Pilot Study of a Novel First-Line Protocol (THOP) for Intermediate–Large B-Cell Lymphoma in Dogs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Patient Selection
2.3. Chemotherapy Protocol
2.4. Evaluation of Response and Monitoring
2.5. Toxicity
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. The THOP Protocol
3.3. Adverse Events
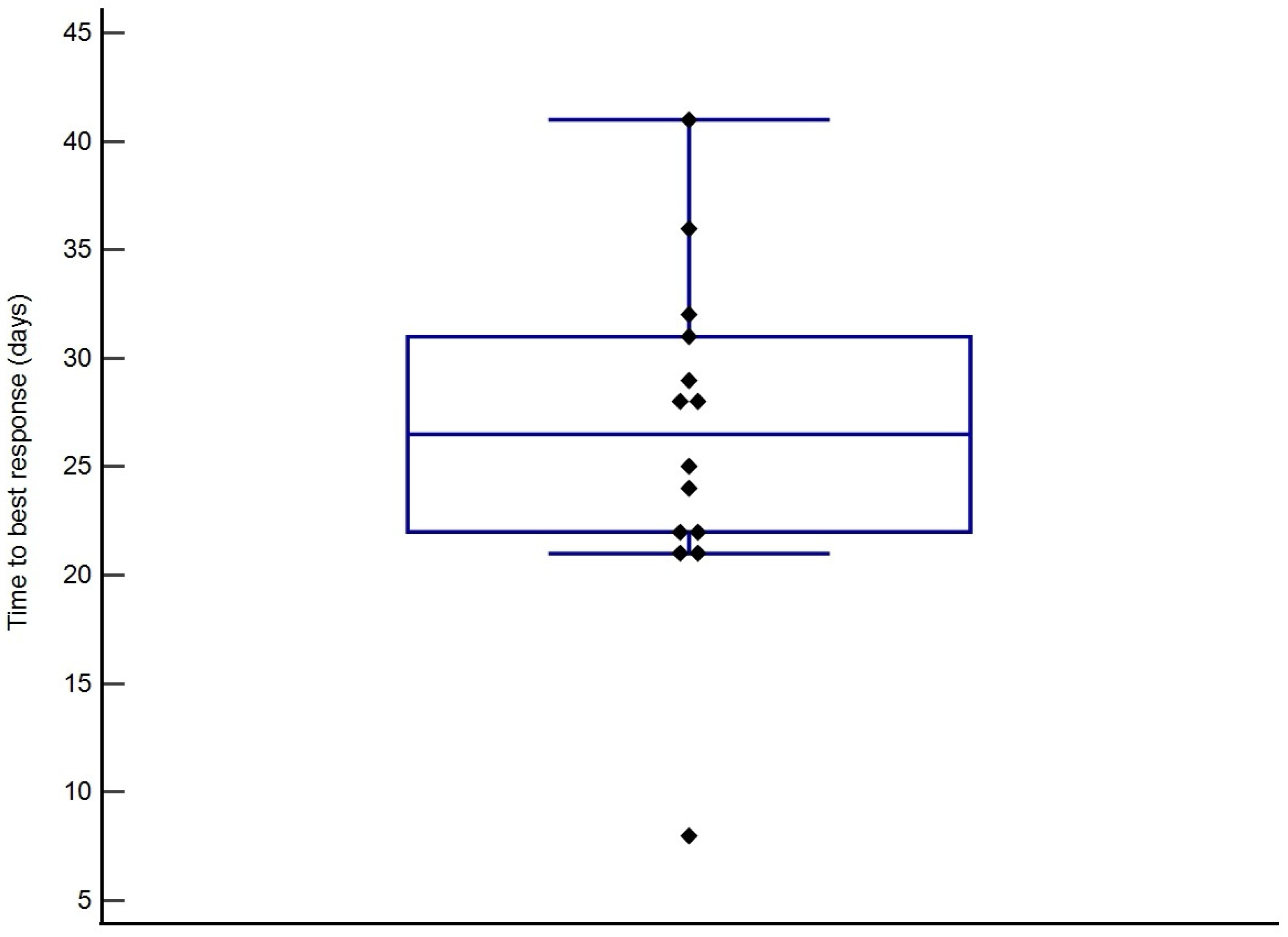
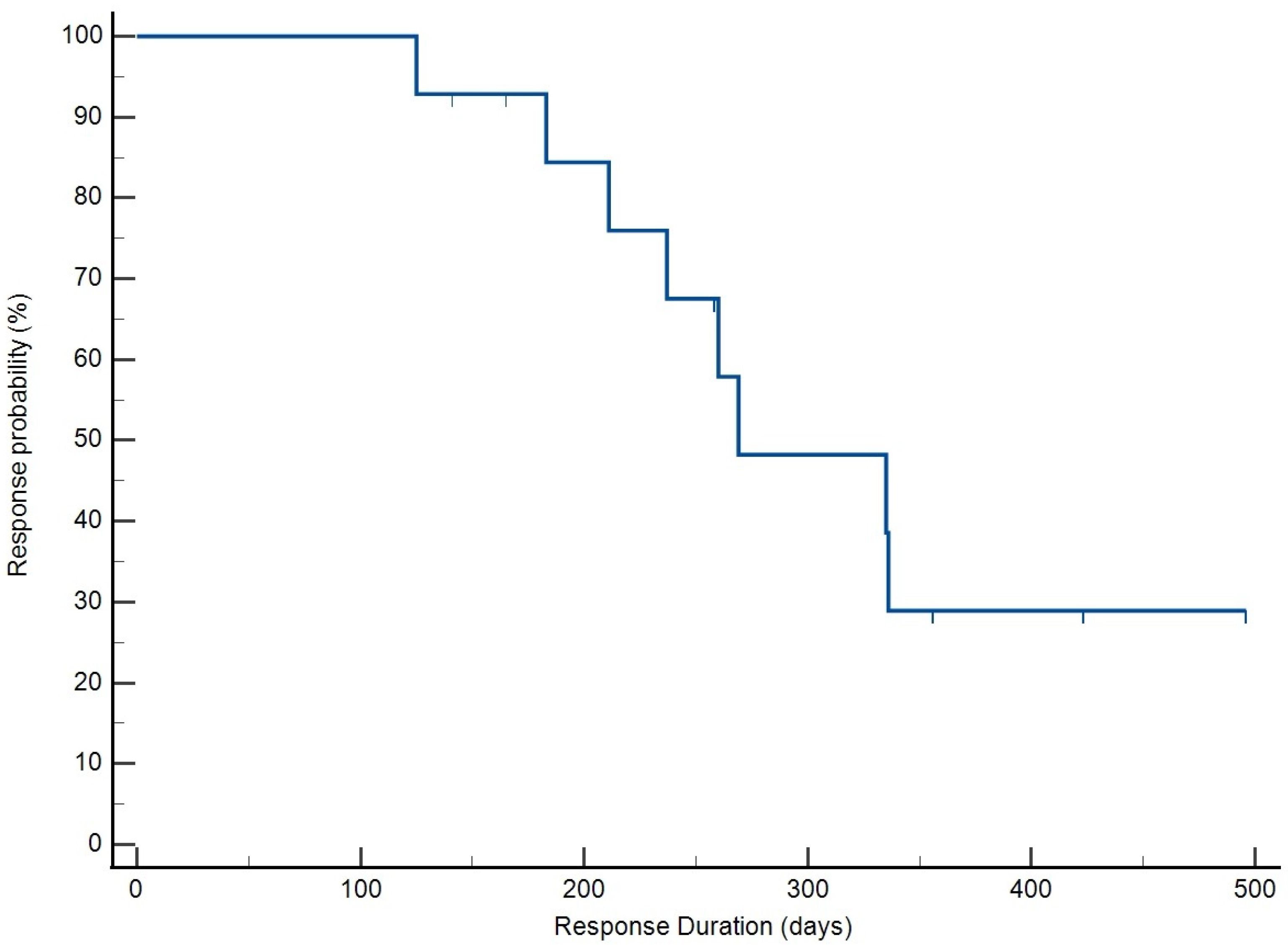
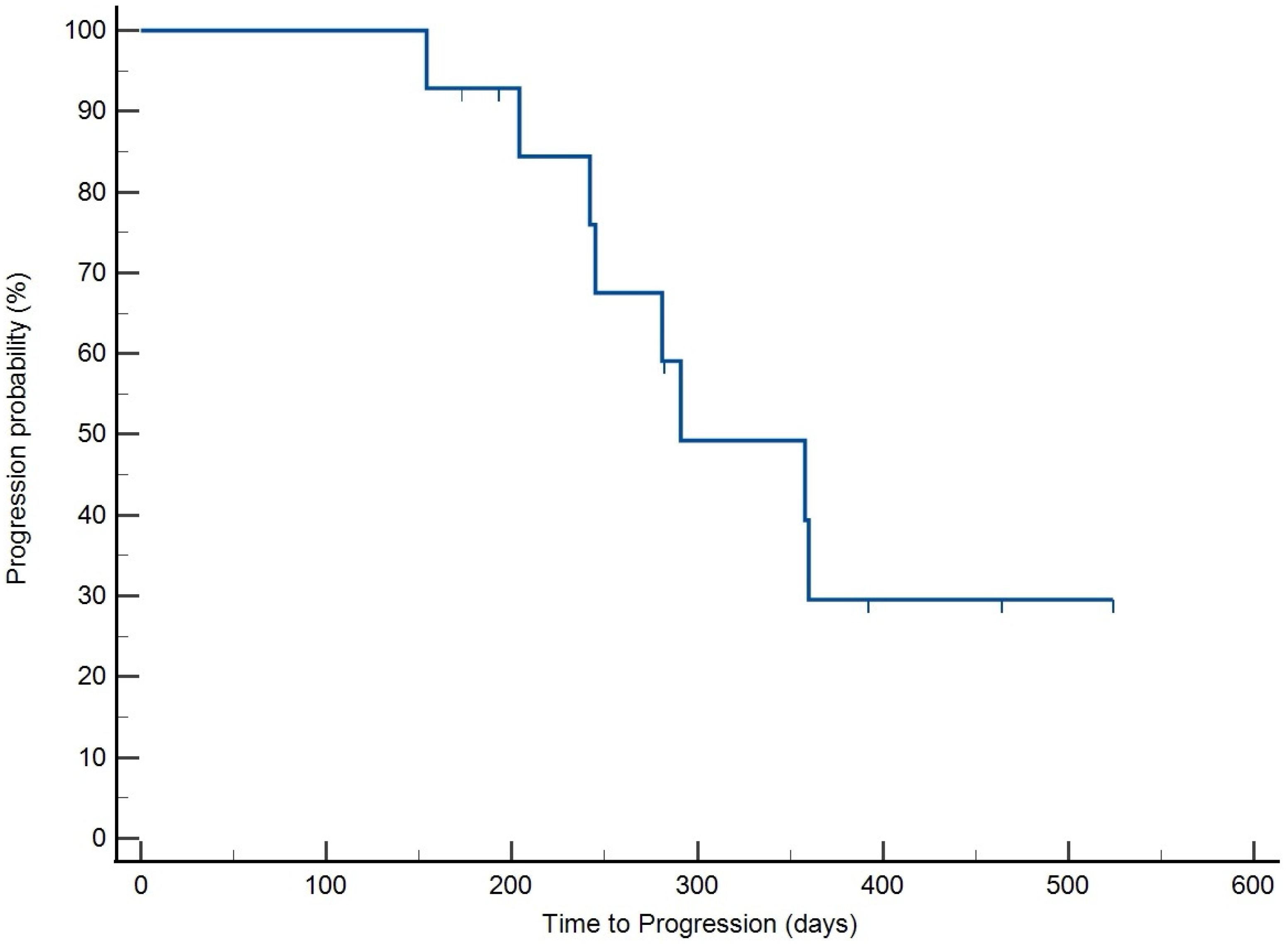
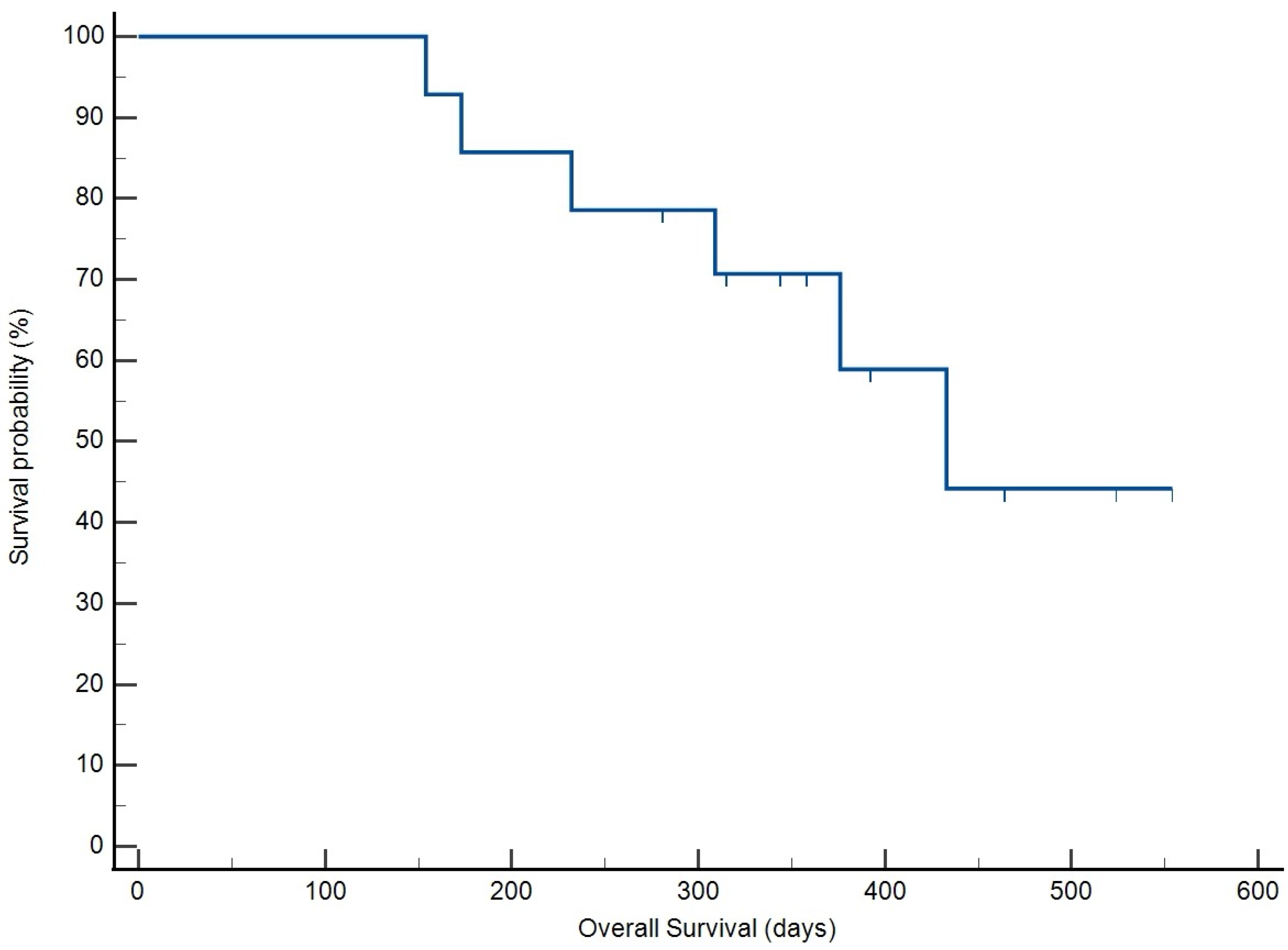
3.4. Treatment Response and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHOP | Chemotherapy protocol of: cyclophosphamide, doxorubicin, vincristine and prednisone |
| THOP | Chemotherapy protocol of: temozolomide, doxorubicin, vincristine and prednisone |
| DOX | Doxorubicin |
| TMZ | Temozolomide |
| TBR | Time to best response |
| RD | Response duration |
| TTP | Time to progression |
| OS | Overall survival |
| CR | Complete response |
| ORR | Overall response rate |
| AE | Adverse event |
References
- David, M.V.; Douglas, H.T.; Julias, M.L. 33—Hematopoietic tumors. In Withrow and Macewen’s Small Animal Clinical Oncology, 6th ed.; Withrow, S.J., Vail, D.M., Page, R.L., Eds.; Elsevier Saunders: St. Louis, MI, USA, 2020; pp. 688–772. [Google Scholar]
- Hawkes, C.; Morris, J.; Bavcar, S.; Wilkie, C.; Ray, S.; Auquier, C.; Benjamin, S.; Massó, J.B.; Bottin, S.; Davies, O.; et al. Comparison of chop-19 and chop-25 for treatment of peripheral nodal b-cell lymphoma in dogs: A european multicenter retrospective cohort study. J. Vet. Intern. Med. 2024, 38, 3193–3205. