Hong-Bai-Lan-Shen Extract Alleviates the CoCl2-Induced Apoptosis in H9C2 Cells by Regulating the AMPK Pathway
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
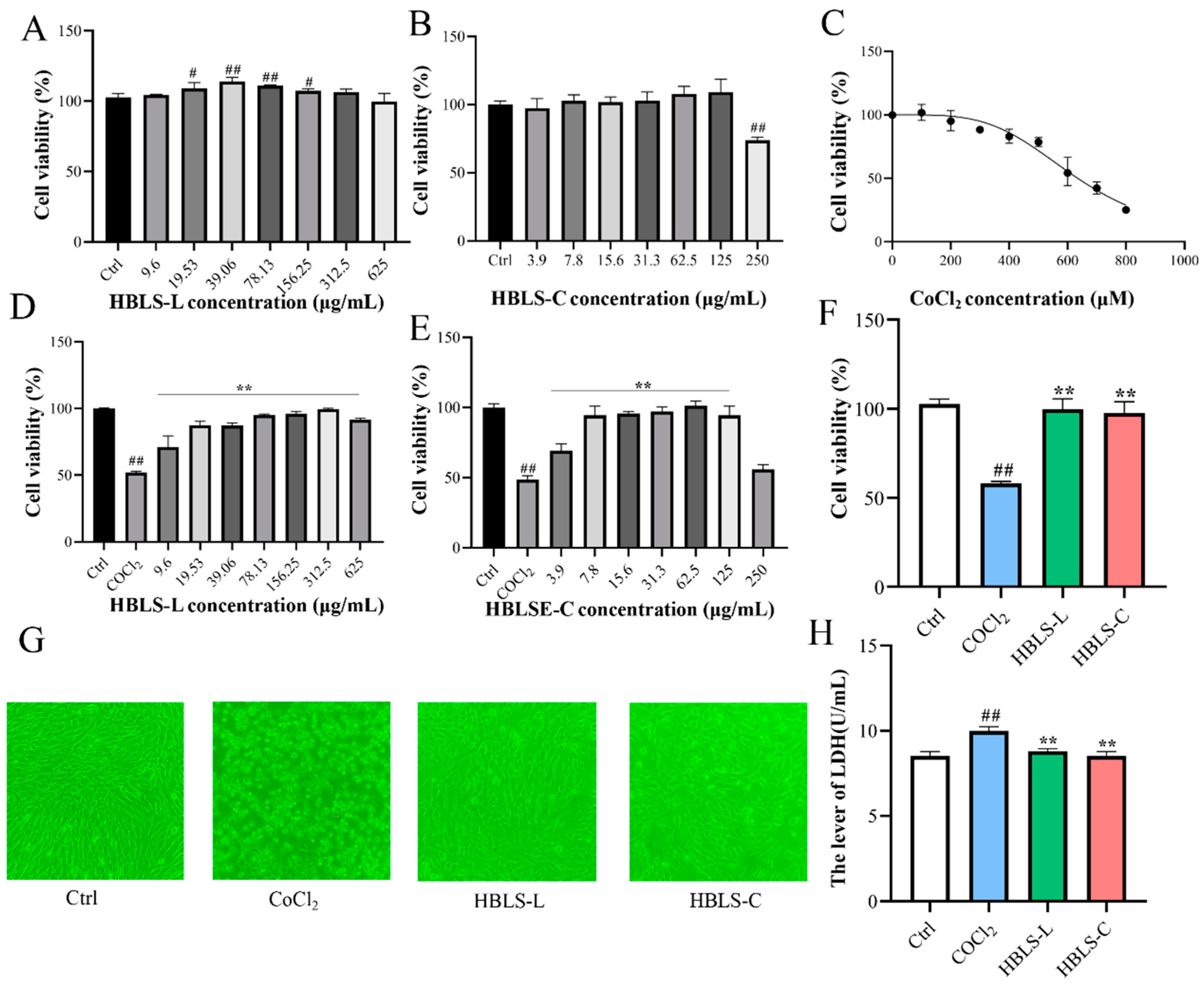
3.1. HBLS Extract Altered the Cell Viability
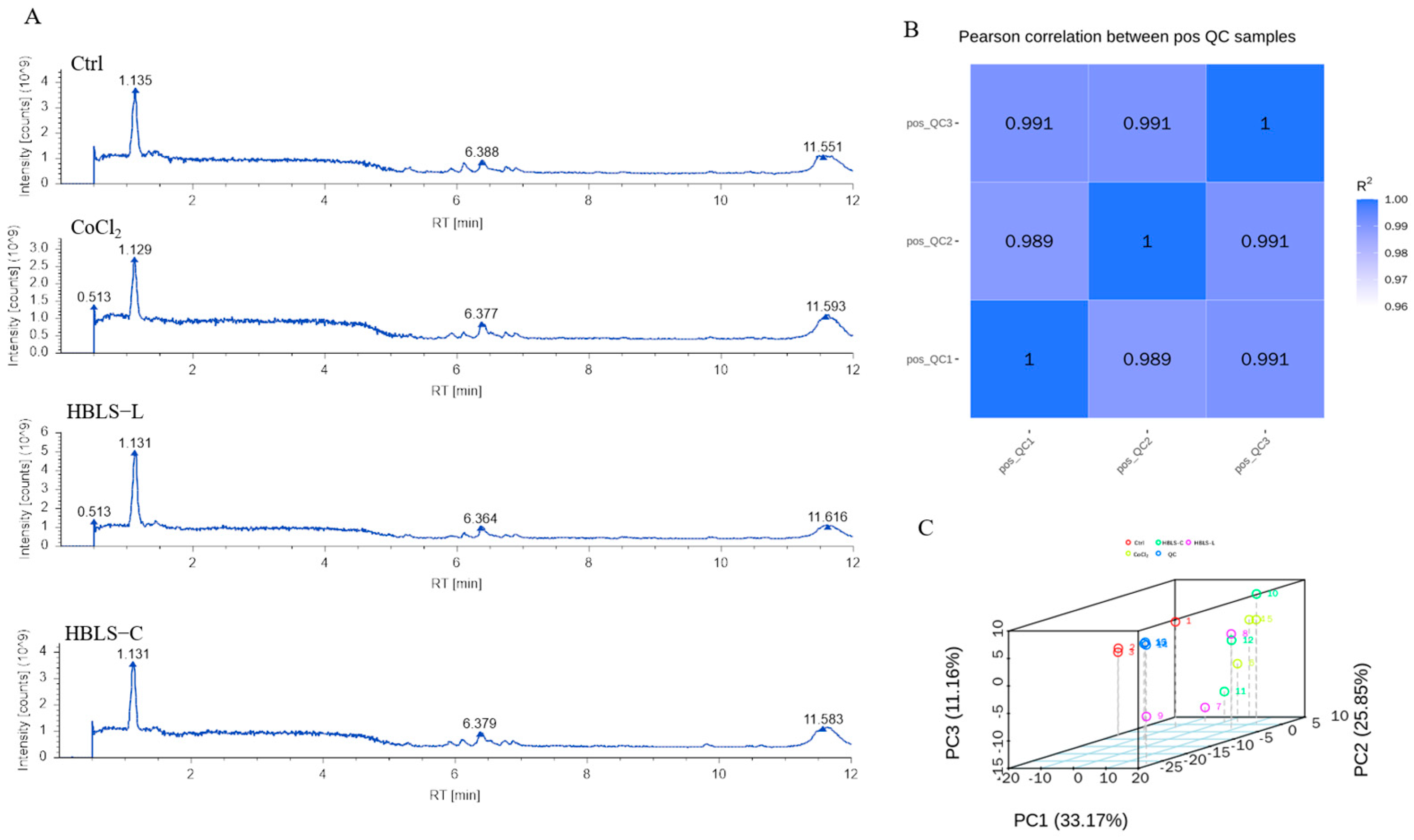
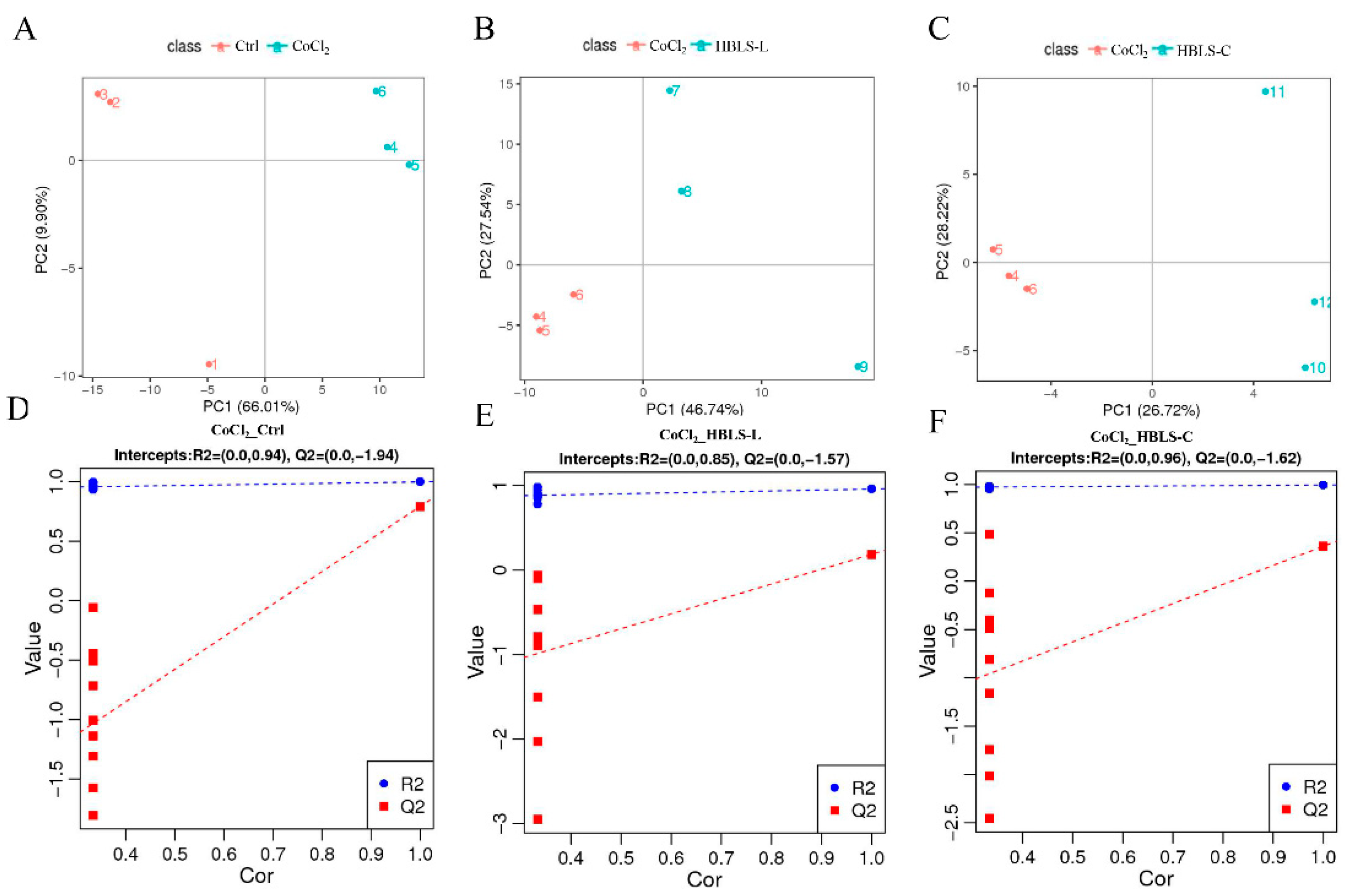
3.2. Metabolomics Analysis Based on LC-MS/MS
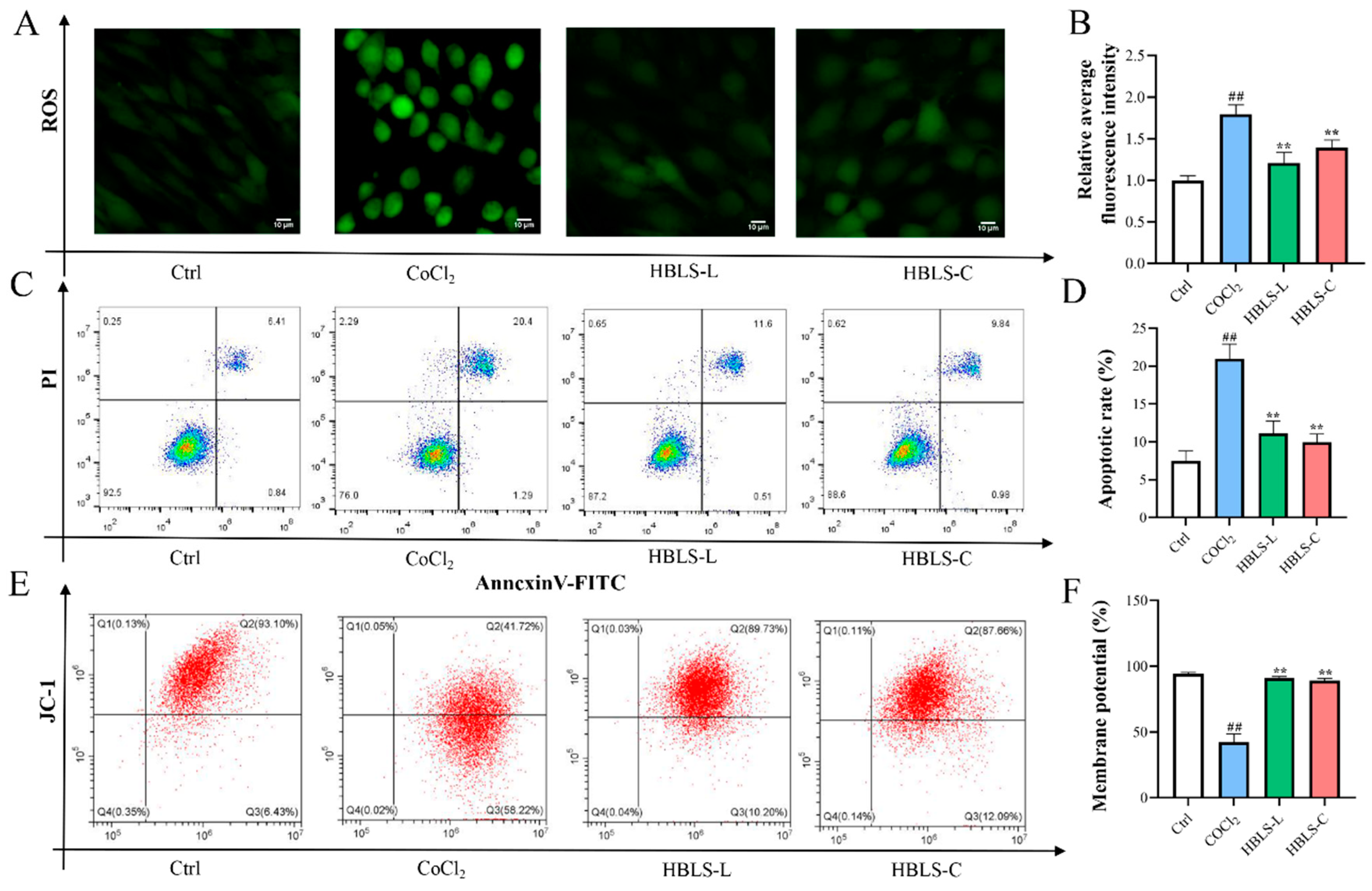
3.3. HBLS Extract on the Effects of CoCl2-Induced H9C2 Cell ROS Generation, Apoptosis, and MMP
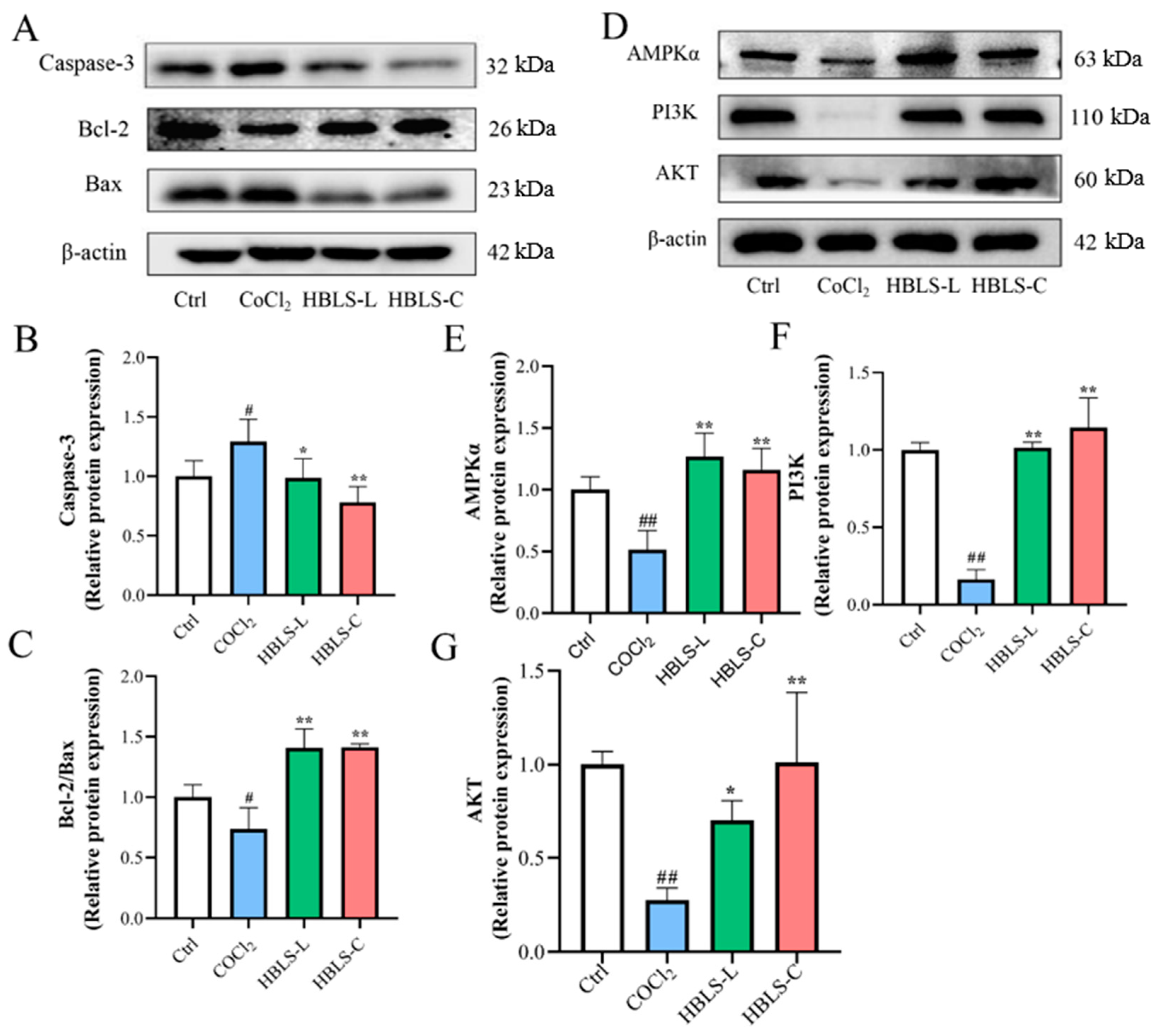
3.4. HBLS Extract Activated Cell Apoptosisthe and AMPK Pathway in CoCl2-Induced H9C2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, P.S.; Chiu, W.T.; Hsu, P.L.; Lin, S.C.; Peng, I.C.; Wang, C.Y.; Tsai, S.J. Pathophysiological implications of hypoxia in human diseases. J. Biomed. Sci. 2020, 27, 63. [Google Scholar] [CrossRef]
- Li, J.Y.; Pan, S.S.; Wang, J.Y.; Lu, J. Changes in Autophagy Levels in Rat Myocardium During Exercise Preconditioning-Initiated Cardioprotective Effects. Int. Heart J. 2019, 60, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, B. General Theory of the Principles of Traditional Chinese Medicine; People’s Medical Publishing House: Beijing, China, 2022. [Google Scholar]
- Chen, Y.; Tang, M.; Yuan, S.; Fu, S.; Li, Y.; Li, Y.; Wang, Q.; Cao, Y.; Liu, L.; Zhang, Q. Rhodiola rosea: A Therapeutic Candidate on Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 1348795. [Google Scholar] [CrossRef]
