Absolute Quantitative Lipidomics Reveals Differences in Lipid Compounds in the Blood of Trained and Untrained Yili Horses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Design and Horse Grouping
2.3. Training Program
- Teach horse to listen with lead exercises;
- Practice exercises to make horse comfortable with touch;
- Train your horse to follow directions through circle work;
- Introducing your horse to a saddle;
- Place the saddle on your horse’s back;
- Tighten the girth in intervals;
- Use a mounting block to climb in the saddle;
- Mount and dismount from the saddle in 10-min intervals.
2.4. Echocardiographic Evaluation
2.5. Quantitative Plasma Lipidomics Analysis by LC/MS-MS
- 1.
- Liquid chromatography column: Thermo Accucore™ C30 column (2.6 μm; 100 mm × 2.1 mm i.d.);
- 2.
- Mobile phases:
- Phase A: acetonitrile/water (60/40, v/v) containing 0.1% formic acid and 10 mmol/L ammonium formate;
- Phase B: acetonitrile/isopropanol (10/90, v/v) containing 0.1% formic acid and 10 mmol/L ammonium formate;
- 3.
- Gradient elution program:
- 0 min: A/B = 80:20 (v/v);
- 2 min: A/B = 70:30 (v/v);
- 4 min: A/B = 40:60 (v/v);
- 9 min: A/B = 15:85 (v/v);
- 14 min: A/B = 10:90 (v/v);
- 15.5 min: A/B = 5:95 (v/v);
- 17.3 min: A/B = 5:95 (v/v);
- 17.5 min: A/B = 80:20 (v/v);
- 20 min: A/B = 80:20 (v/v);
- 4.
- Flow rate: 0.35 mL/min;
- 5.
- Column temperature: 45 °C;
- 6.
- Injection volume: 2 μL.
2.5.1. Data Processing
2.5.2. Screening of Differential Metabolites and KEGG Functional Analysis
2.5.3. Correlation Analysis Between Cardiac Structure, Function, and Plasma Lipid Composition
