Therapeutic Assessment of Diverse Doxycycline-Based Formulations in Promoting Deep Corneal Wound Healing: Evidence from a Rat Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Doxycycline-Loaded Gel Nanoparticle (DNP) and Doxycycline Eyedrop Synthesis
2.2. Animals and Deep Corneal Wound Rat Model
2.3. Treatment Regimen and Clinical Evaluation
2.4. Histopathology and Immunohistochemistry
2.5. Statistical Analysis
3. Results
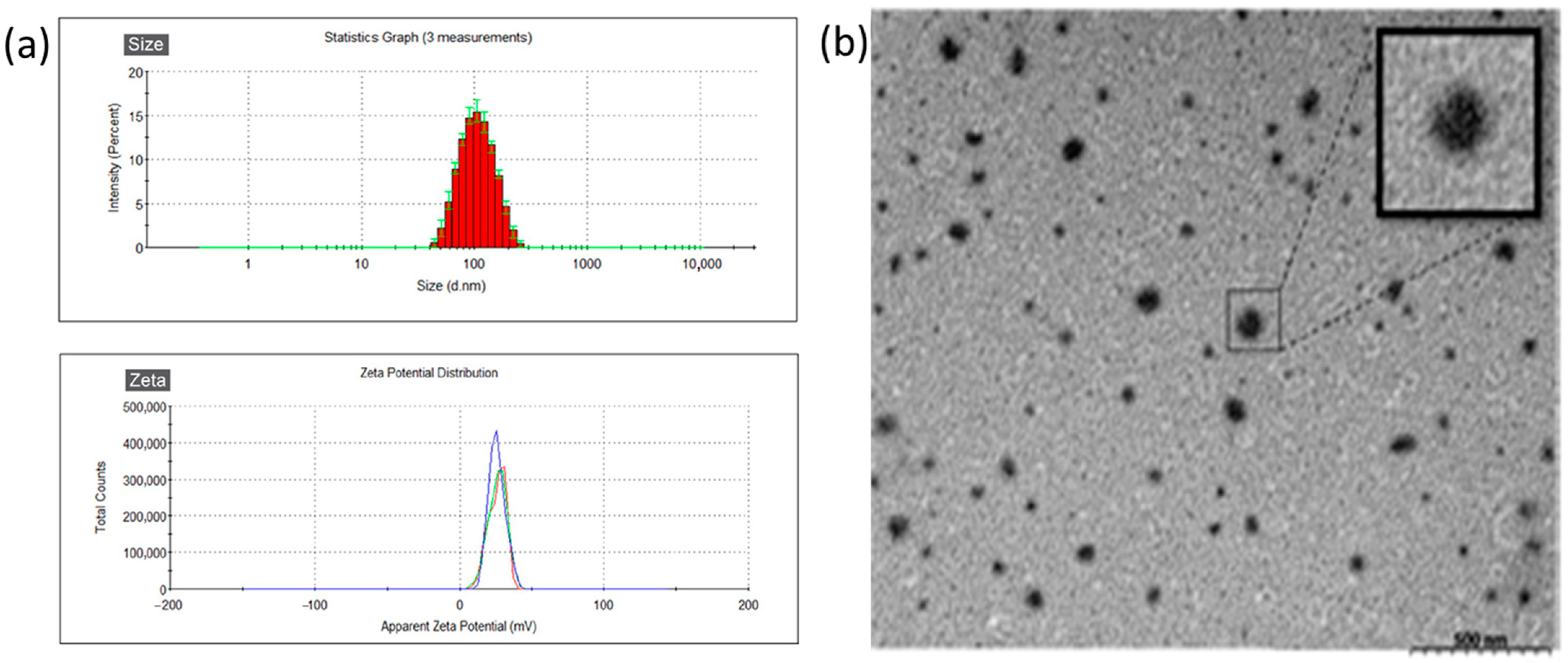
3.1. Characteristics of DNP
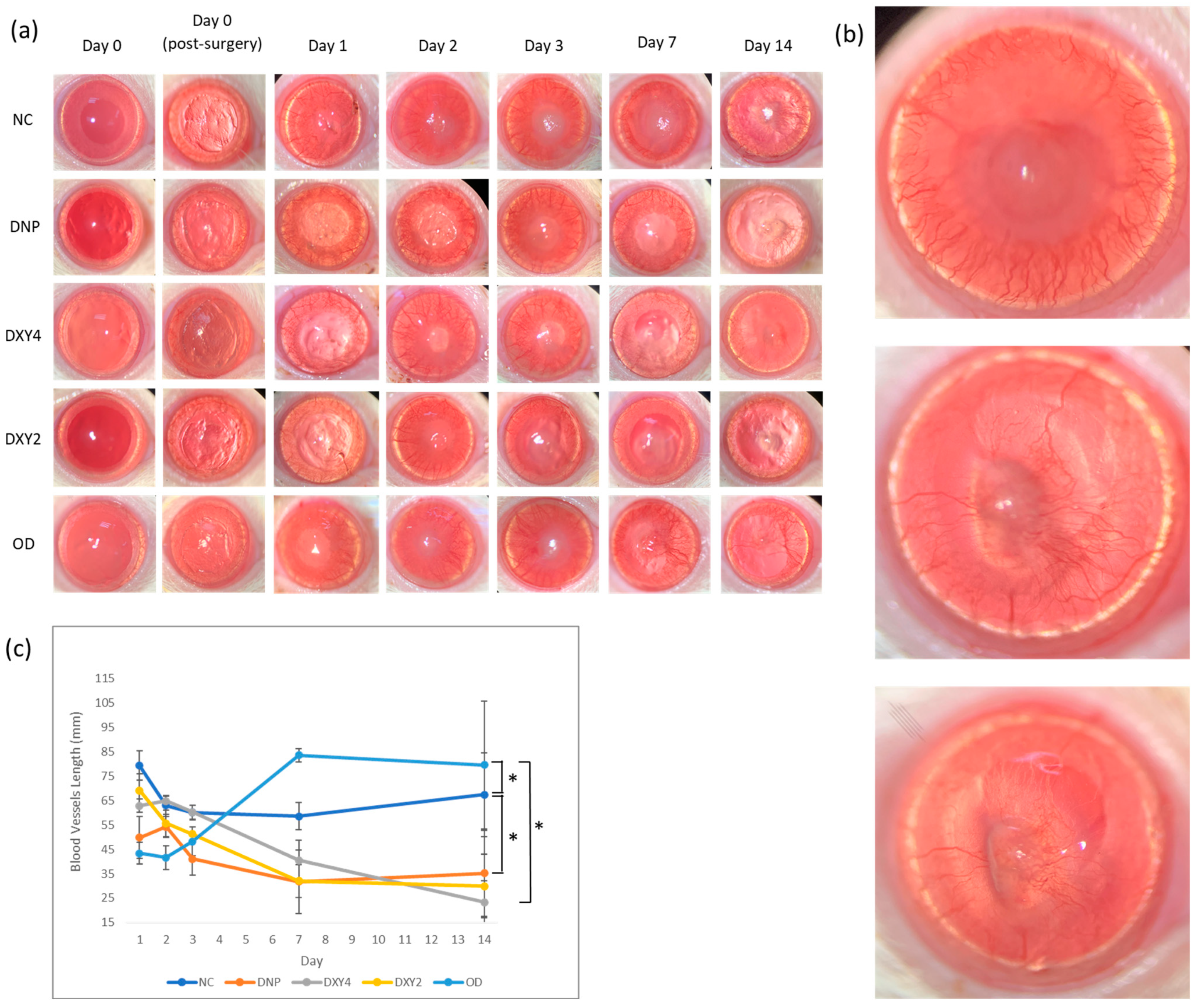
3.2. Effects of Doxycycline on Clinical Findings
3.2.1. Corneal Neovascularization
3.2.2. Corneal Epithelial Wound Healing
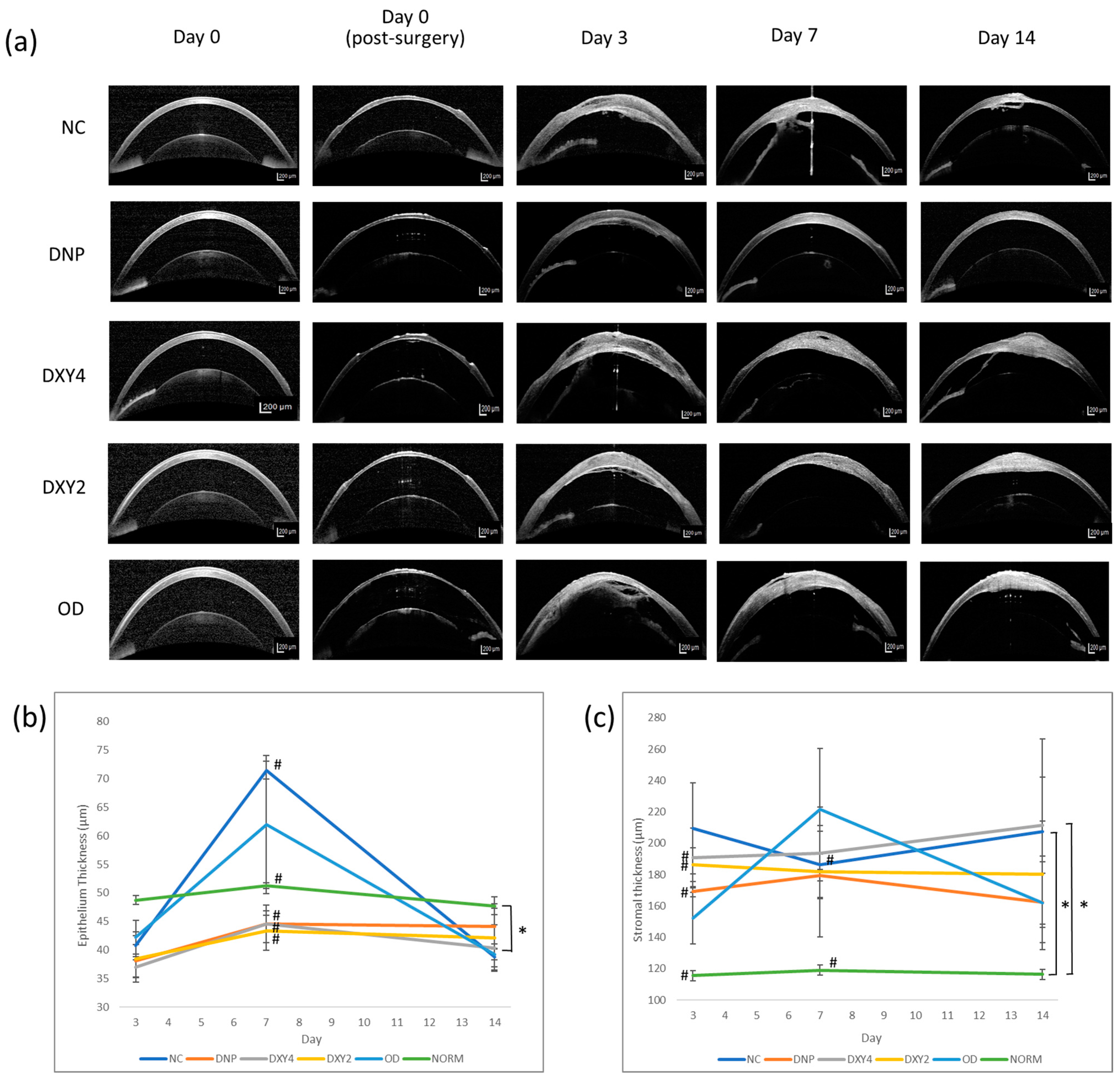
3.2.3. Corneal Epithelial and Stromal Thickness
3.3. Histopathological Findings
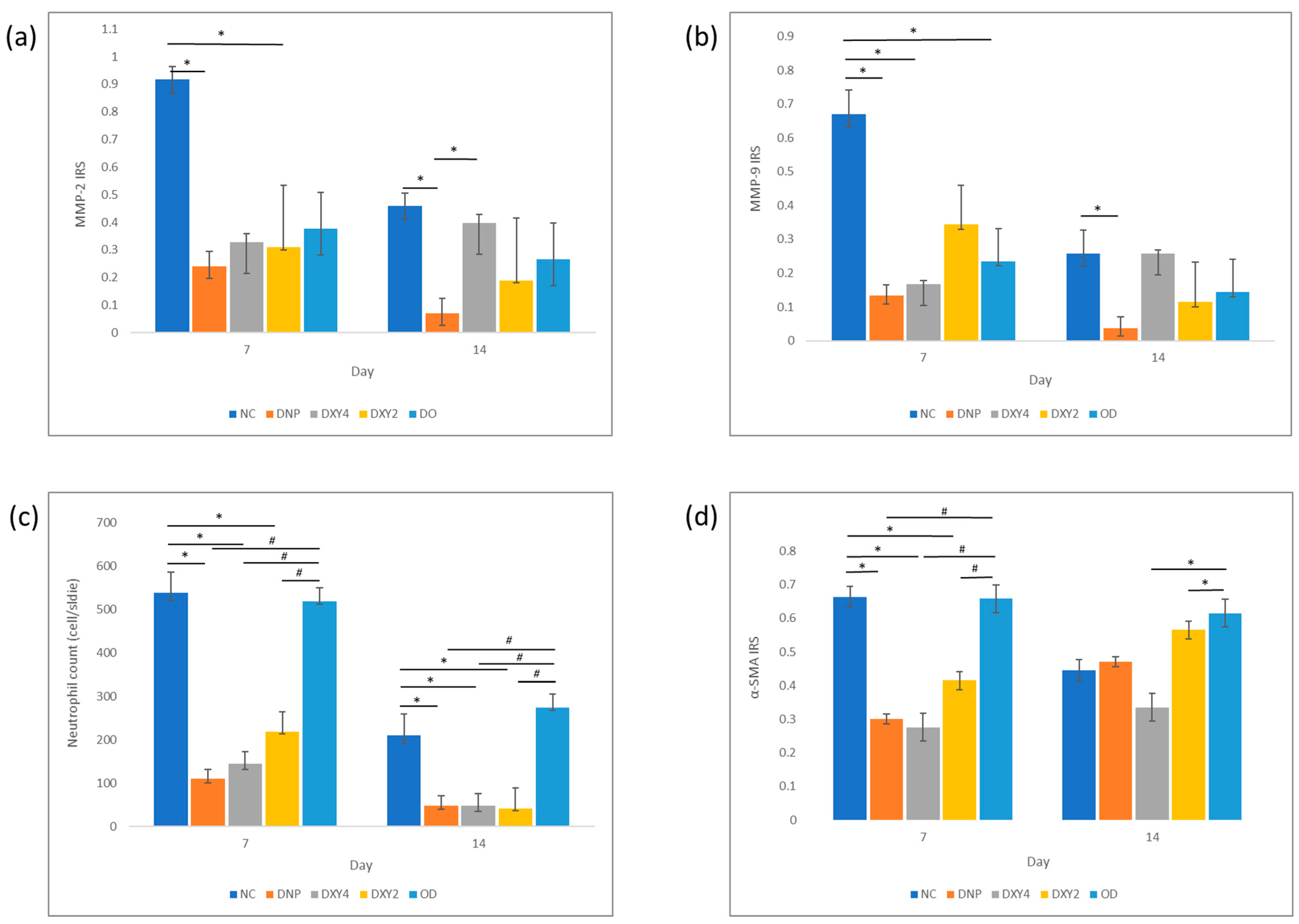
3.3.1. Effects of Doxycycline on the Expression of MMP-2 and MMP-9 and Neutrophil Infiltration
3.3.2. Effects of Doxycycline on Myofibroblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’neill, D.G.; Lee, M.M.; Brodbelt, D.C.; Church, D.B.; Sanchez, R.F. Corneal ulcerative disease in dogs under primary veterinary care in England: Epidemiology and clinical management. Canine Genet. Epidemiol. 2017, 4, 5. [Google Scholar] [CrossRef]
- Brooks, D.E.; Ollivier, F.J. Matrix metalloproteinase inhibition in corneal ulceration. Vet.-Clin. N. Am. Small Anim. Pract. 2004, 34, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Corneal wound healing. Exp. Eye Res. 2020, 197, 108089. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Pelegrino, F.S.; Henriksson, J.T.; Pflugfelder, S.C.; Volpe, E.A.; Li, D.Q.; de Paiva, C.S. Differential Effects of Dexamethasone and Doxycycline on Inflammation and MMP Production in Murine Alkali-Burned Corneas Associated with Dry Eye. Ocul. Surf. 2016, 14, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.S.; Santhiago, M.R. Corneal nerves anatomy, function, injury and regeneration. Exp. Eye Res. 2020, 200, 108243. [Google Scholar] [CrossRef]
- Sivak, J.M.; Fini, M.E. MMPs in the eye: Emerging roles for matrix metalloproteinases in ocular physiology. Prog. Retin. Eye Res. 2002, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.E.; Girard, M.T.; Matsubara, M. Collagenolytic/gelatinolytic enzymes in corneal wound healing. Acta Ophthalmol. 1992, 70, 26–33. [Google Scholar] [CrossRef]
- Fini, M.E.; Parks, W.C.; Rinehart, W.B.; Girard, M.T.; Matsubara, M.; Cook, J.R.; West-Mays, J.A.; Sadow, P.M.; Burgeson, R.E.; Jeffrey, J.J.; et al. Role of matrix metalloproteinases in failure to re-epithelialize after corneal injury. Am. J. Pathol. 1996, 149, 1287–1302. [Google Scholar] [PubMed]
- Agwuh, K.N.; MacGowan, A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 2006, 58, 256–265. [Google Scholar] [CrossRef]
- Smith, V.A.; Cook, S.D. Doxycycline—A role in ocular surface repair. Br. J. Ophthalmol. 2004, 88, 619–625. [Google Scholar] [CrossRef]
- Sapadin, A.N.; Fleischmajer, R. Tetracyclines: Nonantibiotic properties and their clinical implications. J. Am. Acad. Dermatol. 2006, 54, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Korting, H.C.; Schöllmann, C. Tetracycline Actions Relevant to Rosacea Treatment. Skin Pharmacol. Physiol. 2009, 22, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Rosenblatt, M.; Li, D.Q.; Liu, Z.; Monroy, D.; Ji, Z.; Lokeshwar, B.L.; Pflugfelder, S.C. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2544–2557. [Google Scholar] [CrossRef] [PubMed]