- Nusuetrong, P.; Gerdprasert, O. Cardioprotection of Atractylodes lancea against Hypoxia/Reoxygenation-Injured H9c2 Cardiomyoblasts. J. Med. Assoc. Thail. 2016, 99 (Suppl. S8), S179–S186. [Google Scholar]
- Tsai, K.H.; Lee, N.H.; Chen, G.Y.; Hu, W.S.; Tsai, C.Y.; Chang, M.H.; Jong, G.P.; Kuo, C.H.; Tzang, B.S.; Tsai, F.J.; et al. Dung-shen (Codonopsis pilosula) attenuated the cardiac-impaired insulin-like growth factor II receptor pathway on myocardial cells. Food Chem. 2013, 138, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Luo, W.; Luo, Y. Protective effect of alpha-mangostin against CoCl2-induced apoptosis by suppressing oxidative stress in H9C2 rat cardiomyoblasts. Mol. Med. Rep. 2018, 17, 6697–6704. [Google Scholar] [PubMed]
- Zheng, B.; Qi, J.; Liu, P.; Zhang, M.; Zhang, Y.; Xue, Y.; Han, X.; Xu, S.; Chu, L. 10-Gingerol alleviates hypoxia/reoxygenation-induced cardiomyocyte injury through inhibition of the Wnt5a/Frizzled-2 pathway. Food Sci. Nutr. 2021, 9, 3917–3931. [Google Scholar] [CrossRef]
- Li, S.; Jiang, J.; Fang, J.; Li, X.; Huang, C.; Liang, W.; Wu, K. Naringin protects H9C2 cardiomyocytes from chemical hypoxia-induced injury by promoting the autophagic flux via the activation of the HIF-1alpha/BNIP3 signaling pathway. Int. J. Mol. Med. 2021, 47, 102. [Google Scholar] [CrossRef]
- Wu, L.; Wang, J.; Cao, X.; Tian, Y.; Li, J. Effect of acute high-intensity exercise on myocardium metabolic profiles in rat and human study via metabolomics approach. Sci. Rep. 2022, 12, 6791. [Google Scholar] [CrossRef]