2.6. Statistical Analysis
3. Results
3.1. Analysis of Differences in Cardiac Structure Between Trained and Untrained Yili Horses
3.2. Changes in Quantitative Lipidomics Between Trained and Untrained Yili Horses
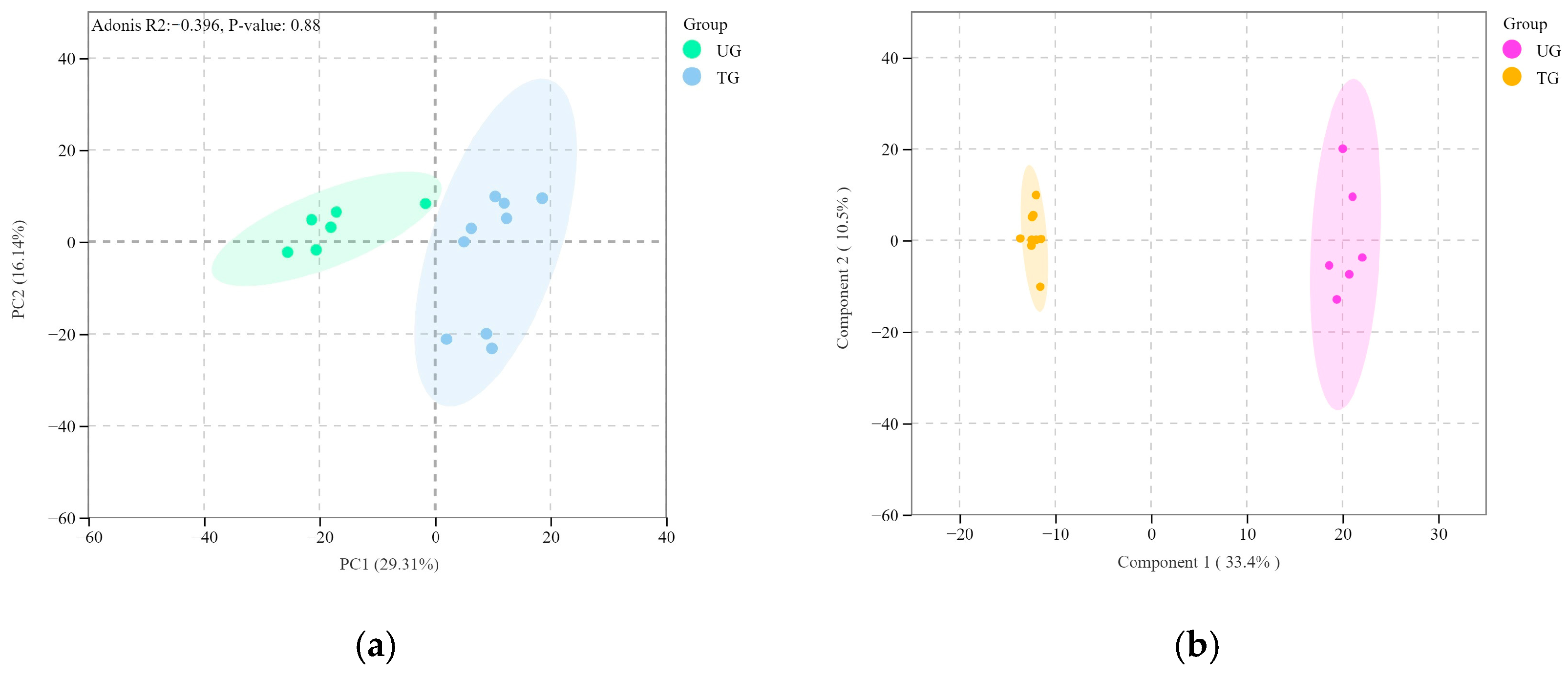
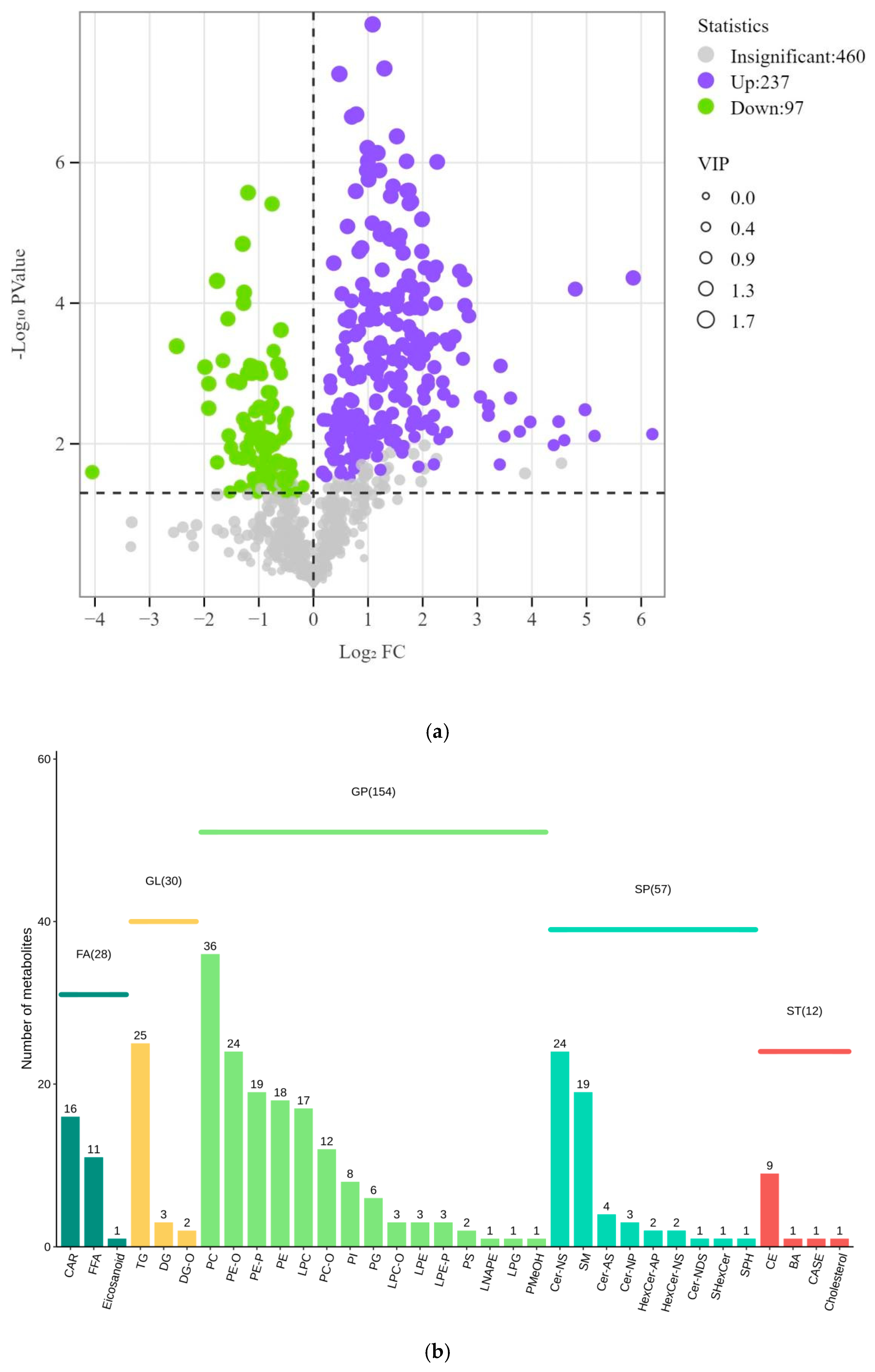
3.2.1. Identification of Differentially Expressed Lipid Metabolites in Plasma
3.2.2. Analysis of Differentially Expressed Lipid Metabolites in Plasma
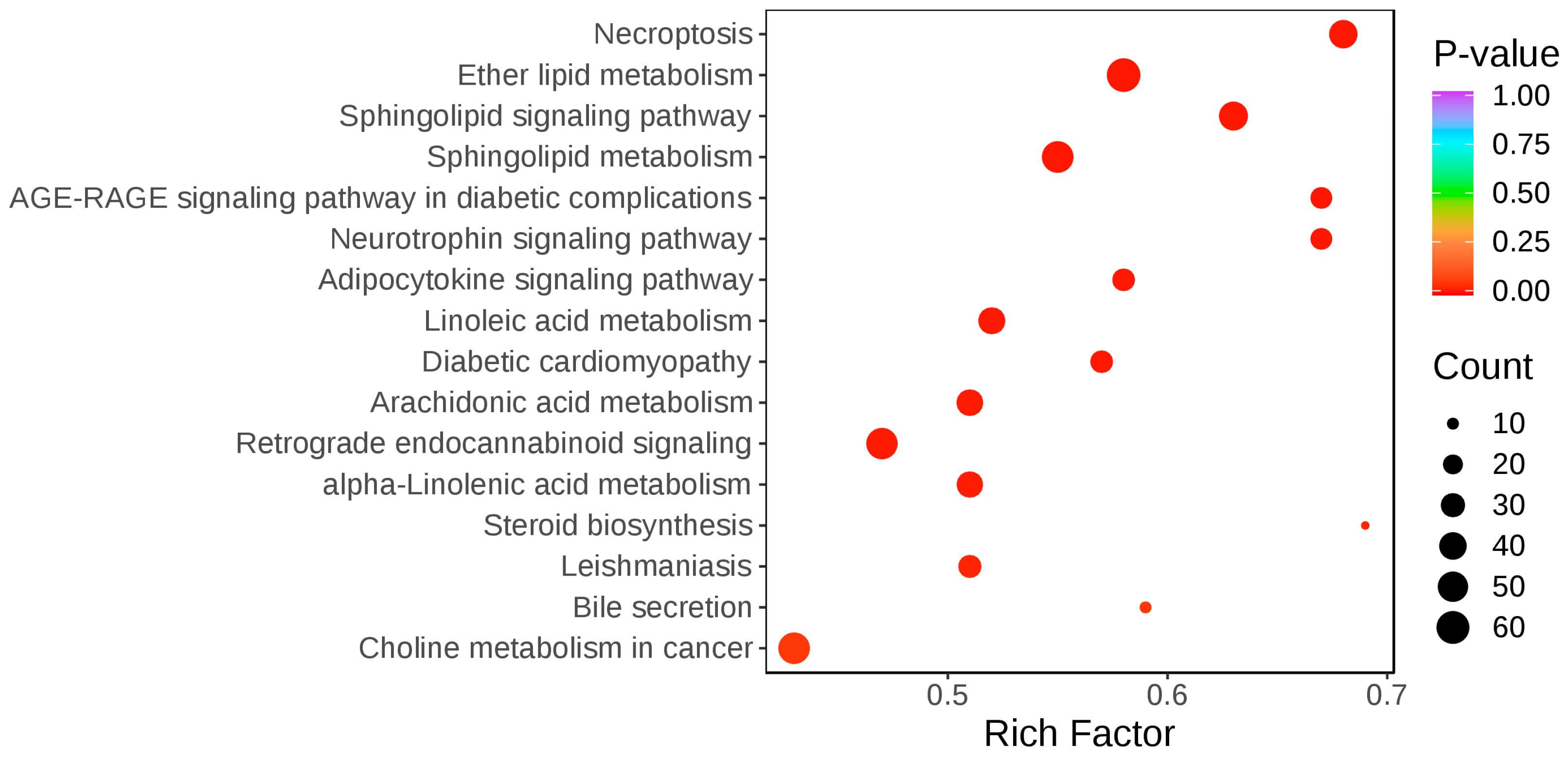
3.2.3. Pathway Enrichment Analysis of Differential Lipid Metabolites
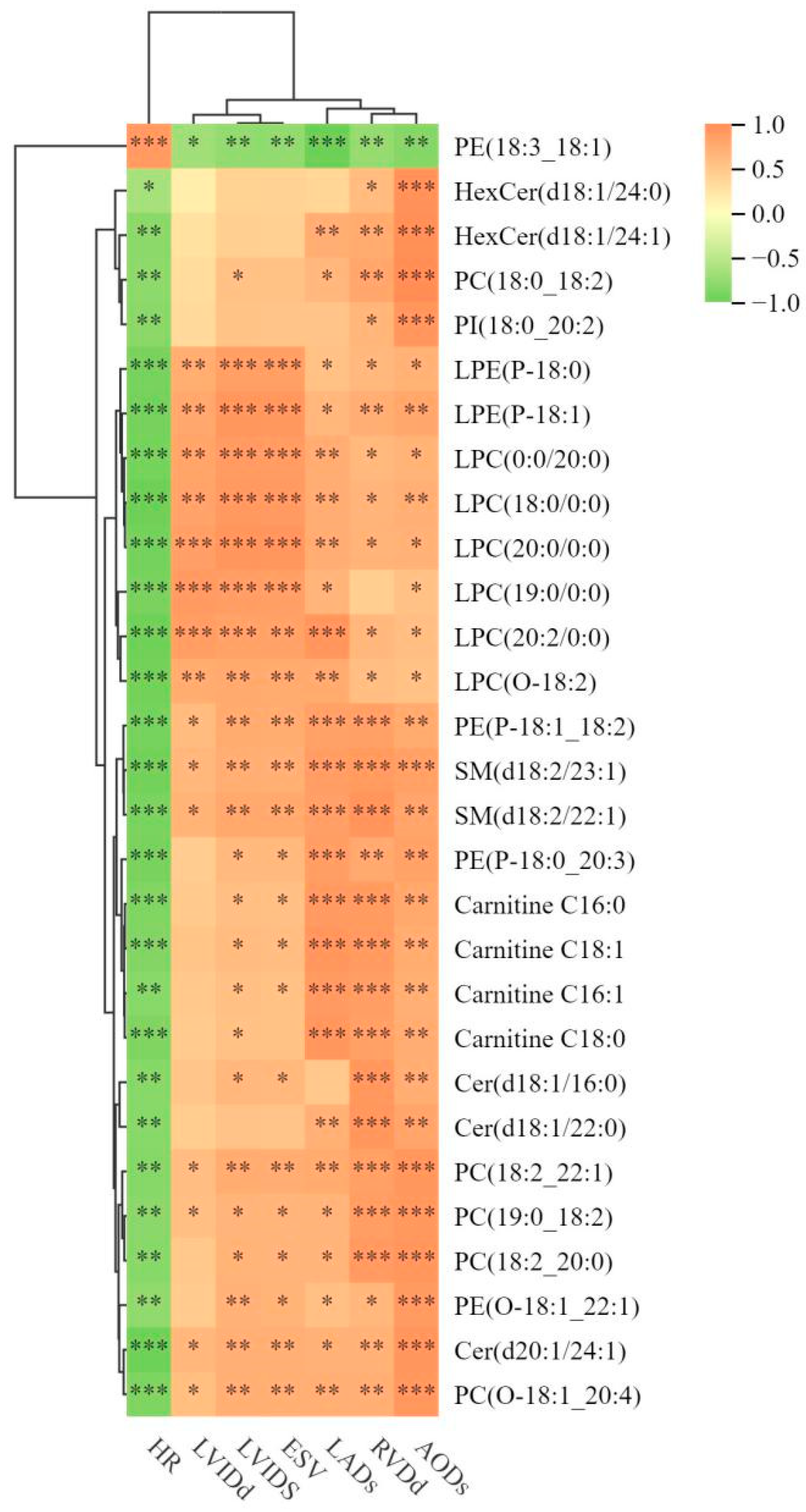
3.2.4. Correlation Analysis Between Differential Metabolites and Cardiac Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shave, R.; Howatson, G.; Dickson, D.; Young, L. Exercise-induced cardiac remodeling: Lessons from humans, horses, and dogs. Vet. Sci. 2017, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G. Horse Anatomy for Performance: A Practical Guide to Training, Riding and Horse Care; David & Charles: Exeter, UK, 2012. [Google Scholar]
- Vernemmen, I.; Vera, L.; Van Steenkiste, G.; van Loon, G.; Decloedt, A. Reference values for 2-dimensional and M-mode echocardiography in Friesian and Warmblood horses. J. Vet. Intern. Med. 2020, 34, 2701–2709. [Google Scholar] [CrossRef]
- Pinar, O.; Sancak, A. Effects of different heart dimensions on race performance in Thorougbred race horses. Acta Sci. Vet. 2018, 46. [Google Scholar] [CrossRef]