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Ringwall, A.; Lopez, L.; Elmslie, R.; Fowler, B.; Lori, J.; Sfiligoi, G.; Skope, A.; Arnold, E.; Hughes, K.L.; Thamm, D.H.; et al. Prospective evaluation of flow cytometric characteristics, histopathologic diagnosis and clinical outcome in dogs with naive b-cell lymphoma treated with a 19-week chop protocol. Vet. Comp. Oncol. 2020, 18, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Childress, M.O.; Ramos-Vara, J.A.; Ruple, A. Retrospective analysis of factors affecting clinical outcome following chop-based chemotherapy in dogs with primary nodal diffuse large b-cell lymphoma. Vet. Comp. Oncol. 2020, 16, E159–E168. [Google Scholar] [CrossRef]
- Curran, K.; Thamm, D.H. Thamm. Retrospective analysis for treatment of naïve canine multicentric lymphoma with a 15-week, maintenance-free chop protocol. Vet. Comp. Oncol. 2020, 14 (Suppl. S1), 147–155. [Google Scholar] [CrossRef]
- Burton, J.H.; Garrett-Mayer, E.; Thamm, D.H. Evaluation of a 15-week chop protocol for the treatment of canine multicentric lymphoma. Vet. Comp. Oncol. 2013, 11, 306–315. [Google Scholar] [CrossRef]
- Parker, A.S.; Burton, J.H.; Curran, K.M.; Wolf-Ringwall, A.; Thamm, D.H. Early progression during or after cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy indicates poor outcome with rescue protocols in dogs with multicentric lymphoma. J. Vet. Intern. Med. 2024, 38, 2282–2292. [Google Scholar] [CrossRef]
