Molecular Detection of Tick-Borne Bacterial Pathogens in Ticks and Rodents from the China–Vietnam Border
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
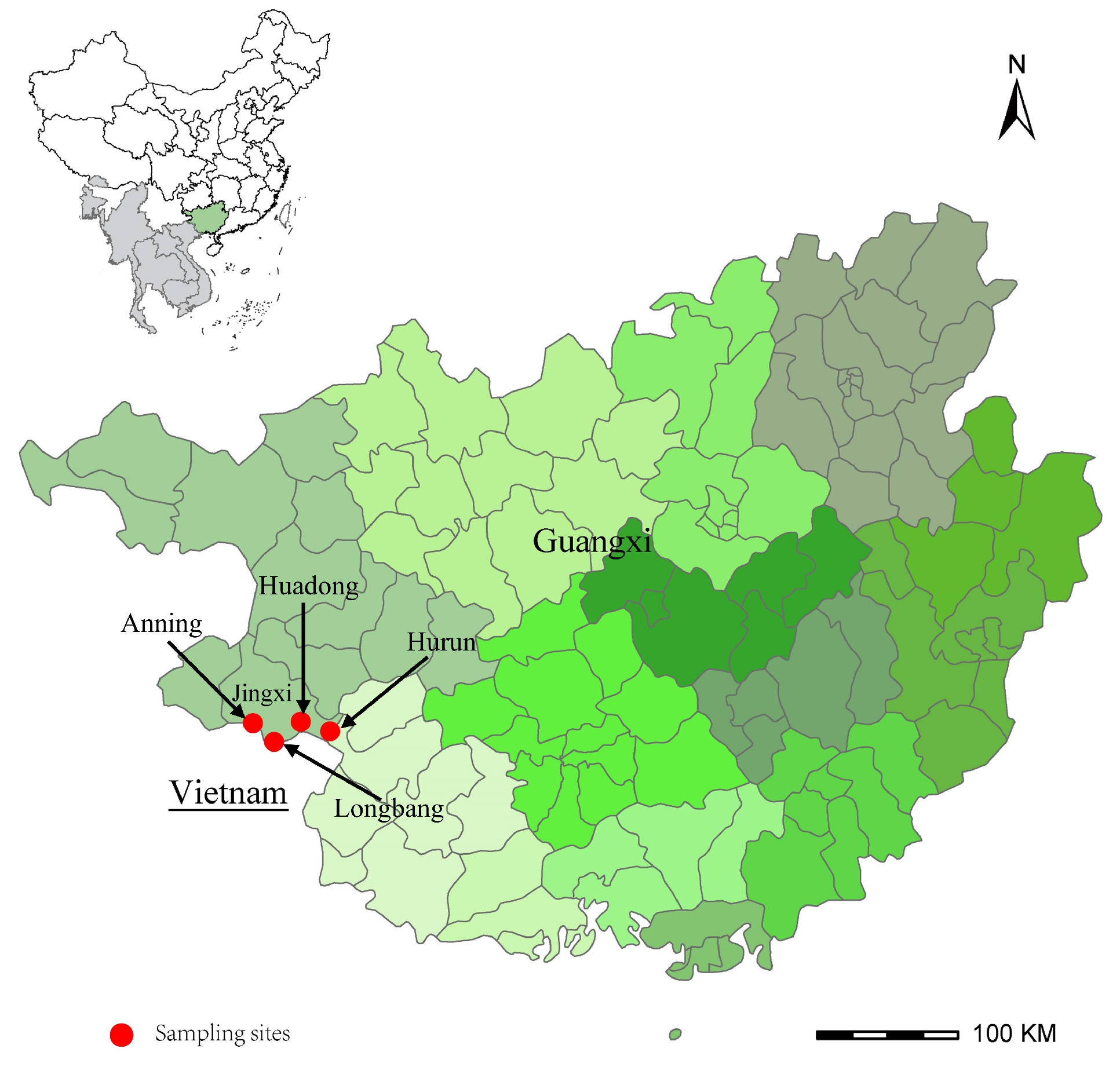
2.1. Sample Collection and Species Identification
2.2. DNA Extraction
2.3. Molecular Detection of Tick-Borne Bacterial Pathogens
2.4. Sequencing and Phylogenetic Analysis
3. Results
3.1. Sample Collection
3.2. Detection and Characterization of SFGR
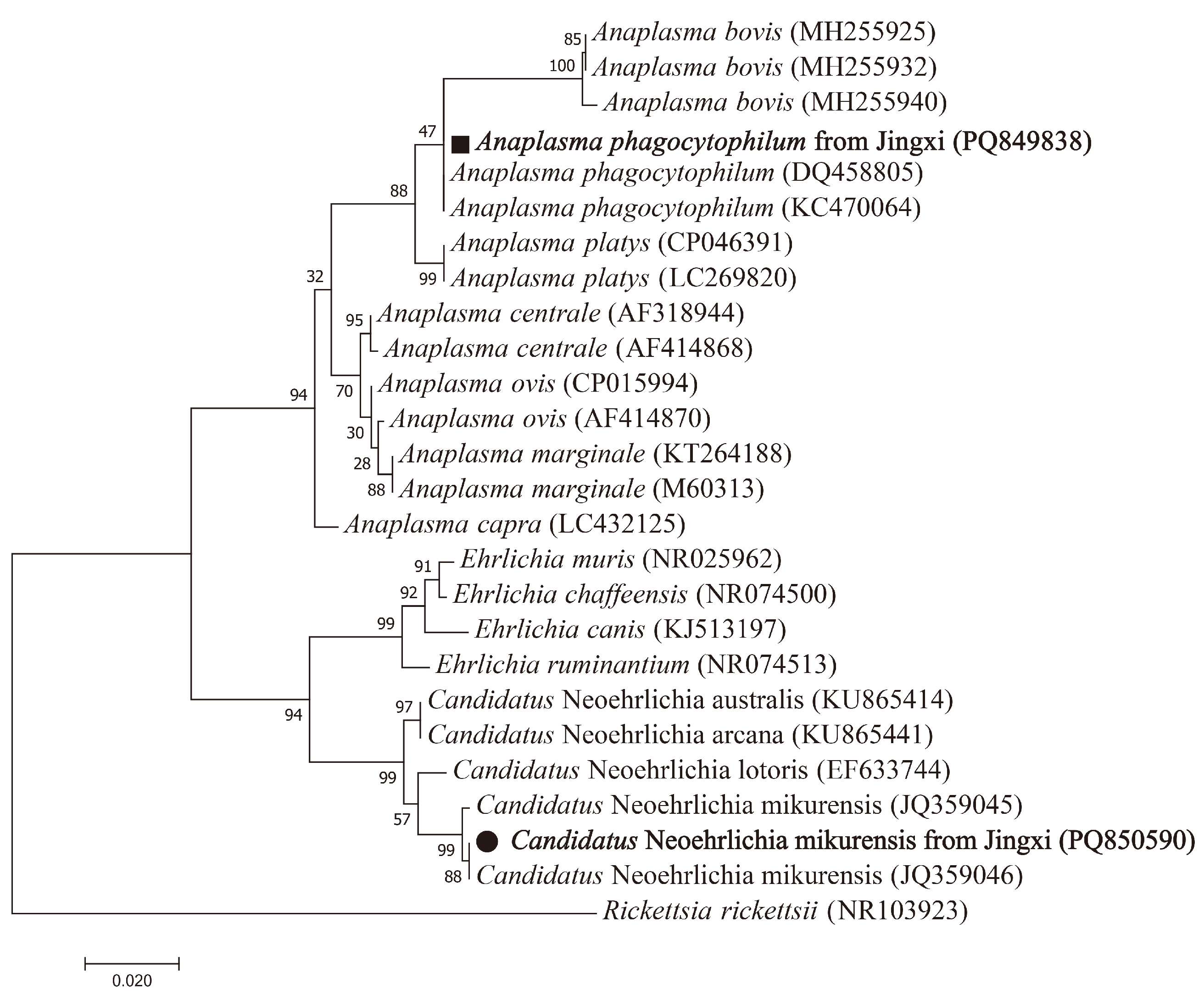
3.3. Detection and Characterization of Anaplasmataceae
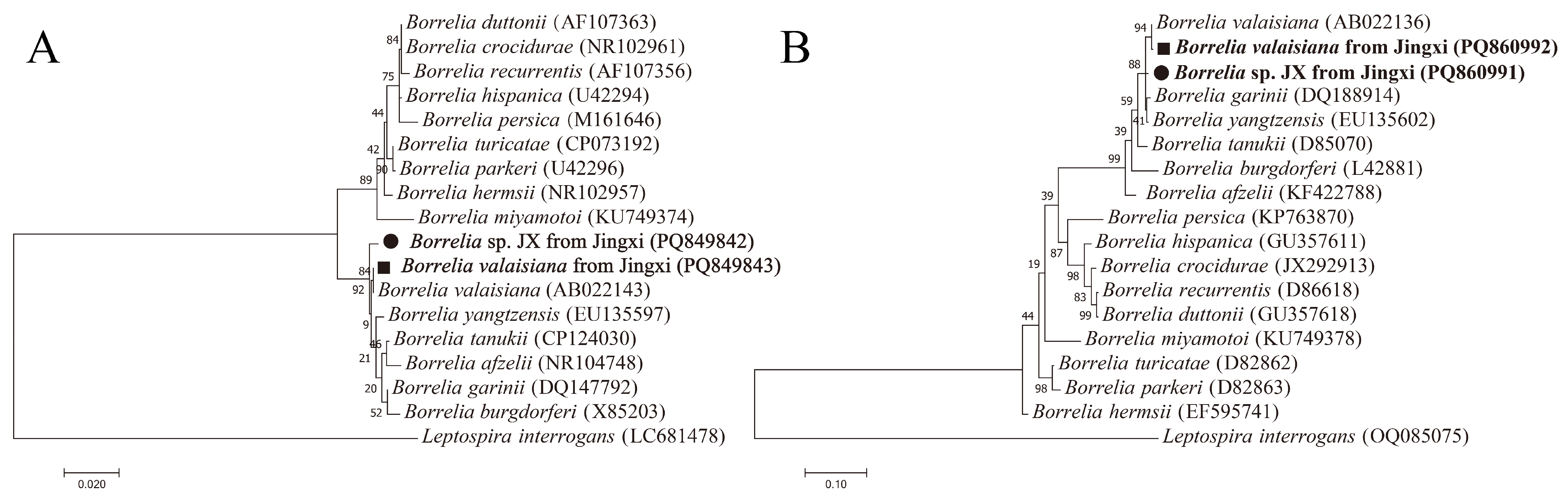
3.4. Detection and Characterization of Borrelia spp.
3.5. Co-Infection in Individual Ticks and Rodents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De la Fuente, J.; Estrada-Pena, A.; Rafael, M.; Almazan, C.; Bermudez, S.; Abdelbaset, A.E.; Kasaija, P.D.; Kabi, F.; Akande, F.A.; Ajagbe, D.O.; et al. Perception of Ticks and Tick-Borne Diseases Worldwide. Pathogens 2023, 12, 1258. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Pustijanac, E.; Bursic, M.; Millotti, G.; Paliaga, P.; Ivesa, N.; Cvek, M. Tick-Borne Bacterial Diseases in Europe: Threats to public health. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 1261–1295. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Leach, S.A. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect. Dis. 2015, 15, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Dibernardo, A.; Koffi, J.; Wood, H.; Leighton, P.A.; Lindsay, L.R. N Increased risk of tick-borne diseases with climate and environmental changes. Can. Commun. Dis. Rep. 2019, 45, 83–89. [Google Scholar] [CrossRef]
- Sonenshine, D.E. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. Int. J. Environ. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef]
- Zhang, X.A.; Ma, Y.D.; Zhang, Y.F.; Hu, Z.Y.; Zhang, J.T.; Han, S.; Wang, G.; Li, S.; Wang, X.; Tang, F.; et al. A New Orthonairovirus Associated with Human Febrile Illness. N. Engl. J. Med. 2024, 391, 821–831. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Bian, C.; Ye, R.Z.; Cui, X.M.; Yao, N.N.; Yang, J.H.; Chu, Y.L.; Su, X.L.; Wu, Y.F.; Ye, J.L.; et al. A series of patients infected with the emerging tick-borne Yezo virus in China: An active surveillance and genomic analysis. Lancet Infect. Dis. 2024. [Google Scholar] [CrossRef]