- Buhl, R.; Ersbøll, A.K.; Eriksen, L.; Koch, J. Changes over time in echocardiographic measurements in young Standardbred racehorses undergoing training and racing and association with racing performance. J. Am. Vet. Med. Assoc. 2005, 226, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Nath, L.; Saljic, A.; Buhl, R.; Elliott, A.; La Gerche, A.; Ye, C.; Schmidt Royal, H.; Lundgren Virklund, K.; Agbaedeng, T.; Stent, A. Histological evaluation of cardiac remodelling in equine athletes. Sci. Rep. 2024, 14, 16709. [Google Scholar] [CrossRef]
- Kadkhodayan, A.; Coggan, A.R.; Peterson, L.R. A “PET” area of interest: Myocardial metabolism in human systolic heart failure. Heart Fail. Rev. 2013, 18, 567–574. [Google Scholar] [CrossRef]
- Actis Dato, V.; Lange, S.; Cho, Y. Metabolic Flexibility of the Heart: The Role of Fatty Acid Metabolism in Health, Heart Failure, and Cardiometabolic Diseases. Int. J. Mol. Sci. 2024, 25, 1211. [Google Scholar] [CrossRef]
- Miao, H.; Li, B.; Wang, Z.; Mu, J.; Tian, Y.; Jiang, B.; Zhang, S.; Gong, X.; Shui, G.; Lam, S.M. Lipidome atlas of the developing heart uncovers dynamic membrane lipid attributes underlying cardiac structural and metabolic maturation. Research 2022, 2022, 0006. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, K.D.; Kim, K.K.; Park, P.J.; Choi, C.J.; Oh, O.J.; Song, S.K.; Kim, K.S.; Cho, C.B. Analysis of metabolomic patterns in thoroughbreds before and after exercise. Asian-Australas. J. Anim. Sci. 2017, 30, 1633–1642. [Google Scholar] [CrossRef]
- Piccione, G.; Arfuso, F.; Fazio, F.; Bazzano, M.; Giannetto, C. Serum lipid modification related to exercise and polyunsaturated fatty acid supplementation in jumpers and thoroughbred horses. J. Equine Vet. Sci. 2014, 34, 1181–1187. [Google Scholar] [CrossRef]