- Chowdhury, S.; Laudicina, D.; Blumenkrantz, N.; Wirth, M.; Alton, K. An lc/ms/ms method for the quantitation of mtic (5-(3-n-methyltriazen-1-yl)-imidazole-4-carboxamide), a bioconversion product of temozolomide, in rat and dog plasma. J. Pharm. Biomed. Anal. 1999, 19, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Bley, C.R.; Leone, V.F.; Finotello, R. An open-label dose escalation study evaluating tolerability and safety of a single 5-days course of temozolomide in dogs with advanced cancer. Vet. Comp. Oncol. 2020, 18, 838–842. [Google Scholar] [CrossRef]
- Treggiari, E.; Elliott, J.W.; Baines, S.J.; Blackwood, L. Temozolomide alone or in combination with doxorubicin as a rescue agent in 37 cases of canine multicentric lymphoma. Vet. Comp. Oncol. 2018, 16, 194–201. [Google Scholar] [CrossRef]
- Dervisis, N.G.; Dominguez, P.A.; Sarbu, L.; Newman, R.G.; Cadile, C.D.; Swanson, C.N.; Kitchell, B.E. Efficacy of temozolomide or dacarbazine in combination with an anthracycline for rescue chemotherapy in dogs with lymphoma. J. Am. Vet. Med. Assoc. 2007, 231, 563–569. [Google Scholar] [CrossRef]
- Martini, V.; Marano, G.; Aresu, L.; Bonfanti, U.; Boracchi, P.; Caniatti, M.; Cian, F.; Gambini, M.; Marconato, L.; Masserdotti, C.; et al. Performance of lymph node cytopathology in diagnosis and characterization of lymphoma in dogs. J. Vet. Intern. Med. 2022, 36, 204–214. [Google Scholar] [CrossRef]
- Thalheim, L.; Williams, L.; Borst, L.; Fogle, J.; Suter, S. Lymphoma immunophenotype of dogs determined by immunohistochemistry, flow cytometry, and polymerase chain reaction for antigen receptor rearrangements. J. Vet. Intern. Med. 2013, 27, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Seelig, D.; Avery, P.; Webb, T.; Yoshimoto, J.; Bromberek, J.; Ehrhart, E.; Avery, A. Canine t-zone lymphoma: Unique immunophenotypic features, outcome, and population characteristics. J. Vet. Intern. Med. 2014, 28, 878–886. [Google Scholar] [CrossRef]
