0.04). The colonies that grew on Dermasel agar appeared as flat, ramified, powdery-like, and pale brown (Figure 1a). The reverse pigment appeared to be dark reddish-brown with some radial grooves (Figure 1b). Numerous large, very thick-walled, elliptical macroconidia with predominantly five to six septa were observed microscopically (Figure 1c). The strain was genetically identified as Paraphyton mirabile (GenBank accession number PV242270).
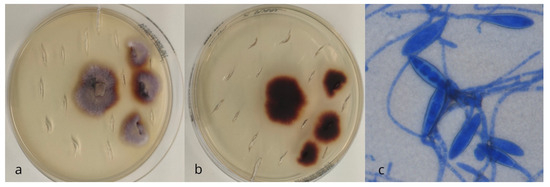
Figure 1.
Recto (a) and versus (b) macroscopic morphology and microscopic (methylene blue stain, 40×) (c) features of Paraphyton spp. colonies.
Yeasts were detected in 22 of the 86 deceased hedgehogs (25.6%, 95% C.I.: 16.4–34.8), and a total of 25 different strains were isolated, as yeasts were detected in both oral and rectal swabs of 3 animals. Twenty-one strains were isolated both on Sabouraud Dextrose agar and on Candida Chromogenic agar, appearing as light green colonies (Figure 2) and resembling the chromatic phenotype of the C. albicans reference strain.
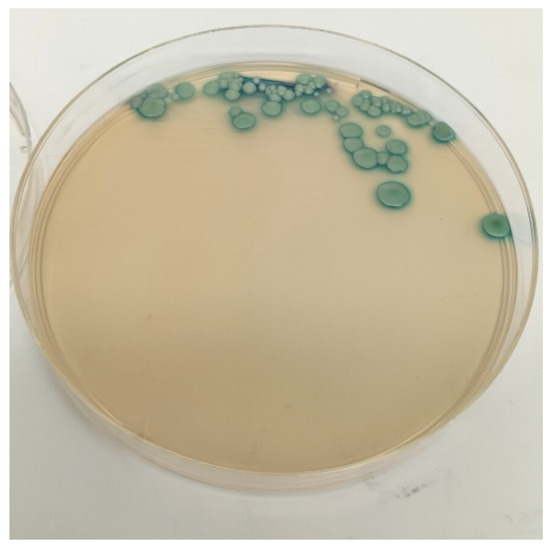
Figure 2.
Candida albicans colonies on Candida Chromogenic agar.
MALDI-TOF confirmed the identification of C. albicans from the chromogenic agar, as well as the identification of other strains, such as two Yarrowia lipolitica, one Rhodotorula mucilaginosa, and one Meyerozyma guilliermondii (the anamorph name of Candida guillermondii) [30] (Table 1).

Table 1.
Fungal isolates obtained through different sampling methods.
Moreover, the 21 C. albicans strains were susceptible to the whole panel of the antimycotics tested, with MIC values tending to fall within the lower end of the reported sensitivity range (Table 2). Among azoles, voriconazole (MICm 0.01; sensitivity range: 0.008–1), posaconazole (MICm 0.03; sensitivity range: 0.008–1) and fluconazole (MICm 0.3; sensitivity range: 0.12–8) exhibited lower MIC values, while itraconazole showed more variability among isolates, with a slightly high MIC value (MICm 0.05; sensitivity range: 0.015–0.12). Echinocandins, such as anidulafungin (MICm 0.02; sensitivity range: 0.015–2) and micafungin (MICm 0.01; sensitivity range: 0.008–2), were effective on the isolates at the lower range value tested. Amphotericin B demonstrated relatively consistent MIC values across isolates (MICm 0.26; sensitivity range: 0.12–1).

Table 2.
Minimum inhibitory concentration (MIC, µg/mL) data of anidulafungin (NDF), micafungin (MCF), caspofungin (CSP), 5-flucytosine (FCT), posaconazole (POS), voriconazole (VOR), itraconazole (ITR), fluconazole (FLU), amphotericin (AMB) for Candida albicans (n. 21 isolates) from European hedgehogs.
4. Discussion
Hedgehog populations have increased in urban areas, becoming synanthropic animals and expanding their opportunity for interactions with other animals and humans. This is evidenced by reports on infectious zoonotic diseases associated with hedgehogs, including their ability to transmit dermatophytes to humans [9]. Dermatophytosis caused by the zoonotic Trichophyton erinacei has been frequently documented in both free-ranging and captive hedgehogs, with prevalence rates as high as 25 to 50% [11,12,31,32,33]. Although to a lesser extent, geophilic dermatophytes such as Nannizzia gypsea, P. cookei and P. mirabile have also been reported, constituting minor zoonotic concerns, generally in asymptomatic animals [11,34]. Thus, the detection of dermatophytes in just one hedgehog was rather unexpected. However, considering that the reservoir for geophilic dermatophytes is the soil, their detection highlights the need for greater attention to soil contamination caused by wildlife, as it can indirectly pose a risk of infection to humans and other animals, including pets [11,34].