- Hu, Y. Traditional Chinese Veterinary Medicine, 1st ed.; Science Press: Beijing, China, 2013. [Google Scholar]
- Cheng, Y.Z.; Chen, L.J.; Lee, W.J.; Chen, M.F.; Jung, L.H.; Cheng, J.T. Increase of myocardial performance by Rhodiola-ethanol extract in diabetic rats. J. Ethnopharmacol. 2012, 144, 234–239. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Guo, L.; Wu, X.; Zhang, T.; Liu, J.; Zhang, J. Atractylodes lactone compounds inhibit platelet activation. Platelets 2017, 28, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Ao, J.; Li, Y.; Zhang, J.; Duan, C. Exploring the protective mechanisms of total tannins from Geum japonicum var. chinense F. Bolle in mice with hematopoietic dysfunction via the JAK2/STAT3/5 signaling pathway. J. Ethnopharmacol. 2022, 296, 15507. [Google Scholar] [CrossRef]
- Wang, J.N.; Kan, C.D.; Lee, L.T.; Huang, L.; Hsiao, Y.L.; Chang, A.H.; Liu, W.; Lin, C.; Lin, C.W. Herbal Extract from Codonopsis pilosula (Franch.) Nannf. Enhances Cardiogenic Differentiation and Improves the Function of Infarcted Rat Hearts. Life 2021, 11, 422. [Google Scholar] [CrossRef]
- Wang, W.; Shi, Y.; Qiu, T.; Meng, J.; Ding, J.; Wang, W.; Wu, D.; Li, K.; Liu, J.; Wu, Y. Modified rougan decoction alleviates lipopolysaccharide-enrofloxacin-induced hepatotoxicity via activating the Nrf2/ARE pathway in chicken. Poult. Sci. 2023, 102, 102404. [Google Scholar] [CrossRef]
- Galgano, F.; Tolve, R.; Scarpa, T.; Caruso, M.C.; Lucini, L.; Senizza, B.; Condelli, N. Extraction Kinetics of Total Polyphenols, Flavonoids, and Condensed Tannins of Lentil Seed Coat: Comparison of Solvent and Extraction Methods. Foods 2021, 10, 1810. [Google Scholar] [CrossRef]