- Federici, T.J. The non-antibiotic properties of tetracyclines: Clinical potential in ophthalmic disease. Pharmacol. Res. 2011, 64, 614–623. [Google Scholar] [CrossRef]
- KuKanich, K.; KuKanich, B.; Slead, T.; Warner, M. Evaluation of drug content (potency) for compounded and FDA–approved formulations of doxycycline on receipt and after 21 days of storage. J. Am. Vet.-Med. Assoc. 2017, 251, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Kogawa, A.; Salgado, H. Doxycycline Hyclate: A Review of Properties, Applications and Analytical Methods. Int. J. Life Sci. Pharm. Res. 2012, 2, 11–25. [Google Scholar]
- Papich, M.G.; Davidson, G.S.; Fortier, L.A. Doxycycline concentration over time after storage in a compounded veterinary preparation. J. Am. Vet.-Med. Assoc. 2013, 242, 1674–1678. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Kompella, U.B.; Kadam, R.S.; Lee, V.H. Recent Advances in Ophthalmic Drug Delivery. Ther. Deliv. 2010, 1, 435–456. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, H.H.; Konráðsdóttir, F.; Loftsson, T.; Stefánsson, E. Topical and systemic absorption in delivery of dexamethasone to the anterior and posterior segments of the eye. Acta Ophthalmol. Scand. 2007, 85, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-H.; Wang, P.-Y.; Lin, I.-C.; Huang, H.; Liu, G.-S.; Tseng, C.-L. Ocular Drug Delivery: Role of Degradable Polymeric Nanocarriers for Ophthalmic Application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Nanoparticles—A historical perspective. Int. J. Pharm. 2007, 331, 1–10. [Google Scholar] [CrossRef]