- Ma, J.; Lv, X.L.; Zhang, X.; Han, S.Z.; Wang, Z.D.; Li, L.; Sun, H.T.; Ma, L.X.; Cheng, Z.L.; Shao, J.W.; et al. Identification of a new orthonairovirus associated with human febrile illness in China. Nat. Med. 2021, 27, 434–439. [Google Scholar] [CrossRef]
- Jia, N.; Liu, H.B.; Ni, X.B.; Bell-Sakyi, L.; Zheng, Y.C.; Song, J.L.; Li, J.; Jiang, B.G.; Wang, Q.; Sun, Y.; et al. Emergence of human infection with Jingmen tick virus in China: A retrospective study. EBioMedicine 2019, 43, 317–324. [Google Scholar] [CrossRef]
- Fang, L.Q.; Liu, K.; Li, X.L.; Liang, S.; Yang, Y.; Yao, H.W.; Sun, R.X.; Sun, Y.; Chen, W.J.; Zuo, S.Q.; et al. Emerging tick-borne infections in mainland China: An increasing public health threat. Lancet Infect. Dis. 2015, 15, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Gou, J.; Zhong, D.; Ma, L.; Yin, C.; Shu, M.; Liu, G.; Lin, Q. The Tick-Borne Pathogens: An Overview of China’s Situation. Acta Parasitol. 2023, 68, 1–20. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, H.; Wang, T.; Sun, W.; Yang, X.; Liu, J. Tick-borne pathogens and the vector potential of ticks in China. Parasites Vectors 2015, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar] [CrossRef]
- Liu, H.B.; Wei, R.; Ni, X.B.; Zheng, Y.C.; Huo, Q.B.; Jiang, B.G.; Ma, L.; Jiang, R.R.; Lv, J.; Liu, Y.X.; et al. The prevalence and clinical characteristics of tick-borne diseases at One Sentinel Hospital in Northeastern China. Parasitology 2019, 146, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Eilbert, W.; Matella, A. Tick-Borne Diseases. Emerg. Med. Clin. N. Am. 2024, 42, 287–302. [Google Scholar] [CrossRef]
- Diop, A.; El Karkouri, K.; Raoult, D.; Fournier, P.E. Genome sequence-based criteria for demarcation and definition of species in the genus Rickettsia. Int. J. Syst. Evol. Microbiol. 2020, 70, 1738–1750. [Google Scholar] [CrossRef]
