Inhibitory Effects of Bovine Lactoferricin-Lactoferrampin on Senecavirus A and Foot-and-Mouth Disease Virus with Recombinant Lactobacillus Oral Treatment in Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Culture Conditions for BHK-21 and PK-15 Cells
2.3. Cell Activity Assay
2.4. Determination of Viral Copy Number by RT-qPCR
2.5. The Half-Maximal Inhibitory Concentration of LFCA Against the Replication of SVA and FMDV
2.6. Detection of Cell Oxidative Stress Index
2.7. The Alterations in the Cellular Antioxidant Gene Expression Levels
2.8. Detection of Antiviral Activity of pPG-A3-LFCA/LRco21 In Vitro
2.9. Protective Effect of LFCA on Virus Infection in Animals
2.10. Statistical Analyses
3. Results
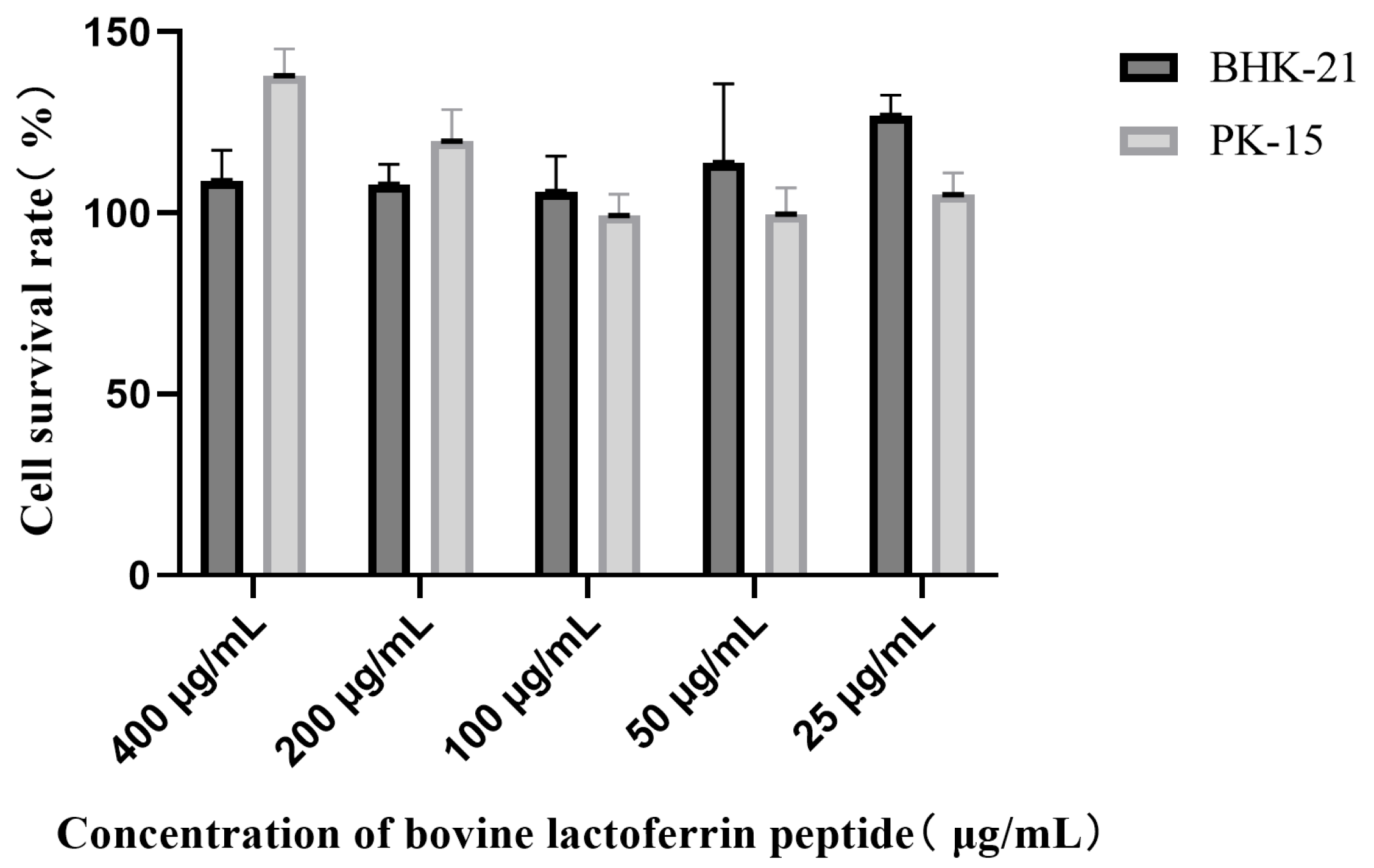
3.1. Cytotoxicity Assessment of LFCA
3.2. LFCA Affected SVA and FMDV Replication
3.3. LFCA Inhibited Virus Replication at 50% Inhibitory Concentration
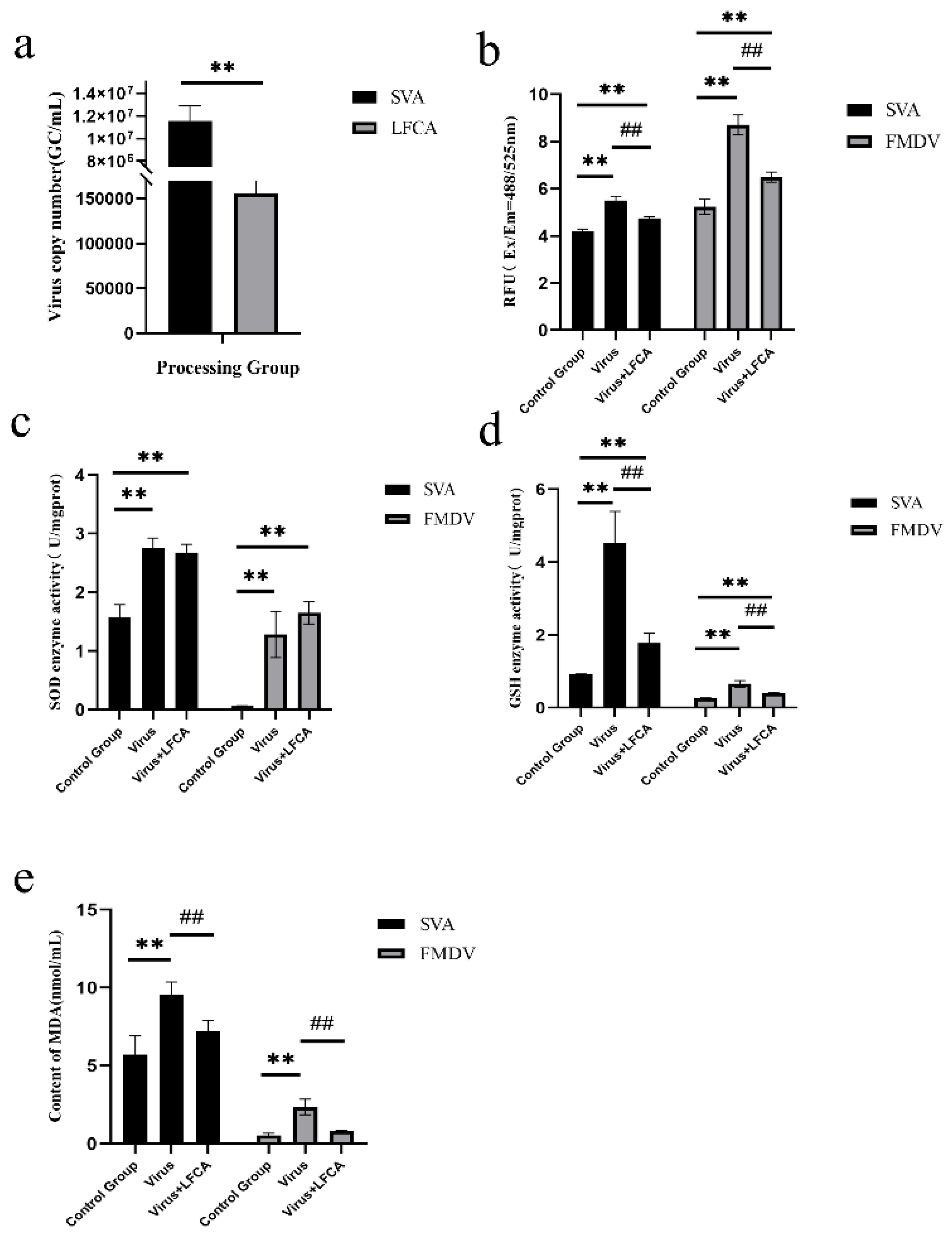
3.4. Effect of LFCA on Oxidative Stress Induced by Virus Infection
3.5. Effect of LFCA on Expression of Antioxidant Genes
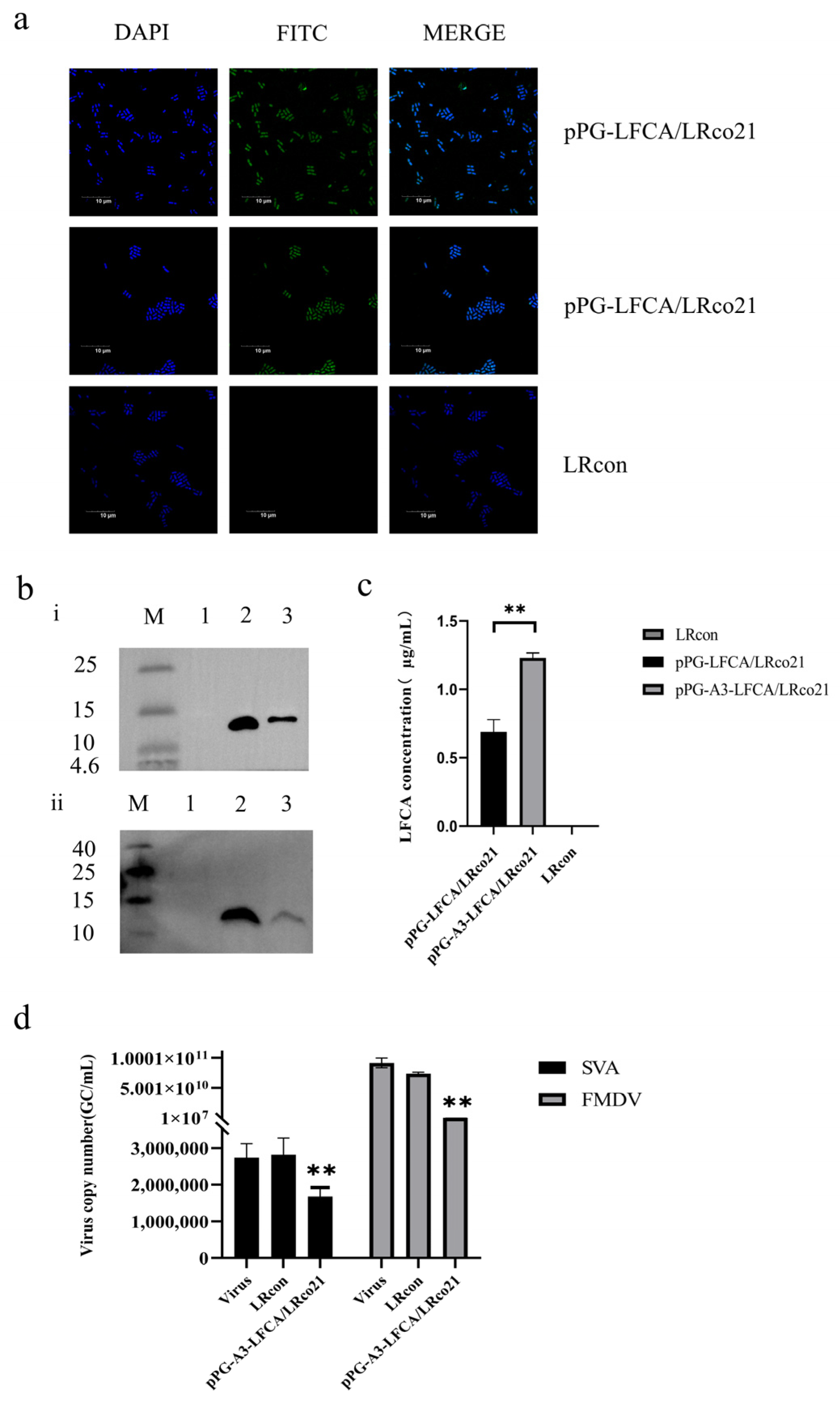
3.6. Identification of LFCA Expression in Recombinant Lactobacillus
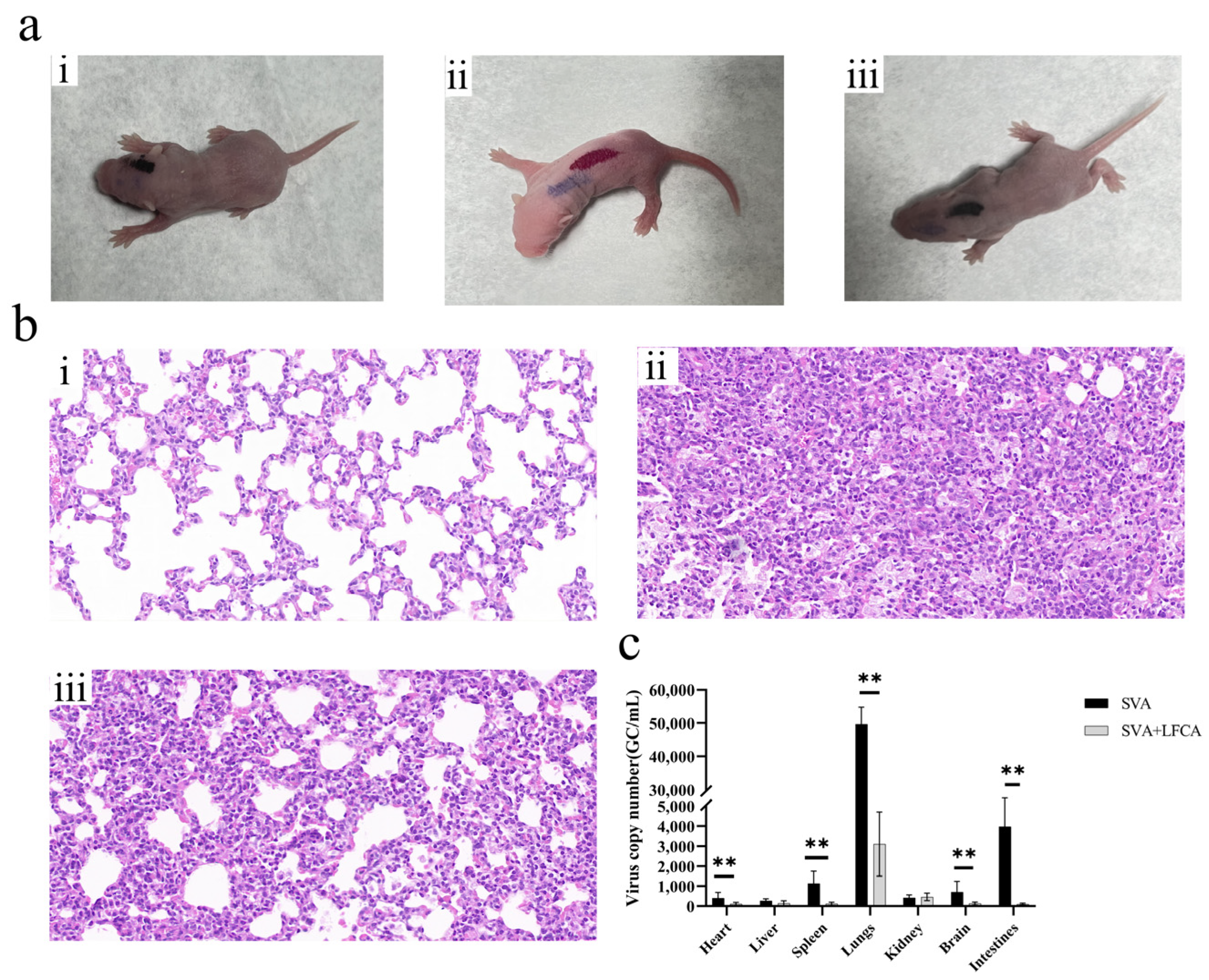
3.7. The Recombinant Lactobacillus Showed a Protective Effect Against SVA Infection in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grubman, M.J.; Baxt, B. Foot-and-mouth disease. Clin. Microbiol. Rev. 2004, 17, 465–493. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K. Foot- and-mouth disease as zoonosis. Arch. Virol. Suppl. 1997, 13, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Alkheraije, K.A. The prevalence of foot-and-mouth disease in Asia. Front. Vet. Sci. 2023, 10, 1201578. [Google Scholar] [CrossRef] [PubMed]