- Pokharkar, V.; Patil, V.; Mandpe, L. Engineering of polymer–surfactant nanoparticles of doxycycline hydrochloride for ocular drug delivery. Drug Deliv. 2015, 22, 955–968. [Google Scholar] [CrossRef]
- Gordon, M.K.; DeSantis, A.; Deshmukh, M.; Lacey, C.J.; Hahn, R.A.; Beloni, J.; Anumolu, S.S.; Schlager, J.J.; Gallo, M.A.; Gerecke, D.R.; et al. Doxycycline Hydrogels as a Potential Therapy for Ocular Vesicant Injury. J. Ocul. Pharmacol. Ther. 2010, 26, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Anumolu, S.S.; DeSantis, A.S.; Menjoge, A.R.; Hahn, R.A.; Beloni, J.A.; Gordon, M.K.; Sinko, P.J. Doxycycline loaded poly(ethylene glycol) hydrogels for healing vesicant-induced ocular wounds. Biomaterials 2010, 31, 964–974. [Google Scholar] [CrossRef]
- Feitosa, R.C.; Ishikawa, E.S.A.; da Silva, M.F.A.; da Silva-Júnior, A.A.; Oliveira-Nascimento, L. Five decades of doxycycline: Does nanotechnology improve its properties? Int. J. Pharm. 2022, 618, 121655. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Wang, M.-C.; Chen, Z.-Y.; Chiu, W.-Y.; Chen, K.-H.; Lin, I.-C.; Yang, W.-C.V.; Wu, C.-C.; Tseng, C.-L. Gelatin–epigallocatechin gallate nanoparticles with hyaluronic acid decoration as eye drops can treat rabbit dry-eye syndrome effectively via inflammatory relief. Int. J. Nanomed. 2018, 13, 7251–7273. [Google Scholar] [CrossRef]
- Chuang, Y.-L.; Fang, H.-W.; Ajitsaria, A.; Chen, K.-H.; Su, C.-Y.; Liu, G.-S.; Tseng, C.-L. Development of Kaempferol-Loaded Gelatin Nanoparticles for the Treatment of Corneal Neovascularization in Mice. Pharmaceutics 2019, 11, 635. [Google Scholar] [CrossRef]
- Tseng, C.-L.; Chen, K.-H.; Su, W.-Y.; Lee, Y.-H.; Wu, C.-C.; Lin, F.-H. Cationic Gelatin Nanoparticles for Drug Delivery to the Ocular Surface: In Vitro and In Vivo Evaluation. J. Nanomater. 2013, 2013, 238351. [Google Scholar] [CrossRef]