- Rao, S.; Lana, S.; Eickhoff, J.; Marcus, E.; Avery, P.; Morley, P.; Avery, A. Class ii major histocompatibility complex expression and cell size independently predict survival in canine b-cell lymphoma. J. Vet. Intern. Med. 2011, 25, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.E.; Kass, P.H.; Myint, M.S.; Scott, F. Canine lymphomas: Association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet. Pathol. 2013, 50, 738–748. [Google Scholar] [CrossRef]
- Childress, M.O.; Ramos-Vara, J.A.; Ruple, A. A randomized controlled trial of the effect of prednisone omission from a multidrug chemotherapy protocol on treatment outcome in dogs with peripheral nodal lymphomas. J. Am. Vet. Med. Assoc. 2016, 249, 1067–1078. [Google Scholar] [CrossRef]
- Hanot, C.C.; Mealey, K.L.; Fidel, J.L.; Burke, N.S.; White, L.A.; Sellon, R.K. Development of prednisone resistance in naive canine lymphoma: Longitudinal evaluation of nr3c1alpha, abcb1, and 11beta-hsd mrna expression. J. Vet. Pharmacol. Ther. 2020, 43, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M.; Michels, G.M.; Khanna, C.; Selting, K.A.; London, C.A.; Veterinary Cooperative Oncology Group. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0)--a veterinary cooperative oncology group (vcog) consensus document. Vet. Comp. Oncol. 2010, 8, 28–37. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary cooperative oncology group-common terminology criteria for adverse events (vcog-ctcae v2) following investigational therapy in dogs and cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar] [CrossRef]