In this survey, parasitic yeasts were also detected. Some of them, such as Rhodotorula mucilaginosa and Meyerozyma guillermondii, are emerging opportunistic pathogens that can cause mycotic infections in both humans and animals [35,36]. An interesting finding is the isolation and the identification of C. albicans, which was previously identified as a commensal organism in the digestive tracts of hedgehogs [9]. Finding C. albicans in oral swabs suggests the potential for transmission through bites or contamination of the hedgehog’s integument due to self-anointing behavior [37]. Since C. albicans is the causative agent of candidiasis in humans and animals, its detection in the oral mucosa or integument suggests that these animals should be considered potential carriers or reservoirs for this yeast. This highlights the importance of implementing proper management practices, particularly in immunosuppressed individuals (e.g., elderly people and infants), who may accidentally handle these synanthropic animals. Despite the potential hazard, the MIC values for C. albicans obtained in this study are encouraging, showing that all isolates are susceptible to the antifungal drugs that were tested. Regular and more extensive fungal monitoring is encouraged to confirm this trend. Nevertheless, it must be considered that pathogenic fungi possess numerous resistance mechanisms to antifungal drugs, facilitated by their genetic adaptability and versatile homeostatic responses to environmental stressors. Currently, the available literature data highlight the decreased efficacy of certain antifungal drugs against some dermatophytes and yeasts of the genus Malassezia in domestic animals [14,38,39]. Wildlife has recently been recognized as a significant bioindicator not only of environmental quality, but also as a sentinel for many zoonotic pathogens, as well as for assessing the presence of antimicrobial resistance genes contamination in the environment [40,41,42,43,44]. Further research on larger samples and, possibly, more species of wild animals should be carried out to assess the diffusion of fungal diseases and their resistance to antifungal treatments. Monitoring the presence of antifungal resistance is particularly important, especially in animals that are rescued and rehabilitated for reintroduction into the wild, as this practice can promote the spreading of antifungal resistant strains, posing a risk to people who work in wildlife rehabilitation centers and come into daily contact with potentially infected animals.
These data highlight the critical importance of passive surveillance efforts in detecting zoonotic fungi in wild animals, thereby increasing awareness of the complex interactions between wildlife, public health, and the environment, following the One Health approach [44,45]. Considering wild animals as sentinels, investigating their health status is essential to prevent potential outbreaks of zoonotic infections and monitor the drug resistance phenomenon, which is an issue of increasing concern in both human and veterinary medicine. Effective strategies, such as discouraging wildlife habituation to human presence and implementing measures to reduce accessible food sources, are crucial in regions where the urban and natural environments overlap. Additionally, attention should be paid to those who closely interact with wildlife, not only wildlife rehabilitators, veterinarians, and technicians, but also and especially ordinary citizens who might come in contact with wild animals inhabiting gardens or public parks. Dedicated educational initiatives should then be promoted to highlight the health risks associated with improper wildlife handling.
Author Contributions
Conceptualization, L.B., G.M. and S.C.; Data curation, L.B., D.C. and S.C.; Funding acquisition, M.G.; Investigation, L.B., M.G., A.R., G.R., I.M., M.D., N.D., D.C., M.C. and F.R.M.; Methodology, L.B., D.C. and S.C.; Project administration, M.G. and S.C.; Resources, M.G. and S.C.; Supervision, G.M. and S.C.; Validation, L.B. and S.C.; Visualization, L.B., G.M. and S.C.; Writing—original draft, L.B., G.M., D.C. and S.C.; Writing—review and editing, M.G., A.R., G.R., I.M., M.D., N.D., M.C. and F.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Health (RC002/2021 IZSUM).
Institutional Review Board Statement
This study did not require any approval by an ethics committee as the procedures carried out on live animals were not likely to produce pain, suffering, distress equivalent or higher than that caused by the introduction of a needle, in accordance with good veterinary practices. Most of the animals were dead at the time of sampling for causes independent from this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Giulia Morganti, upon reasonable request.