- Coester, C.J.; Langer, K.; Von Briesen, H.; Kreuter, J. Gelatin nanoparticles by two step desolvation—A new preparation method, surface modifications and cell uptake. J. Microencapsul. 2000, 17, 187–193. [Google Scholar] [PubMed]
- Tamgadge, S.; Mudaliar, U.; Tamgadge, A.; Pereira, T.; Dhouskar, S.; Rajhans, S.; Salunke, G. Immunohistochemical expression of myofibroblasts using alpha-smooth muscle actin (SMA) to assess the aggressive potential of various clinical subtypes of ameloblastoma. J. Microsc. Ultrastruct. 2019, 7, 130–135. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, K.; Long, Q. Intraperitoneal Injection of MCC950 Inhibits the Progression of Myopia in Form-Deprivation Myopic Mice. Int. J. Mol. Sci. 2023, 24, 15839. [Google Scholar] [CrossRef]
- Dhahir, R.K.; Al-Nima, A.M.; Al-Bazzaz, F. Nanoemulsions as Ophthalmic Drug Delivery Systems. Turk. J. Pharm. Sci. 2021, 18, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.-S. Successfully Improving Ocular Drug Delivery Using the Cationic Nanoemulsion, Novasorb. J. Drug Deliv. 2012, 2012, 604204. [Google Scholar] [CrossRef]
- Liu, L.-R.; Sheng, S.-T.; Xu, J.-W.; Xu, W. Research progress on animal models of corneal epithelial-stromal injury. Int. J. Ophthalmol. 2023, 16, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Hutcheon, A.E.K.; Zieske, J.D. Mouse Models of Corneal Scarring. In Fibrosis: Methods and Protocols; Rittié, L., Ed.; Springer: New York, NY, USA, 2017; pp. 117–122. [Google Scholar] [CrossRef]
- Koulikovska, M.; Rafat, M.; Petrovski, G.; Veréb, Z.; Akhtar, S.; Fagerholm, P.; Lagali, N. Enhanced Regeneration of Corneal Tissue via a Bioengineered Collagen Construct Implanted by a Nondisruptive Surgical Technique. Tissue Eng. Part A 2014, 21, 1116–1130. [Google Scholar] [CrossRef]