- Malhotra, M.; Chandrasekahran, A.; Tonse, R.; Jalali, R.; Patil, V. Cross-sectional study of temozolomide-induced chemotherapy-induced nausea and vomiting in patients with glioma. Clin. Oncol. (R. Coll. Radiol.) 2019, 31, e85. [Google Scholar] [CrossRef]
- Rozzi, A.; Nardoni, C.; Corona, M.; Restuccia, M.R.; Fabi, A.; Bria, E.; Minniti, G.; Lanzetta, G. Palonosetron for the prevention of chemotherapy-induced nausea and vomiting in glioblastoma patients treated with temozolomide: A phase ii study. Support. Care Cancer 2011, 19, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.P.; Woodring, S.; Randazzo, D.M.; Friedman, H.S.; Desjardins, A.; Healy, P.; Herndon, J.E.; McSherry, F.; Lipp, E.S.; Miller, E.; et al. Randomized open-label phase ii trial of 5-day aprepitant plus ondansetron compared to ondansetron alone in the prevention of chemotherapy-induced nausea-vomiting (cinv) in glioma patients receiving adjuvant temozolomide. Support. Care Cancer 2020, 28, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Crespo, E.H.; Mariné, A.F.; i Battle, M.P.; Massó, J.F.B.; Feliu-Pascual, A.L. Survival time after surgical debulking and temozolomide adjuvant chemotherapy in canine intracranial gliomas. Vet. Sci. 2022, 9, 427. [Google Scholar] [CrossRef]
- Hicks, J.; Platt, S.; Stewart, G.; Senneca, C.; Holmes, S.; Kent, M.; Howerth, E.; Kaplan, J.; Kaplan, E. Intratumoral temozolomide in spontaneous canine gliomas: Feasibility of a novel therapy using implanted microcylinders. Vet. Med. Sci. 2019, 5, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Denies, S.; Cicchelero, L.; de Rooster, H.; Daminet, S.; Polis, I.; Van de Maele, I.; Sanders, N.N. Immunological and angiogenic markers during metronomic temozolomide and cyclophosphamide in canine cancer patients. Vet. Comp. Oncol. 