- Arzt, J.; Pacheco, J.M.; Stenfeldt, C.; Rodriguez, L.L. Pathogenesis of virulent and attenuated foot-and-mouth disease virus in cattle. Virol. J. 2017, 14, 89. [Google Scholar] [CrossRef]
- Leme, R.A.; Zotti, E.; Alcântara, B.K.; Oliveira, M.V.; Freitas, L.A.; Alfieri, A.F.; Alfieri, A.A. Senecavirus A: An Emerging Vesicular Infection in Brazilian Pig Herds. Transbound. Emerg. Dis. 2015, 62, 603–611. [Google Scholar] [CrossRef]
- Segalés, J.; Barcellos, D.; Alfieri, A.; Burrough, E.; Marthaler, D. Senecavirus A. Vet. Pathol. 2017, 54, 11–21. [Google Scholar] [CrossRef]
- Ran, X.; Hu, Z.; Wang, J.; Yang, Z.; Li, Z.; Wen, X. Prevalence of Senecavirus A in pigs from 2014 to 2020: A global systematic review and meta-analysis. J. Vet. Sci. 2023, 24, e48. [Google Scholar] [CrossRef]
- Preis, G.; Sanhueza, J.M.; Vilalta, C.; Vannucci, F.A.; Culhane, M.R.; Corzo, C.A. Senecavirus A seroprevalence and risk factors in United States pig farms. Front. Vet. Sci. 2022, 9, 1011975. [Google Scholar] [CrossRef]
- Wu, H.; Li, C.; Ji, Y.; Mou, C.; Chen, Z.; Zhao, J. The Evolution and Global Spatiotemporal Dynamics of Senecavirus A. Microbiol. Spectr. 2022, 10, e0209022. [Google Scholar] [CrossRef]
- Dee, S.; Havas, K.; Spronk, G. Detection of Senecavirus A in pigs from a historically negative national swine herd and associated with feed imports from endemically infected countries. Transbound. Emerg. Dis. 2022, 69, 3147–3149. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Campos-Rodriguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin and Peptide-derivatives: Antimicrobial Agents with Potential Use in Nonspecific Immunity Modulation. Curr. Pharm. Des. 2018, 24, 1067–1078. [Google Scholar] [CrossRef]