- Nolazco Sassot, L.; Villarino, N.; Dasgupta, N.; Morrison, J.; Bayly, W.; Gang, D.; Sanz, M. The lipidome of Thoroughbred racehorses before and after supramaximal exercise. Equine Vet. J. 2019, 51, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Le Moyec, L.; Robert, C.; Triba, M.N.; Billat, V.L.; Mata, X.; Schibler, L.; Barrey, E. Protein catabolism and high lipid metabolism associated with long-distance exercise are revealed by plasma NMR metabolomics in endurance horses. PLoS ONE 2014, 9, e90730. [Google Scholar] [CrossRef] [PubMed]
- Le Moyec, L.; Robert, C.; Triba, M.N.; Bouchemal, N.; Mach, N.; Riviere, J.; Zalachas-Rebours, E.; Barrey, E. A first step toward unraveling the energy metabolism in endurance horses: Comparison of plasma nuclear magnetic resonance metabolomic profiles before and after different endurance race distances. Front. Mol. Biosci. 2019, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zeng, Y.; Ma, C.; Meng, J.; Wang, J.; Ren, W.; Wang, C.; Yuan, X.; Yang, X.; Yao, X. Plasma Non-targeted Metabolomics Analysis of Yili Horses Raced on Tracks with Different Surface Hardness. J. Equine Vet. Sci. 2023, 121, 104197. [Google Scholar] [CrossRef]
- Schirone, L.; Forte, M.; Palmerio, S.; Yee, D.; Nocella, C.; Angelini, F.; Pagano, F.; Schiavon, S.; Bordin, A.; Carrizzo, A. A review of the molecular mechanisms underlying the development and progression of cardiac remodeling. Oxidative Med. Cell. Longev. 