- Park, C.K.; Kim, J.H. Comparison of wound healing after photorefractive keratectomy and laser in situ keratomileusis in rabbits. J. Cataract. Refract. Surg. 1999, 25, 842–850. [Google Scholar] [CrossRef]
- Sumioka, T.; Kitano, A.; Flanders, K.C.; Okada, Y.; Yamanaka, O.; Fujita, N.; Iwanishi, H.; Kao, W.W.-Y.; Saika, S. Impaired cornea wound healing in a tenascin C-deficient mouse model. Mod. Pathol. 2013, 93, 207–217. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Y.; Gao, X.-W.; Liu, Y.; Lei, D.-K. Experimental study of trabecular tissue repair for corneal defect in rabbits. Int. J. Ophthalmol. 2020, 13, 1356–1360. [Google Scholar] [CrossRef]
- Liu, C.Y.; Kao, W.W.Y. Corneal Epithelial Wound Healing. In Progress in Molecular Biology and Translational Science; Elsevier, B.V.: Amsterdam, The Netherlands, 2015; pp. 61–71. [Google Scholar]
- Hamrah, P.; Sahin, A. 5-Limbus and Corneal Epithelium. In Ocular Surface Disease: Cornea, Conjunctiva and Tear Film; Holland, E.J., Mannis, M.J., Lee, W.B., Eds.; W.B. Saunders: London, UK, 2013; pp. 29–33. [Google Scholar] [CrossRef]
- Zagon, I.S.; Sassani, J.W.; Mclaughlin, P.J. Cellular dynamics of corneal wound re-epithelialization in the rat II. DNA synthesis of the ocular surface epithelium following wounding. Brain Res. 1999, 839, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Sassani, J.W.; McLaughlin, P.J. Cellular dynamics of corneal wound re-epithelialization in the rat: I. Fate of ocular surface epithelial cells synthesizing DNA prior to wounding. Brain Res. 1999, 822, 149–163. [Google Scholar] [CrossRef]