- Masson, P.L.; Heremans, J.F. Lactoferrin in milk from different species. Comp. Biochem. Physiol. B Comp. Biochem. 1971, 39, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Hennart, P.F.; Brasseur, D.J.; Delogne-Desnoeck, J.B.; Dramaix, M.M.; Robyn, C.E. Lysozyme, lactoferrin, and secretory immunoglobulin A content in breast milk: Influence of duration of lactation, nutrition status, prolactin status, and parity of mother. Am. J. Clin. Nutr. 1991, 53, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Tomita, M.; Giehl, T.J.; Ellison, R.T., 3rd. Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect. Immun. 1993, 61, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Avery, T.M.; Boone, R.L.; Lu, J.; Spicer, S.K.; Guevara, M.A.; Moore, R.E.; Chambers, S.A.; Manning, S.D.; Dent, L.; Marshall, D.; et al. Analysis of Antimicrobial and Antibiofilm Activity of Human Milk Lactoferrin Compared to Bovine Lactoferrin against Multidrug Resistant and Susceptible Acinetobacter baumannii Clinical Isolates. ACS Infect. Dis. 2021, 7, 2116–2126. [Google Scholar] [CrossRef]
- Flores-Villaseñor, H.; Canizalez-Román, A.; Reyes-Lopez, M.; Nazmi, K.; de la Garza, M.; Zazueta-Beltrán, J.; León-Sicairos, N.; Bolscher, J.G. Bactericidal effect of bovine lactoferrin, LFcin, LFampin and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2010, 23, 569–578. [Google Scholar] [CrossRef]
- Pietrantoni, A.; Di Biase, A.M.; Tinari, A.; Marchetti, M.; Valenti, P.; Seganti, L.; Superti, F. Bovine lactoferrin inhibits adenovirus infection by interacting with viral structural polypeptides. Antimicrob. Agents Chemother. 2003, 47, 2688–2691. [Google Scholar] [CrossRef]