2017, 2017, 3920195. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Hanson, C.; Kline, K.; Foreman, J. Measurements of heart scores and heart weights in horses of two different morphic body types. Comp. Biochem. Physiol. Part A Physiol. 1994, 108, 175–178. [Google Scholar] [CrossRef]
- José Lopes, V.; Barbosa da Costa, G.; Garcia Pereira, A.; de Freitas Pereira, L.; Fernandes Magalhães, L.; dos Santos, D.; Paulino Junior, D. Morphoquantitative evaluation of the heart of equine athletes. Pubvet 2021, 15, a826. [Google Scholar]
- Young, L.E. Cardiac responses to training in 2-year-old thoroughbreds: An echocardiographic study. Equine Vet. J. 1999, 31, 195–198. [Google Scholar] [CrossRef]
- Naeije, R.; Badagliacca, R. The overloaded right heart and ventricular interdependence. Cardiovasc. Res. 2017, 113, 1474–1485. [Google Scholar] [CrossRef]
- Maskhuliva, L.; Akhalkatsi, V.; Chelidze, K.; Kakhabrishvili, Z.; Matiashvili, M.; Chabashvili, N.; Chutkerashvili, T. Echocardiographic Study of Right Ventricular Remodeling in Top-Level Georgian Athletes. Br. J. Sports Med. 2014, 48, 634. [Google Scholar] [CrossRef]
- Jacob, M.; Chappell, D.; Becker, B.F. Regulation of blood flow and volume exchange across the microcirculation. Crit. Care 2016, 20, 1–13. [Google Scholar] [CrossRef]
- Morra, E.A.; Zaniqueli, D.; Rodrigues, S.L.; El-Aouar, L.M.; Lunz, W.; Mill, J.G.; Carletti, L. Long-term intense resistance training in men is associated with preserved cardiac structure/function, decreased aortic stiffness, and lower central augmentation pressure. J. Hypertens. 2014, 32, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.A.; Hill, B.G. Metabolic coordination of physiological and pathological cardiac remodeling. Circ. Res. 2018, 123, 107–128. [Google Scholar] [CrossRef]