- Xue, J.; Chen, S.S.; Jian, R.; Chen, G.Q.; Xie, G.; Du, L.; Guo, W.P. Molecular evidence of Rickettsia canadensis in ticks, Hebei, China. Infect. Genet. Evol. 2023, 115, 105506. [Google Scholar] [CrossRef]
- Zhao, G.P.; Wang, Y.X.; Fan, Z.W.; Ji, Y.; Liu, M.J.; Zhang, W.H.; Li, X.L.; Zhou, S.X.; Li, H.; Liang, S.; et al. Mapping ticks and tick-borne pathogens in China. Nat. Commun. 2021, 12, 1075. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, W.B.; Pan, Y.S.; Jiang, B.G.; Du, C.H.; Que, T.C.; Zhan, L.; Wu, J.H.; Yu, M.H.; Cui, X.M.; et al. Detection of Novel Spotted Fever Group Rickettsiae (Rickettsiales: Rickettsiaceae) in Ticks (Acari: Ixodidae) in Southwestern China. J. Med. Entomol. 2021, 58, 1363–1369. [Google Scholar] [CrossRef]
- Rar, V.; Tkachev, S.; Tikunova, N. Genetic diversity of Anaplasma bacteria: Twenty years later. Infect. Genet. Evol. 2021, 91, 104833. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, Y.C.; Ma, L.; Jia, N.; Jiang, B.G.; Jiang, R.R.; Huo, Q.B.; Wang, Y.W.; Liu, H.B.; Chu, Y.L.; et al. Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet Infect. Dis. 2015, 15, 663–670. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Mu, J.; Yu, X.; Fei, Y.; Chang, J.; Bi, Y.; Zhou, Y.; Ding, Z.; Yin, R. Emergence of a Novel Ehrlichia minasensis Strain, Harboring the Major Immunogenic Glycoprotein trp36 with Unique Tandem Repeat and C-Terminal Region Sequences, in Haemaphysalis hystricis Ticks Removed from Free-Ranging Sheep in Hainan Province, China. Microorganisms 2019, 7, 369. [Google Scholar] [CrossRef]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; Zweygarth, E.; Vancova, M.; Broniszewska, M.; Grubhoffer, L.; Passos, L.M.F.; Ribeiro, M.F.B.; Alberdi, P.; de la Fuente, J. Ehrlichia minasensis sp. nov., isolated from the tick Rhipicephalus microplus. Int. J. Syst. Evol. Microbiol. 2016, 66, 1426–1430. [Google Scholar] [CrossRef]