- Jeong, Y.Y.; Lee, G.Y.; Yoo, Y.C. Bovine Lactoferricin Induces Intestinal Epithelial Cell Activation through Phosphorylation of FAK and Paxillin and Prevents Rotavirus Infection. J. Microbiol. Biotechnol. 2021, 31, 1175–1182. [Google Scholar] [CrossRef]
- Bellamy, W.; Wakabayashi, H.; Takase, M.; Kawase, K.; Shimamura, S.; Tomita, M. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med. Microbiol. Immunol. 1993, 182, 97–105. [Google Scholar] [CrossRef]
- Nakamura, A.; Kimura, F.; Tsuji, S.; Hanada, T.; Takebayashi, A.; Takahashi, A.; Kitazawa, J.; Morimune, A.; Amano, T.; Kushima, R.; et al. Bovine lactoferrin suppresses inflammatory cytokine expression in endometrial stromal cells in chronic endometritis. J. Reprod. Immunol. 2022, 154, 103761. [Google Scholar] [CrossRef]
- Yoo, Y.C.; Watanabe, S.; Watanabe, R.; Hata, K.; Shimazaki, K.; Azuma, I. Bovine lactoferrin and Lactoferricin inhibit tumor metastasis in mice. Adv. Exp. Med. Biol. 1998, 443, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lönnerdal, B. Bovine lactoferrin and lactoferricin exert antitumor activities on human colorectal cancer cells (HT-29) by activating various signaling pathways. Biochem. Cell Biol. 2017, 95, 99–109. [Google Scholar] [CrossRef]
- Arredondo-Beltrán, I.G.; Ramírez-Sánchez, D.A.; Zazueta-García, J.R.; Canizalez-Roman, A.; Angulo-Zamudio, U.A.; Velazquez-Roman, J.A.; Bolscher, J.G.M.; Nazmi, K.; León-Sicairos, N. Antitumor activity of bovine lactoferrin and its derived peptides against HepG2 liver cancer cells and Jurkat leukemia cells. BioMetals Int. J. Role Met. Ions Biol. Biochem. Med. 2023, 36, 639–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. CMLS 2005, 62, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Liu, X.; Su, B.; Lin, J. Improved Antimicrobial Activity of Bovine Lactoferrin Peptide (LFcinB) Based on Rational Design. Protein J. 2023, 42, 633–644. [Google Scholar] [CrossRef]