- Schugar, R.C.; Gliniak, C.M.; Osborn, L.J.; Massey, W.; Sangwan, N.; Horak, A.; Banerjee, R.; Orabi, D.; Helsley, R.N.; Brown, A.L. Gut microbe-targeted choline trimethylamine lyase inhibition improves obesity via rewiring of host circadian rhythms. eLife 2022, 11, e63998. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, R.; Liang, Y.; Shi, J.; Li, J.; Shang, F.; Mao, X.; Sun, J. Changes of myocardial lipidomics profiling in a rat model of diabetic cardiomyopathy using UPLC/Q-TOF/MS analysis. Diabetol. Metab. Syndr. 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Drechsler, R.; Chen, S.-W.; Dancy, B.C.; Mehrabkhani, L.; Olsen, C.P. HPLC-based mass spectrometry characterizes the phospholipid alterations in ether-linked lipid deficiency models following oxidative stress. PLoS ONE 2016, 11, e0167229. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishimura, A.; Nishiyama, K.; Goto, T.; Numaga-Tomita, T.; Nishida, M. Mitochondrial dynamics in exercise physiology. Pflügers Arch.-Eur. J. Physiol. 2020, 472, 137–153. [Google Scholar] [CrossRef]
- Amorese, A.J.; Ryan, A.S. Home-based tele-exercise in musculoskeletal conditions and chronic disease: A literature review. Front. Rehabil. Sci. 2022, 3, 811465. [Google Scholar] [CrossRef]
- Feng, L.T.; Chen, Z.N.; Bian, H. Skeletal muscle: Molecular structure, myogenesis, biological functions, and diseases. MedComm 2024, 5, e649. [Google Scholar] [CrossRef]