2017, 15, 594–605. [Google Scholar] [CrossRef]
- Cancedda, S.; Bley, C.R.; Aresu, L.; Dacasto, M.; Leone, V.F.; Pizzoni, S.; Gracis, M.; Marconato, L. Efficacy and side effects of radiation therapy in comparison with radiation therapy and temozolomide in the treatment of measurable canine malignant melanoma. Vet. Comp. Oncol. 2016, 14, e146–e157. [Google Scholar] [CrossRef]
- Gagnon, J.; Dervisis, N.G.; E Kitchell, B. Treatment-related toxicities in tumor-bearing cats treated with temozolomide alone or in combination with doxorubicin: A pilot assessment. J. Feline Med. Surg. 2012, 14, 560–565. [Google Scholar] [CrossRef]
| Week | Screening | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Procedure | |||||||||||||||||
| Vincristine 0.7 mg/m2 IV | X | X | X | X | X | ||||||||||||
| Prednisone 40 mg/m2 PO q24 h for 7 days | X | X | X | X | X | ||||||||||||
| Doxorubicin 30 mg/m2 IV | X | X | X | X | X | ||||||||||||
| Temozolomide 100 mg/m2 PO q24 for 5 days | X | X | X | X | X | ||||||||||||
| CBC | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Chemistry | X | X | X | X | X | X | |||||||||||
| Pathologist review of CBC | X | X | |||||||||||||||
| Urinalysis | X | ||||||||||||||||
| Cytology or histopathology | X | ||||||||||||||||
| Flow cytometry or immunohistochemistry | X | ||||||||||||||||
| Thoracic radiographs | X | X | |||||||||||||||
| Abdominal ultrasound | X | X | |||||||||||||||