- Muller, A.; Monti, G.; Otth, C.; Sepulveda, P.; Bittencourt, P.; Nachum-Biala, Y.; Gutierrez, R.; Harrus, S. “Candidatus Neoehrlichia chilensis” sp. nov.: Molecular detection and characterization of a novel Anaplasmataceae in wild rodents from Valdivia, southern Chile. Transbound. Emerg. Dis. 2018, 65, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Schwameis, M.; Auer, J.; Mitteregger, D.; Simonitsch-Klupp, I.; Ramharter, M.; Burgmann, H.; Lagler, H. Anaplasmataceae-Specific PCR for Diagnosis and Therapeutic Guidance for Symptomatic Neoehrlichiosis in Immunocompetent Host. Emerg. Infect. Dis. 2016, 22, 281–284. [Google Scholar] [CrossRef]
- Li, H.; Jiang, J.F.; Liu, W.; Zheng, Y.C.; Huo, Q.B.; Tang, K.; Zuo, S.Y.; Liu, K.; Jiang, B.G.; Yang, H.; et al. Human infection with Candidatus Neoehrlichia mikurensis, China. Emerg. Infect. Dis. 2012, 18, 1636–1639. [Google Scholar] [CrossRef] [PubMed]
- Che, T.L.; Jiang, B.G.; Xu, Q.; Zhang, Y.Q.; Lv, C.L.; Chen, J.J.; Tian, Y.J.; Yang, Y.; Hay, S.I.; Liu, W.; et al. Mapping the risk distribution of Borrelia burgdorferi sensu lato in China from 1986 to 2020: A geospatial modelling analysis. Emerg. Microbes Infect. 2022, 11, 1215–1226. [Google Scholar] [CrossRef]
- Nguyen, V.L.; Colella, V.; Iatta, R.; Bui, K.L.; Dantas-Torres, F.; Otranto, D. Ticks and associated pathogens from dogs in northern Vietnam. Parasitol. Res. 2019, 118, 139–142. [Google Scholar] [CrossRef]
- Do, T.; Bui, K.L.; Zafar, I.; Inpankaew, T.; Galon, M.E.; Ta, P.A.; Tran, K.T.; Hasan, T.; Shengwei, J.; Ma, Z.; et al. Molecular detection, risk factors, and phylogenetic analysis of tick-borne pathogens in dogs from northern Vietnam. Trop. Biomed. 2024, 41, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, S.S.; Liu, J.Z. Illustrated keys to families and genera of the superfamily Ixodoidea under new taxonomic system. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2011, 29, 302–304, 309. [Google Scholar]
- Chitimia, L.; Lin, R.Q.; Cosoroaba, I.; Wu, X.Y.; Song, H.Q.; Yuan, Z.G.; Zhu, X.Q. Genetic characterization of ticks from southwestern Romania by sequences of mitochondrial cox1 and nad5 genes. Exp. Appl. Acarol. 2010, 52, 305–311. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Cao, W.; Pan, H. Ehrlichiae and ehrlichial diseases in China. Ann. N. Y. Acad. Sci. 2003, 990, 45–53. [Google Scholar] [CrossRef]