- Pang, B.; Shi, L.W.; Du, L.J.; Li, Y.C.; Zhang, M.Z.; Ni, Q. Sheng Mai San protects H9C2 cells against hyperglycemia-induced apoptosis. BMC Complement. Altern. Med. 2019, 19, 309. [Google Scholar] [CrossRef] [PubMed]
- Sellick, C.A.; Hansen, R.; Stephens, G.M.; Goodacre, R.; Dickson, A.J. Metabolite extraction from suspension-cultured mammalian cells for global metabolite profiling. Nat. Protoc. 2011, 6, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, H.; Sun, Y.; Li, X.; Chen, L.; Jin, X.; Lyu, S.; Wen, W.; Liao, J. Integration of multi-omics in investigations on the mechanisms of action of Chinese herbal medicine interventions in metabolic diseases. Tradit. Med. Res. 2022, 7, 31. [Google Scholar] [CrossRef]
- Fiehn, O.; Kopka, J.; Dormann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef]
- Wishart, D.S. Current progress in computational metabolomics. Brief. Bioinform. 2007, 8, 279–293. [Google Scholar] [CrossRef]
- Liu, A.; Chu, Y.J.; Wang, X.; Yu, R.; Jiang, H.; Li, Y.; Zhou, H.; Gong, L.L.; Yang, W.Q.; Ju, J. Serum Metabolomics Study Based on LC-MS and Antihypertensive Effect of Uncaria on Spontaneously Hypertensive Rats. Evid. Based Complement. Altern. Med. 2018, 2018, 9281946. [Google Scholar] [CrossRef]