| Grade I | Grade II | Grade III | Grade IV | |
|---|---|---|---|---|
| Neutropenia | 9 | 3 | 4 | 4 |
| Anemia | 13 | |||
| Thrombocytopenia | 2 | 1 | ||
| ALP elevation | 13 | 1 | 1 | |
| ALT elevation | 7 | 3 | ||
| BUN elevation | 5 | 2 | ||
| Nausea | 2 | 2 | 1 | |
| Inappetence | 11 | 6 | ||
| Diarrhea | 1 | 3 |
| Drug | Neutropenia | Thrombocytopenia | Treatment Delays | Dose Reductions |
|---|---|---|---|---|
| Vincristine | Grade III: 3 Grade IV:1 | None | 10 | 3 |
| Doxorubicin/Temozolomide | Grade III: 1 Grade IV:3 | Grade III: 1 | 5 | Doxorubicin: 7 Temozolomide: 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tellez Silva, A.; Yang, E.; Nightengale, M.; Dervisis, N.; Klahn, S. Pilot Study of a Novel First-Line Protocol (THOP) for Intermediate–Large B-Cell Lymphoma in Dogs. Vet. Sci. 2025, 12, 251. https://doi.org/10.3390/vetsci12030251
Tellez Silva A, Yang E, Nightengale M, Dervisis N, Klahn S. Pilot Study of a Novel First-Line Protocol (THOP) for Intermediate–Large B-Cell Lymphoma in Dogs. Veterinary Sciences. 2025; 12(3):251. https://doi.org/10.3390/vetsci12030251
Chicago/Turabian StyleTellez Silva, Alejandra, Ester Yang, Marlie Nightengale, Nikolaos Dervisis, and Shawna Klahn. 2025. "Pilot Study of a Novel First-Line Protocol (THOP) for Intermediate–Large B-Cell Lymphoma in Dogs" Veterinary Sciences 12, no. 3: 251. https://doi.org/10.3390/vetsci12030251
APA StyleTellez Silva, A., Yang, E., Nightengale, M., Dervisis, N., & Klahn, S. (2025). Pilot Study of a Novel First-Line Protocol (THOP) for Intermediate–Large B-Cell Lymphoma in Dogs. Veterinary Sciences, 12(3), 251. https://doi.org/10.3390/vetsci12030251