- Masuzawa, T.; Takada, N.; Kudeken, M.; Fukui, T.; Yano, Y.; Ishiguro, F.; Kawamura, Y.; Imai, Y.; Ezaki, T. Borrelia sinica sp. nov., a lyme disease-related Borrelia species isolated in China. Int. J. Syst. Evol. Microbiol. 2001, 51, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.B.; Jia, N.; Jiang, B.G.; Sun, T.; Zheng, Y.C.; Huo, Q.B.; Liu, K.; Ma, L.; Zhao, Q.M.; Yang, H.; et al. Lyme borreliosis caused by diverse genospecies of Borrelia burgdorferi sensu lato in northeastern China. Clin. Microbiol. Infect. 2014, 20, 808–814. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef]
- He, K.; Chen, S.; Yu, L.; Wei, D.; Xu, X. Japanese spotted fever complicated with pleural effusion in Zhejiang province, China: A case report and literature review. J. Infect. Dev. Ctries. 2024, 18, 1135–1140. [Google Scholar] [CrossRef]
- Li, H.; Zhang, P.H.; Du, J.; Yang, Z.D.; Cui, N.; Xing, B.; Zhang, X.A.; Liu, W. Rickettsia japonica Infections in Humans, Xinyang, China, 2014–2017. Emerg. Infect. Dis. 2019, 25, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.H.; Lee, S.H.; Kim, M.J.; Lee, J.H.; Kim, E.S.; Lee, J.S.; Kim, M.K.; Park, M.Y.; Kang, J.S. Japanese spotted fever, South Korea. Emerg. Infect. Dis. 2006, 12, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Gaywee, J.; Sunyakumthorn, P.; Rodkvamtook, W.; Ruang-areerate, T.; Mason, C.J.; Sirisopana, N. Human infection with Rickettsia sp. related to R. japonica, Thailand. Emerg. Infect. Dis. 2007, 13, 657–659. [Google Scholar] [CrossRef]
- Fournier, P.E.; Dumler, J.S.; Greub, G.; Zhang, J.; Wu, Y.; Raoult, D. Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 2003, 41, 5456–5465. [Google Scholar] [CrossRef]
- Tsui, P.Y.; Tsai, K.H.; Weng, M.H.; Hung, Y.W.; Liu, Y.T.; Hu, K.Y.; Lien, J.C.; Lin, P.R.; Shaio, M.F.; Wang, H.C.; et al. Molecular detection and characterization of spotted fever group rickettsiae in Taiwan. Am. J. Trop. Med. Hyg. 2007, 77, 883–890. [Google Scholar] [CrossRef]