- Zou, H.M.; Zhang, B.; Xu, X.C.; Su, J.; Sun, Y.N.; Xue, S.; Wang, X.Y.; Qiu, M.F. Urinary metabolomic strategy to evaluate Compound Danshen Dripping Pills for myocardial ischaemia in rats. J. Pharm. Biomed. Anal. 2015, 112, 98–105. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Arif, T.; Shteinfer-Kuzmine, A. Apoptotic proteins with non-apoptotic activity: Expression and function in cancer. Apoptosis 2023, 28, 730–753. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.R.; Li, S.; Tan, X.C.; Huang, T.; Dong, H.J.; Xue, R.; Li, J.C.; Zhang, Y.; Zhang, Y.Z.; Wang, X. Xinyang Tablet attenuates chronic hypoxia-induced right ventricular remodeling via inhibiting cardiomyocytes apoptosis. Chin. Med. 2022, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Wang, T.; Li, Y.; Wang, L.; Yang, J.; Dong, H.; Li, S. Liraglutide ameliorates COCl2-induced oxidative stress and apoptosis in H9C2 cells via regulating cell autophagy. Exp. Ther. Med. 2020, 19, 3716–3722. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, H.; Shu, Q.; Chen, M.; Xie, L. Green Tea Polyphenols Modulated Cerebral SOD Expression and Endoplasmic Reticulum Stress in Cardiac Arrest/Cardiopulmonary Resuscitation Rats. Biomed. Res. Int. 2020, 2020, 5080832. [Google Scholar] [CrossRef]
- Niu, N.; Li, Z.; Zhu, M.; Sun, H.; Yang, J.; Xu, S.; Zhao, W.; Song, R. Effects of nuclear respiratory factor-1 on apoptosis and mitochondrial dysfunction induced by cobalt chloride in H9C2 cells. Mol. Med. Rep. 2019, 19, 2153–2163. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, G.; Liu, H.; Xing, N.; Sun, Y.; Zhai, Y.; Yang, B.; Kong, A.T.; Kuang, H.; Wang, Q. Cardioprotective effect of the xanthones from Gentianella acuta against myocardial ischemia/reperfusion injury in isolated rat heart. Biomed. Pharmacother. 