- Da, Y.S.; Takagi, H.; Hiroshima, M.; Matsuoka, S.; Ueda, M. Sphingomyelin metabolism underlies Ras excitability for efficient cell migration and chemotaxis. Cell Struct. Funct. 2023, 48, 145–160. [Google Scholar]
- Ji, X.; Chen, Z.; Wang, Q.; Li, B.; Wei, Y.; Li, Y.; Lin, J.; Cheng, W.; Guo, Y.; Wu, S. Sphingolipid metabolism controls mammalian heart regeneration. Cell Metab. 2024, 36, 839–856.e8. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Ulu, A.; Mukherjee, M.; Yuil-Valdes, A.G.; Thoene, M.; Van Ormer, M.; Slotkowski, R.; Mauch, T.; Anderson-Berry, A.; Hanson, C.K. Something Smells Fishy: How Lipid Mediators Impact the Maternal–Fetal Interface and Neonatal Development. Biomedicines 2023, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
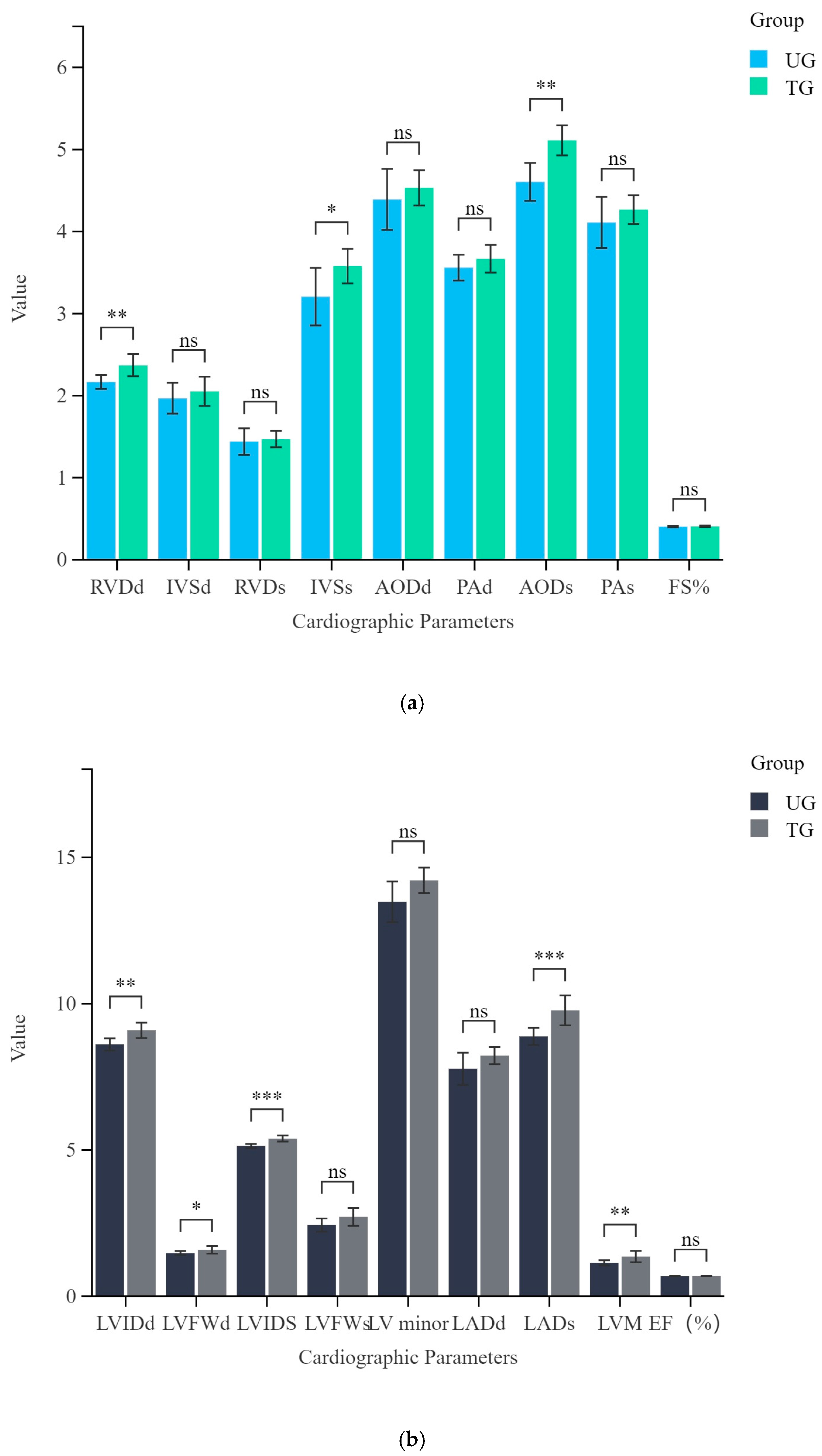
| TERM | Untrained Group (UG) | Trained Group (TG) |
|---|---|---|
| RVDd (cm) | 2.17 ± 0.09 | 2.37 ± 0.13 |
| IVSd (cm) | 1.97 ± 0.19 | 2.05 ± 0.18 |
| LVIDd (cm) | 8.61 ± 0.21 | 9.09 ± 0.26 |
| LVFWd (cm) | 1.48 ± 0.07 | 1.59 ± 0.13 |
| RVDs (cm) | 1.44 ± 0.16 | 1.47 ± 0.1 |
| IVSs (cm) | 3.21 ± 0.35 | 3.58 ± 0.21 |
| LVIDs (cm) | 5.14 ± 0.07 | 5.39 ± 0.1 |
| LVFWs (cm) | 2.43 ± 0.22 | 2.71 ± 0.31 |
| LV minor (cm) | 13.48 ± 0.7 | 14.22 ± 0.43 |
| LADd (cm) | 7.78 ± 0.55 | 8.23 ± 0.29 |
| LADs (cm) | 8.88 ± 0.3 | 9.77 ± 0.51 |
| AODd (cm) | 4.39 ± 0.37 | 4.53 ± 0.22 |
| PAd (cm) | 3.56 ± 0.16 | 3.67 ± 0.17 |
| AODs (cm) | 4.61 ± 0.23 | 5.11 ± 0.18 |
| PAs (cm) | 4.11 ± 0.31 | 4.27 ± 0.17 |