- Shi, P.; Fan, F.; Chen, H.; Xu, Z.; Cheng, S.; Lu, W.; Du, M. A bovine lactoferrin-derived peptide induced osteogenesis via regulation of osteoblast proliferation and differentiation. J. Dairy Sci. 2020, 103, 3950–3960. [Google Scholar] [CrossRef]
- Arias, M.; Hilchie, A.L.; Haney, E.F.; Bolscher, J.G.; Hyndman, M.E.; Hancock, R.E.; Vogel, H.J. Anticancer activities of bovine and human lactoferricin-derived peptides. Biochem. Cell Biol. 2017, 95, 91–98. [Google Scholar] [CrossRef]
- Bolscher, J.G.; Adão, R.; Nazmi, K.; van den Keybus, P.A.; van ’t Hof, W.; Nieuw Amerongen, A.V.; Bastos, M.; Veerman, E.C. Bactericidal activity of LFchimera is stronger and less sensitive to ionic strength than its constituent lactoferricin and lactoferrampin peptides. Biochimie 2009, 91, 123–132. [Google Scholar] [CrossRef]
- Ligtenberg, A.J.M.; Bikker, F.J.; Bolscher, J.G.M. LFchimera: A synthetic mimic of the two antimicrobial domains of bovine lactoferrin. Biochem. Cell Biol. 2021, 99, 128–137. [Google Scholar] [CrossRef]
- León-Sicairos, N.; Angulo-Zamudio, U.A.; Vidal, J.E.; López-Torres, C.A.; Bolscher, J.G.; Nazmi, K.; Reyes-Cortes, R.; Reyes-López, M.; de la Garza, M.; Canizalez-Román, A. Bactericidal effect of bovine lactoferrin and synthetic peptide lactoferrin chimera in Streptococcus pneumoniae and the decrease in luxS gene expression by lactoferrin. BioMetals Int. J. Role Met. Ions Biol. Biochem. Med. 2014, 27, 969–980. [Google Scholar] [CrossRef]