Acknowledgments
The authors would like to acknowledge the precious work of the volunteers of WildUmbria Wildlife Rescue Center, the students and veterinarians of the University Teaching Hospital of University of Perugia and the technical assistants of the Istituto Zooprofilattico Sperimentale dell’Umbria e delle Marche.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mitchell-Jones, A.J.; Amori, G.; Bogdanowicz, W.; Krystufek, B.; Reijnders, P.J.H.; Spitzenberger, F.; Stubbe, M.; Thissen, J.B.M.; Vohralik, V.; Zima, J. The Atlas of European Mammals; PR Academy Ltd.: London, UK, 1999. [Google Scholar]
- Rasmussen, S.L.; Berg, T.B.; Dabelsteen, T.; Jones, O.R. The ecology of suburban juvenile European hedgehogs (Erinaceus europaeus) in Denmark. Ecol. Evol. 2019, 9, 13174–13187. [Google Scholar] [CrossRef]
- Rondinini, C.; Doncaster, C.P. Roads as barriers to movement for hedgehogs. Funct. Ecol. 2002, 16, 504–509. [Google Scholar] [CrossRef]
- Mariacher, A.; Santini, A.; Del Lesto, I.; Tonon, S.; Cardini, E.; Barone, A.; Eleni, C.; Fichi, G.; Perrucci, S. Endoparasite Infections of the European Hedgehog (Erinaceus europaeus) in Central Italy. Animals 2021, 11, 3171. [Google Scholar] [CrossRef]
- Gaglio, G.; Allen, S.; Bowden, L.; Bryant, M.; Morgan, E.R. Parasites of European hedgehogs (Erinaceus europaeus) in Britain: Epidemiological study and coprological test evaluation. Eur. J. Wildl. Res. 2010, 56, 839–844. [Google Scholar] [CrossRef]
- Rasmussen, S.L.; Hallig, J.; van Wijk, R.E.; Petersen, H.H. An investigation of endoparasites and the determinants of parasite infection in European hedgehogs (Erinaceus europaeus) from Denmark. Int. J. Parasitol. Parasites Wildl. 2021, 16, 217–227. [Google Scholar] [CrossRef]
- Dyachenko, V.; Kuhnert, Y.; Schmaeschke, R.; Etzold, M.; Pantchev, N.; Daugschies, A. Occurrence and molecular characterization of Cryptosporidium spp. genotypes in European hedgehogs (Erinaceus europaeus L.) in Germany. Parasitology 2010, 137, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, A.I.; van Leeuwen, A.D.; Jacobs-Reitsma, W.; Wijnands, L.M.; Bouw, E.; Jahfari, S.; van Hoek, A.H.A.M.; van der Giessen, J.W.B.; Roelfsema, J.H.; Kroes, M.; et al. Presence of zoonotic agents in engorged ticks and hedgehog faeces from Erinaceus europaeus in (sub)urban areas. Parasit. Vectors 2015, 8, 210. [Google Scholar] [CrossRef]
- Ruszkowski, J.J.; Hetman, M.; Turlewicz-Podbielska, H.; Pomorska-Mól, M. Hedgehogs as a Potential Source of Zoonotic Pathogens—A Review and an Update of Knowledge. Animals 2021, 11, 1754. [Google Scholar] [CrossRef]
- Garcias, B.; Aguirre, L.; Seminati, C.; Reyes, N.; Allepuz, A.; Obón, E.; Molina-Lopez, R.A.; Darwich, L. Extended-Spectrum β-Lactam Resistant Klebsiella pneumoniae and Escherichia coli in Wild European Hedgehogs (Erinaceus europeus) Living in Populated Areas. Animals 2021, 11, 2837. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Dyląg, M.; Nowakiewicz, A. European Hedgehogs (Erinaceus europaeus L.) as a Reservoir of Dermatophytes in Poland. Microb. Ecol. 2022, 84, 363–375. [Google Scholar] [CrossRef]
- Le Barzic, C.; Cmokova, A.; Denaes, C.; Arné, P.; Hubka, V.; Guillot, J.; Risco-Castillo, V. Detection and Control of Dermatophytosis in Wild European Hedgehogs (Erinaceus europaeus) Admitted to a French Wildlife Rehabilitation Centre. J. Fungi 2021, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- de Hoog, G.S.; Dukik, K.; Monod, M.; Packeu, A.; Stubbe, D.; Hendrickx, M.; Kupsch, C.; Stielow, J.B.; Freeke, J.; Göker, M.; et al. Toward a Novel Multilocus Phylogenetic Taxonomy for the Dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Osińska, M.; Kopiński, Ł. Population Differentiation, Antifungal Susceptibility, and Host Range of Trichophyton mentagrophytes Isolates Causing Recalcitrant Infections in Humans and Animals. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2099–2113. [Google Scholar] [CrossRef]