| EF (%) | 0.69 ± 0.01 | 0.69 ± 0.01 |
| FS% | 0.4 ± 0.01 | 0.41 ± 0.01 |
| LVM (kg) | 1.14 ± 0.09 | 1.36 ± 0.19 |
| EDV (mL) | 405.77 ± 21.99 | 457.75 ± 29.38 |
| ESV (mL) | 125.85 ± 3.96 | 140.92 ± 6.41 |
| SV (mL) | 279.92 ± 18.39 | 316.83 ± 23.98 |
| HR | 45.03 ± 2.24 | 43.93 ± 3.96 |
| CO (L/min) | 15.42 + 1.41 | 13.89 + 1.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Meng, J.; Wang, J.; Ren, W.; Yang, X.; Adina, W.; Bao, Y.; Zeng, Y.; Yao, X. Absolute Quantitative Lipidomics Reveals Differences in Lipid Compounds in the Blood of Trained and Untrained Yili Horses. Vet. Sci. 2025, 12, 255. https://doi.org/10.3390/vetsci12030255
Wang T, Meng J, Wang J, Ren W, Yang X, Adina W, Bao Y, Zeng Y, Yao X. Absolute Quantitative Lipidomics Reveals Differences in Lipid Compounds in the Blood of Trained and Untrained Yili Horses. Veterinary Sciences. 2025; 12(3):255. https://doi.org/10.3390/vetsci12030255
Chicago/Turabian StyleWang, Tongliang, Jun Meng, Jianwen Wang, Wanlu Ren, Xixi Yang, Wusiman Adina, Yike Bao, Yaqi Zeng, and Xinkui Yao. 2025. "Absolute Quantitative Lipidomics Reveals Differences in Lipid Compounds in the Blood of Trained and Untrained Yili Horses" Veterinary Sciences 12, no. 3: 255. https://doi.org/10.3390/vetsci12030255
APA StyleWang, T., Meng, J., Wang, J., Ren, W., Yang, X., Adina, W., Bao, Y., Zeng, Y., & Yao, X. (2025). Absolute Quantitative Lipidomics Reveals Differences in Lipid Compounds in the Blood of Trained and Untrained Yili Horses. Veterinary Sciences, 12(3), 255. https://doi.org/10.3390/vetsci12030255