- Ghafar, N.A.; Jalil, N.A.A.; Kamarudin, T.A. Wound healing of the corneal epithelium: A review. Asian Biomed. 2021, 15, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Sagga, N.; Kuffová, L.; Vargesson, N.; Erskine, L.; Collinson, J.M. Limbal epithelial stem cell activity and corneal epithelial cell cycle parameters in adult and aging mice. Stem Cell Res. 2018, 33, 185–198. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
- Mauris, J.; Woodward, A.M.; Cao, Z.; Panjwani, N.; Argüeso, P. Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3. J. Cell Sci. 2014, 127, 3141–3148. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Corrales, R.M.; Villarreal, A.L.; Farley, W.J.; Li, D.-Q.; Stern, M.E.; Pflugfelder, S.C. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp. Eye Res. 2006, 83, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.L.; Cornelius, L.A. Matrix Metalloproteinases: Pro- and Anti-Angiogenic Activities. J. Investig. Dermatol. Symp. Proc. 2000, 5, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Sivak, J.; Ashton, P.; Russo, L.A.; Pham, B.Q.; Kasahara, N.; Raizman, M.B.; Fini, M.E. Curcuminoids Inhibit the Angiogenic Response Stimulated by Fibroblast Growth Factor-2, Including Expression of Matrix Metalloproteinase Gelatinase, B. J. Biol. Chem. 2000, 275, 10405–10412. [Google Scholar] [CrossRef]
- Schnaper, H.W.; Grant, D.S.; Stetler-Stevenson, W.G.; Fridman, R.; D’Orazi, G.; Murphy, A.N.; Bird, R.E.; Hoythya, M.; Fuerst, T.R.; French, D.L.; et al. Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. J. Cell. Physiol. 1993, 156, 235–246. [Google Scholar] [CrossRef]