- Xue, J.; Chen, S.S.; Jian, R.; Chen, G.Q.; Qin, X.; Lu, M.; Wang, W.; Xie, G.C.; Du, L.; Li, K.; et al. Great genetic diversity of vector-borne bacteria and protozoan in wild rodents from Guangxi, China. PLoS Negl. Trop. Dis. 2024, 18, e0012159. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, J.; Tang, F.; Sun, Y.; Li, Z.; Zhang, W.; Gong, Z.; Liu, K.; Yang, H.; Liu, W.; et al. Wide distribution and genetic diversity of “Candidatus Neoehrlichia mikurensis” in rodents from China. Appl. Environ. Microbiol. 2013, 79, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.X.; Wen, Y.X.; Zhang, Y.G.; Wang, S.S.; Qiu, Q.C.; Shi, Z.X.; Li, D.Y.; Chen, D.Q.; Liu, X.D.; Zhao, J.H. Clinical manifestations and epidemiological characteristics of Lyme disease in Hailin county, Heilongjiang Province, China. Ann. N. Y. Acad. Sci. 1988, 539, 302–313. [Google Scholar] [CrossRef]
- Chu, C.Y.; Liu, W.; Jiang, B.G.; Wang, D.M.; Jiang, W.J.; Zhao, Q.M.; Zhang, P.H.; Wang, Z.X.; Tang, G.P.; Yang, H.; et al. Novel genospecies of Borrelia burgdorferi sensu lato from rodents and ticks in southwestern China. J. Clin. Microbiol. 2008, 46, 3130–3133. [Google Scholar] [CrossRef]
- Hou, J.; Ling, F.; Chai, C.; Lu, Y.; Yu, X.; Lin, J.; Sun, J.; Chang, Y.; Ye, X.; Gu, S.; et al. Prevalence of Borrelia burgdorferi sensu lato in ticks from eastern China. Am. J. Trop. Med. Hyg. 2015, 92, 262–266. [Google Scholar] [CrossRef]

| Pathogens | Gene | Primer Name | Expected Size (bp) | Sequence (5′-3′) | Reference |
|---|---|---|---|---|---|
| SFGR | ompA | Rr190.70f | 532 | ATGGCGAATATTTCTCCAAAA | |
| Rr190.602r | AGTGCAGCATTCGCTCCCCCT | ||||
| gltA | CS2d | 1256 | ATGACCAATGAAAATAATAAT | [34] | |
| RpCS1258r | ATTGCAAAAAGTACAGTGAACA | ||||
| CSEndr | 750 | CTTATACTCTCTATGTACA | |||
| RpCS877f | GGGGACCTGCTCACGGCGG | ||||
| SFGR and Anaplasmataceae | 16S rRNA | Eh-out1 | 1438 | TTGAGAGTTTGATCCTGGCTCAGAACG | |
| Eh-3-17 | GATAGCGGAATTCCTAGTGTAGAGGTG | ||||