2017, 93, 626–635. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Liu, L.; Kaushal, V.; Hong, X.; Melnyk, O.; Seth, R.; Safirstein, R.; Shah, S.V. Regulation of caspase-3 and -9 activation in oxidant stress to RTE by forkhead transcription factors, Bcl-2 proteins, and MAP kinases. Am. J. Physiol. Renal Physiol. 2004, 287, F1258–F1268. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Klimentova, E.A.; Suchkov, I.A.; Shchulkin, A.V.; Glazkova, A.P.; Kalinin, R.E. Expression of Apoptotic Markers Bcl-2 and Bax in the Vascular Wall. Sovrem. Tekhnologii Med. 2021, 13, 46–50. [Google Scholar] [CrossRef]
- Peng, N.; Jin, L.; He, A.; Deng, C.; Wang, X. Effect of sulphoraphane on newborn mouse cardiomyocytes undergoing is chaemia/reperfusion injury. Pharm. Biol. 2019, 57, 753–759. [Google Scholar] [CrossRef]
- Raj, S.R.; ND, D.; Mondal, S.; Ashokan, M.; Thota, L.N.; Karuthadurai, T.; Ramesha, K.P. Expression analysis of pro-apoptotic BAX and anti-apoptotic BCL-2 genes in relation to lactation performance in Deoni and Holstein Friesian crossbred cows. Anim. Biotechnol. 2023, 34, 1354–1361. [Google Scholar] [CrossRef]
- Qi, D.; Young, L.H. AMPK: Energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol. Metab. 2015, 26, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.R.; Li, J.; Coven, D.L.; Pypaert, M.; Zechner, C.; Palmeri, M.; Giordano, F.J.; Mu, J.; Birnbaum, M.J.; Young, L.H. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J. Clin. Investig. 2004, 114, 495–503. [Google Scholar] [CrossRef]