- Di Biase, A.M.; Pietrantoni, A.; Tinari, A.; Siciliano, R.; Valenti, P.; Antonini, G.; Seganti, L.; Superti, F. Heparin-interacting sites of bovine lactoferrin are involved in anti-adenovirus activity. J. Med. Virol. 2003, 69, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Trybala, E.; Superti, F.; Johansson, M.; Bergström, T. Inhibition of herpes simplex virus infection by lactoferrin is dependent on interference with the virus binding to glycosaminoglycans. Virology 2004, 318, 405–413. [Google Scholar] [CrossRef]
- Andersen, J.H.; Jenssen, H.; Sandvik, K.; Gutteberg, T.J. Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. J. Med. Virol. 2004, 74, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Andersen, J.H.; Uhlin-Hansen, L.; Gutteberg, T.J.; Rekdal, Ø. Anti-HSV activity of lactoferricin analogues is only partly related to their affinity for heparan sulfate. Antivir. Res. 2004, 61, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Marr, A.K.; Jenssen, H.; Moniri, M.R.; Hancock, R.E.; Panté, N. Bovine lactoferrin and lactoferricin interfere with intracellular trafficking of Herpes simplex virus-1. Biochimie 2009, 91, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, M.G.; Marchetti, M.; Superti, F. Bovine lactoferrin prevents the entry and intercellular spread of herpes simplex virus type 1 in Green Monkey Kidney cells. Antivir. Res. 2007, 76, 252–262. [Google Scholar] [CrossRef]
- Wang, W.Y.; Wong, J.H.; Ip, D.T.; Wan, D.C.; Cheung, R.C.; Ng, T.B. Bovine Lactoferrampin, Human Lactoferricin, and Lactoferrin 1-11 Inhibit Nuclear Translocation of HIV Integrase. Appl. Biochem. Biotechnol. 2016, 179, 1202–1212. [Google Scholar] [CrossRef]