- Marples, M.J.; Smith, J.M.B. The Hedgehog as a Source of Human Ringworm. Nature 1960, 188, 867–868. [Google Scholar] [CrossRef] [PubMed]
- Concha, M.; Nicklas, C.; Balcells, E.; Guzmán, A.M.; Poggi, H.; León, E.; Fich, F. The First Case of Tinea Faciei Caused by Trichophyton mentagrophytes var. erinacei Isolated in Chile. Int. J. Dermatol. 2012, 51, 283–285. [Google Scholar] [CrossRef]
- Lee, D.; Yang, J.; Choi, S.; Won, C.; Chang, S.; Lee, M.; Choi, J.; Moon, K.; Kim, M. An Unusual Clinical Presentation of Tinea Faciei Caused by Trichophyton mentagrophytes var. erinacei. Pediatr. Dermatol. 2011, 28, 210–212. [Google Scholar] [CrossRef]
- Nenoff, P.; Herrmann, J.; Gräser, Y. Trichophyton mentagrophytes sive interdigitale? A Dermatophyte in the Course of Time. JDDG J. Dtsch. Dermatol. Ges. 2007, 5, 198–202. [Google Scholar] [CrossRef]
- Fairley, R.A. The Histological Lesions of Trichophyton mentagrophytes var. erinacei Infection in Dogs. Vet. Dermatol. 2001, 12, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Kurtdede, A.; Haydardedeoglu, A.E.; Alihosseini, H.; Colakoglu, E.C. Dermatophytosis Caused by Trichophyton mentagrophytes var. erinacei in a Dog: A Case Report. Vet. Med. 2014, 59, 349–351. [Google Scholar] [CrossRef]
- Iacob, O.; Iftinca, A. The Dermatitis by Caparinia tripilis and Microsporum, in African Pygmy Hedgehog (Atelerix albiventris) in Romania–First Report. Rev. Bras. Parasitol. Vet. 2018, 27, 584–588. [Google Scholar] [CrossRef]
- Smith, J.M.B.; Marples, M.J. Trichophyton mentagrophytes var. erinacei. Med. Mycol. 1964, 3, 1–10. [Google Scholar] [CrossRef]
- Barlow, A.; Partridge, T.; Clark, C.; Everest, D. Oral Candidiasis in European Hedgehogs. Vet. Rec. 2012, 171, 602–603. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T. Intestinal Candidiasis in an African Hedgehog (Atelerix albiventris). Exot. Pet Pract. 1997, 2, 79. [Google Scholar]
- Moriello, K.A. Diagnostic techniques for dermatophytosis. Clin. Tech. Small Anim. Pract. 2001, 16, 219–224. [Google Scholar] [CrossRef]
- Ngo, T.M.C.; Ton Nu, P.A.; Le, C.C.; Ha, T.N.T.; Do, T.B.T.; Tran Thi, G. First Detection of Trichophyton indotineae Causing Tinea Corporis in Central Vietnam. Med. Mycol. Case Rep. 2022, 36, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- CLSI. Clinical and Laboratory Standards Institute. In Performance Standards for Antifungal Susceptibility Testing of Yeasts, 1st ed.; CLSI: Malvern, PA, USA, 2017. [Google Scholar]
- Xiao, J.L.; Xu, G.C.; de Hoog, S.; Qiao, J.J.; Fang, H.; Li, Y.L. Oral Prevalence of Candida Species in Patients Undergoing Systemic Glucocorticoid Therapy and the Antifungal Sensitivity of the Isolates. Infect. Drug Resist. 2020, 13, 2601–2607. [Google Scholar] [CrossRef]
- Daniel, H.-M.; Lachance, M.-A.; Kurtzman, C.P. On the Reclassification of Species Assigned to Candida and Other Anamorphic Ascomycetous Yeast Genera Based on Phylogenetic Circumscription. Antonie Van Leeuwenhoek 2014, 106, 67–84. [Google Scholar] [CrossRef]
- Abarca, M.L.; Castellá, G.; Martorell, J.; Cabañes, F.J. Trichophyton erinacei in Pet Hedgehogs in Spain: Occurrence and Revision of Its Taxonomic Status. Med. Mycol. 2017, 55, 164–172. [Google Scholar] [CrossRef]