- Hu, M.; Han, M.; Zhang, H.; Li, Z.; Xu, K.; Kang, H.; Zong, J.; Zhao, F.; Liu, Y.; Liu, W. Curcumin (CUMINUP60(R)) mitigates exercise fatigue through regulating PI3K/Akt/AMPK/mTOR pathway in mice. Aging 2003, 15, 2308–2320. [Google Scholar] [CrossRef] [PubMed]
- Torrealba, N.; Rodriguez-Berriguete, G.; Fraile, B.; Olmedilla, G.; Martinez-Onsurbe, P.; Sanchez-Chapado, M.; Paniagua, R.; Royuela, M. PI3K pathway and Bcl-2 family. Clinicopathological features in prostate cancer. Aging Male 2018, 21, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Wang, Y.; Chen, D.; Xiang, P.; Shen, J.; Li, Y.; Wang, S. Protective Effects of Notoginsenoside R1 via Regulation of the PI3K-Akt-mTOR/JNK Pathway in Neonatal Cerebral Hypoxic-Ischemic Brain Injury. Neurochem. Res. 2018, 43, 1210–1226. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt Signaling and Redox Metabolism in Cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef]

| Medicinal Plant | Voucher Specimen Number | Local Name | Ratio | Origin (China) | Key Phytochemical Compounds | Effect | Ref. |
|---|---|---|---|---|---|---|---|
| Rhodiola rosea (rhizome of Rhodiola rosea L.) | NJAU-CVM-2022005 | Hong Jingtian | 4 | Xizang | Salidroside, Kaempferol, Quercetin et al. | The monarch herb increases the cardiac output in heart failure. | [12] |
| Atractylodes (radix of Atractylodes macrocephala Koidz.) | NJAU-CVM-2022006 | Bai Zhu | 3 | Sichuan province | Biatractylenolide II, Luteolin, Isoscopoletin, et al. | The minister herb is widely used in the treatment of coronary artery disease | [13] |