- Jenssen, H.; Sandvik, K.; Andersen, J.H.; Hancock, R.E.; Gutteberg, T.J. Inhibition of HSV cell-to-cell spread by lactoferrin and lactoferricin. Antivir. Res. 2008, 79, 192–198. [Google Scholar] [CrossRef]
- Ho, Y.H.; Sung, T.C.; Chen, C.S. Lactoferricin B inhibits the phosphorylation of the two-component system response regulators BasR and CreB. Mol. Cell. Proteom. MCP 2012, 11, M111.014720. [Google Scholar] [CrossRef]
- Chang, C.K.; Kao, M.C.; Lan, C.Y. Antimicrobial Activity of the Peptide LfcinB15 against Candida albicans. J. Fungi 2021, 7, 519. [Google Scholar] [CrossRef] [PubMed]
- Mouritzen, M.V.; Petkovic, M.; Qvist, K.; Poulsen, S.S.; Alarico, S.; Leal, E.C.; Dalgaard, L.T.; Empadinhas, N.; Carvalho, E.; Jenssen, H. Improved diabetic wound healing by LFcinB is associated with relevant changes in the skin immune response and microbiota. Mol. Ther. Methods Clin. Dev. 2021, 20, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Toscanelli, W.; Fracella, M.; De Angelis, M.; Scagnolari, C.; Sorrentino, L.; Piselli, E.; Marcocci, M.E.; Midulla, F.; Mancino, E.; Nenna, R.; et al. NRF2 Antioxidant Response and Interferon-Stimulated Genes Are Differentially Expressed in SARS-CoV-2-Positive Young Subjects. Immun. Inflamm. Dis. 2025, 13, e70109. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Isolation and Identification of Porcine Senecavirus A and Experimental Infection of ICR Mice. Master’s Thesis, Heilongjiang Bayi Agricultural University, Daqing, China, 2019. Available online: https://kns.cnki.net/kcms2/article/abstract?v=YBmesx2FU7mJqM5lnxyM2mSBL6ixLgkCmlnk9jhX16wz7dLUT0VUX4z1R-v0_dHlSnjtmWKkhwPZ01tCsVNUFCbP5ncMUUa09HSxRdhKl-VvnCZ3VZAkUUHycctLAhN4u8wb_9h3yoCTq9GgXROKVm0Oq55XdbSXW2XFxnTj3W9PZw04ZqCcfDdOcMmLfEHg1ADnIASYJfk=&uniplatform=NZKPT&language=CHS (accessed on 16 August 2019).