- Čmoková, A.; Kolařík, M.; Guillot, J.; Risco-Castillo, V.; Cabañes, F.J.; Nenoff, P.; Uhrlaß, S.; Dobiáš, R.; Mallátová, N.; Yaguchi, T.; et al. Host-driven subspeciation in the hedgehog fungus, Trichophyton erinacei, an emerging cause of human dermatophytosis. Persoonia 2022, 48, 203–218. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sano, A.; Takizawa, K.; Fukushima, K.; Miyaji, M.; Nishimura, K. The epidemiology and mating behavior of Arthroderma benhamiae var. erinacei in household four-toed hedgehogs (Atelerix albiventris) in Japan. Nippon Ishinkin Gakkai Zasshi 2003, 44, 31–38. [Google Scholar] [CrossRef][Green Version]
- Illner, A.; Michl, C.; Sunderkötter, C.; Uhrlaß, S.; Krüger, C.; Baert, F.; Stubbe, D.; Nenoff, P. Paraphyton mirabile-A rare geophilic derma-tophyte-with unclear relation to nail disease. J. Eur. Acad. Dermatol. Venereol. 2023, 27, E991–E993. [Google Scholar] [CrossRef]
- Pal, M. Rhodotoruliosis: An Emerging Opportunistic Mycosis of Humans and Animals. Open Access J. Mycol. Mycol. Sci. 2021, 4. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Mendez, M.; Kibbler, C.; Erzsebet, P.; Chang, S.-C.; Gibbs, D.L.; Newell, V.A. Candida guilliermondii, an Opportunistic Fungal Pathogen with Decreased Susceptibility to Fluconazole: Geographic and Temporal Trends from the ARTEMIS DISK Antifungal Surveillance Program. J. Clin. Microbiol. 2006, 44, 3551–3556. [Google Scholar] [CrossRef] [PubMed]
- D’Havé, H.; Scheirs, J.; Verhagen, R.; De Coen, W. Gender, age and seasonal dependent self-anointing in the European hedgehog Erinaceus europaeus. Acta Theriol. 2005, 50, 167–173. [Google Scholar] [CrossRef]
- Begum, J.; Kumar, R. Prevalence of Dermatophytosis in Animals and Antifungal Susceptibility Testing of Isolated Trichophyton and Microsporum Species. Trop. Anim. Health Prod. 2021, 53, 3. [Google Scholar] [CrossRef]
- Yurayart, C.; Niae, S.; Limsivilai, O.; Thengchaisri, N.; Sattasathuchana, P. Comparative Analysis of the Distribution and Antifungal Susceptibility of Yeast Species in Cat Facial Hair and Human Nails. Sci. Rep. 2024, 14, 14726. [Google Scholar] [CrossRef]
- Marenzoni, M.L.; Morganti, G.; Moretta, I.; Crotti, S.; Agnetti, F.; Moretti, A.; Pitzurra, L.; Casagrande Proietti, P.; Sechi, P.; Cenci Goga, B.; et al. Microbiological and parasitological survey of zoonotic agents in apparently healthy feral pigeons. Pol. J. Vet. Sci. 2016, 19, 309–315. [Google Scholar] [CrossRef]
- Pascucci, I.; Antognini, E.; Canonico, C.; Montalbano, M.G.; Necci, A.; di Donato, A.; Moriconi, M.; Morandi, B.; Morganti, G.; Crotti, S.; et al. One Health Approach to Rickettsiosis: A Five-Year Study on Spotted Fever Group Rickettsiae in Ticks Collected from Humans, Animals and Environment. Microorganisms 2022, 10, 35. [Google Scholar] [CrossRef]
- Musa, L.; Stefanetti, V.; Casagrande Proietti, P.; Grilli, G.; Gobbi, M.; Toppi, V.; Brustenga, L.; Magistrali, C.F.; Franciosini, M.P. Antimicrobial Susceptibility of Commensal E. coli Isolated from Wild Birds in Umbria (Central Italy). Animals 2023, 13, 1776. [Google Scholar] [CrossRef]
- López-Islas, J.J.; Méndez-Olvera, E.T.; Reyes, C.T.; Martínez-Gómez, D. Identification of Antimicrobial Resistance Genes in Intestinal Content from Coyote (Canis latrans). Pol. J. Vet. Sci. 2023, 26, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Rhimi, W.; Sgro, G.; Aneke, C.I.; Annoscia, G.; Latrof, M.S.; Mosca, A.; Venezian, V.; Otranto, D.; Alastruey-Izquierdo, A.; Cafarchia, C. Wild Boar (Sus scrofa) as Reservoir of Zoonotic Yeasts: Bioindicator of Environmental Quality. Mycopathologia 2022, 187, 235–248. [Google Scholar] [PubMed]
- Jota Baptista, C.V.; Seixas, F.; Gonzalo-Orden, J.M.; Oliveira, P.A. Can the European Hedgehog (Erinaceus europaeus) Be a Sentinel for One Health Concerns? Biologics 2021, 1, 61–69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).