| Gei Herba (Geum aleppicum Jacq.) | NJAU-CVM-2022007 | Lan Buzheng | 1 | Xizang | Flavonoids, Phenylpropanoids, Tannins, et al. | The assistant herb improves peripheral hemogram promotes hematopoiesis | [14] |
| Codonopsis (radix of Codonopsis pilosula (Franch.) Nannf.) | NJAU-CVM-2022008 | Dang Shen | 2 | Xizang | Polysaccharides, Alkaloids, Flavonoids, et al. | The assistant herb improves cardiac function of infarcted hearts | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Meng, J.; Wang, W.; Gu, B.; Hu, M.; Liu, J. Hong-Bai-Lan-Shen Extract Alleviates the CoCl2-Induced Apoptosis in H9C2 Cells by Regulating the AMPK Pathway. Vet. Sci. 2025, 12, 267. https://doi.org/10.3390/vetsci12030267
Ding J, Meng J, Wang W, Gu B, Hu M, Liu J. Hong-Bai-Lan-Shen Extract Alleviates the CoCl2-Induced Apoptosis in H9C2 Cells by Regulating the AMPK Pathway. Veterinary Sciences. 2025; 12(3):267. https://doi.org/10.3390/vetsci12030267
Chicago/Turabian StyleDing, Jinxue, Jinwu Meng, Wenjia Wang, Bolin Gu, Mengxin Hu, and Jiaguo Liu. 2025. "Hong-Bai-Lan-Shen Extract Alleviates the CoCl2-Induced Apoptosis in H9C2 Cells by Regulating the AMPK Pathway" Veterinary Sciences 12, no. 3: 267. https://doi.org/10.3390/vetsci12030267
APA StyleDing, J., Meng, J., Wang, W., Gu, B., Hu, M., & Liu, J. (2025). Hong-Bai-Lan-Shen Extract Alleviates the CoCl2-Induced Apoptosis in H9C2 Cells by Regulating the AMPK Pathway. Veterinary Sciences, 12(3), 267. https://doi.org/10.3390/vetsci12030267