- Liu, C.; Liu, Y.; Li, X.; Liang, L.; Cui, S. Pathogenicity Analysis of Weaned Piglets Challenged With Novel Emerging Senecavirus A in Fujian, China. Front. Vet. Sci. 2021, 8, 694110. [Google Scholar] [CrossRef]

| Experimental Group | Earliest Onset of Symptoms (Days) | Number of First Symptoms (Only) | Mean Body Weight of Mice After Challenge (g) | Time to Symptom Relief (Days) | Number of Symptoms Relieved (Only) | Mean Body Weight of Mice at End of Experiment (g) | Changes in Body Weight of Mice (g) |
|---|---|---|---|---|---|---|---|
| CON | - | - | 2.95 | - | - | 5.53 | 2.58 ± 0.114 |
| SVA | 1 | 7 | 2.97 | 4 | 7 | 5.23 | 2.26 ± 0.121 |
| LFCA | 2 | 8 | 2.83 | 4 | 8 | 5.30 | 2.47 ± 0.119 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Zhang, S.; Sui, L.; Wang, X.; Li, J.; Cui, W.; Jiang, Y.; Qiao, X.; Tang, L. Inhibitory Effects of Bovine Lactoferricin-Lactoferrampin on Senecavirus A and Foot-and-Mouth Disease Virus with Recombinant Lactobacillus Oral Treatment in Mice. Vet. Sci. 2025, 12, 199. https://doi.org/10.3390/vetsci12030199
Zhao W, Zhang S, Sui L, Wang X, Li J, Cui W, Jiang Y, Qiao X, Tang L. Inhibitory Effects of Bovine Lactoferricin-Lactoferrampin on Senecavirus A and Foot-and-Mouth Disease Virus with Recombinant Lactobacillus Oral Treatment in Mice. Veterinary Sciences. 2025; 12(3):199. https://doi.org/10.3390/vetsci12030199
Chicago/Turabian StyleZhao, Wenyue, Senhao Zhang, Ling Sui, Xiaona Wang, Jiaxuan Li, Wen Cui, Yanping Jiang, Xinyuan Qiao, and Lijie Tang. 2025. "Inhibitory Effects of Bovine Lactoferricin-Lactoferrampin on Senecavirus A and Foot-and-Mouth Disease Virus with Recombinant Lactobacillus Oral Treatment in Mice" Veterinary Sciences 12, no. 3: 199. https://doi.org/10.3390/vetsci12030199
APA StyleZhao, W., Zhang, S., Sui, L., Wang, X., Li, J., Cui, W., Jiang, Y., Qiao, X., & Tang, L. (2025). Inhibitory Effects of Bovine Lactoferricin-Lactoferrampin on Senecavirus A and Foot-and-Mouth Disease Virus with Recombinant Lactobacillus Oral Treatment in Mice. Veterinary Sciences, 12(3), 199. https://doi.org/10.3390/vetsci12030199