| Eh-out1 | 660 | TTGAGAGTTTGATCCTGGCTCAGAACG | [35] | ||
| Eh-out2 | TAAGGTGGTAATCCAGC | ||||
| Eh-out2f | 890 | CACCTCTACACTAGGAATTCCGCTATC | |||
| Eh-3-17 | GATAGCGGAATTCCTAGTGTAGAGGTG | ||||
| Borrelia. spp. | 16S rRNA | 16S1 | 1523 | ATAACGAAGAGTTTGATCCTGGC | |
| 16S2 | CAGCCGCACTTTCCAGTACG | [36] | |||
| fla | FlaF | 588 | TTAGGTTTTCAATAGCATACTCAG | ||
| FlaR | GCAGTTCAATCAGGTAACGG | [37] |
| Tick | Host | No. of Ticks (%) | Number of SFGR (%, 95% CI) | ||
|---|---|---|---|---|---|
| Rickettsia japonica | Rickettsia sp. JX | Total | |||
| Ha. cornigera | cattle | 80 (90.9) | 37 (46.3, 35.2–57.7) | 33 (41.3, 30.5–52.8) | 70 (87.5, 77.8–93.5) |
| R. microplus | cattle | 5 (5.7) | 0 | 1 (20, 1.1–70.1) | 1 (20, 1.1–70.1) |
| I. granulatus | R. norvegicus | 3 (3.4) | 0 | 3 (100, 31–100) | 3 (100, 31–100) |
| Total | 88 | 37 (42.1, 31.8–53.1) | 37 (42.1, 31.8–53.1) | 74 (84.1, 74.4–90.7) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Xiao, W.; Du, X.; Xue, J.; Wang, H.; Wang, Q.; Wang, Y.; Jia, H.; Song, H.; Qiu, S. Molecular Detection of Tick-Borne Bacterial Pathogens in Ticks and Rodents from the China–Vietnam Border. Vet. Sci. 2025, 12, 256. https://doi.org/10.3390/vetsci12030256
Liu H, Xiao W, Du X, Xue J, Wang H, Wang Q, Wang Y, Jia H, Song H, Qiu S. Molecular Detection of Tick-Borne Bacterial Pathogens in Ticks and Rodents from the China–Vietnam Border. Veterinary Sciences. 2025; 12(3):256. https://doi.org/10.3390/vetsci12030256
Chicago/Turabian StyleLiu, Hongbo, Wenwei Xiao, Xinying Du, Jingzhuang Xue, Hui Wang, Qi Wang, Yule Wang, Huiqun Jia, Hongbin Song, and Shaofu Qiu. 2025. "Molecular Detection of Tick-Borne Bacterial Pathogens in Ticks and Rodents from the China–Vietnam Border" Veterinary Sciences 12, no. 3: 256. https://doi.org/10.3390/vetsci12030256
APA StyleLiu, H., Xiao, W., Du, X., Xue, J., Wang, H., Wang, Q., Wang, Y., Jia, H., Song, H., & Qiu, S. (2025). Molecular Detection of Tick-Borne Bacterial Pathogens in Ticks and Rodents from the China–Vietnam Border. Veterinary Sciences, 12(3), 256. https://doi.org/10.3390/vetsci12030256