- Kim, H.-S.; Luo, L.; Pflugfelder, S.C.; Li, D.-Q. Doxycycline Inhibits TGF-β1–Induced MMP-9 via Smad and MAPK Pathways in Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2021, 46, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Hoda, S.A.; Hoda, S.A.; Hoda, R.S.; Hoda, R.S. Robbins and Cotran Pathologic Basis of Disease. Am. J. Clin. Pathol. 2020, 154, 869. [Google Scholar] [CrossRef]
- Zonneveld, R.; Molema, G.; Plötz, F.B. Analyzing Neutrophil Morphology, Mechanics, and Motility in Sepsis: Options and Challenges for Novel Bedside Technologies. Crit. Care Med. 2016, 44, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Santhiago, M.; Barbosa, F.; Agrawal, V.; Singh, N.; Ambati, B.; Wilson, S. Effect of TGFβ and PDGF-B blockade on corneal myofibroblast development in mice. Exp. Eye Res. 2011, 93, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Corneal myofibroblasts and fibrosis. Exp. Eye Res. 2020, 201, 108272. [Google Scholar] [CrossRef]
- Wilson, S.E.; Torricelli, A.A.; Marino, G.K. Corneal epithelial basement membrane: Structure, function and regeneration. Exp. Eye Res. 2020, 194, 108002. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.A.; Santhanam, A.; Wu, J.; Singh, V.; Wilson, S.E. The corneal fibrosis response to epithelial-stromal injury. Exp. Eye Res. 2016, 142, 110–118. [Google Scholar] [CrossRef]
- Santhanam, A.; Marino, G.K.; Torricelli, A.A.M.; Wilson, S.E. EBM regeneration and changes in EBM component mRNA expression in stromal cells after corneal injury. Mol. Vis. 2017, 23, 39. [Google Scholar] [PubMed]
- Hutcheon, A.E.; Choi, R.; Hong, J.; Lee, J.; Mohan, R.R.; Ambrósio, R.; Zieske, J.D.; Wilson, S.E. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp. Eye Res. 2003, 76, 71–87. [Google Scholar] [CrossRef]


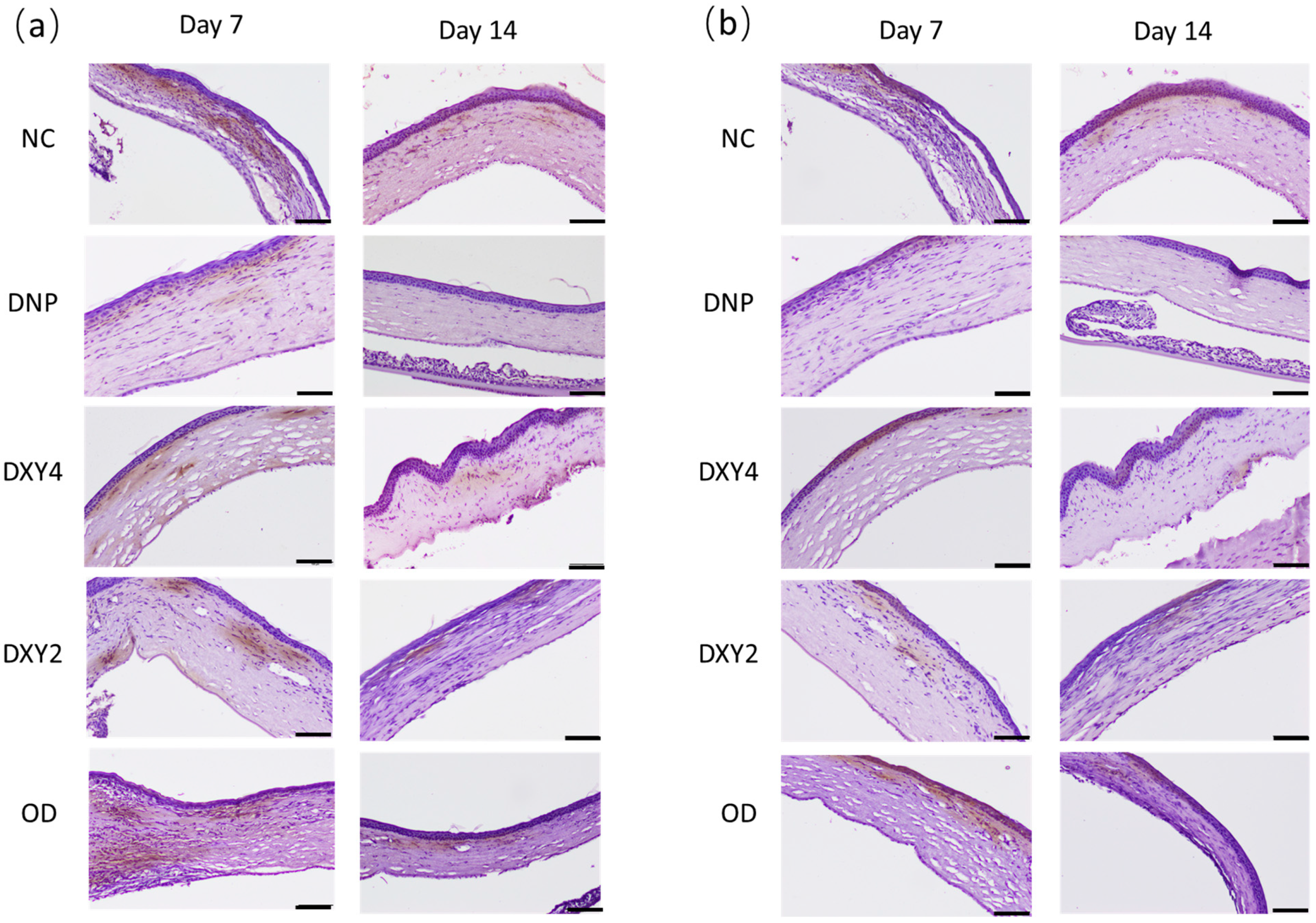
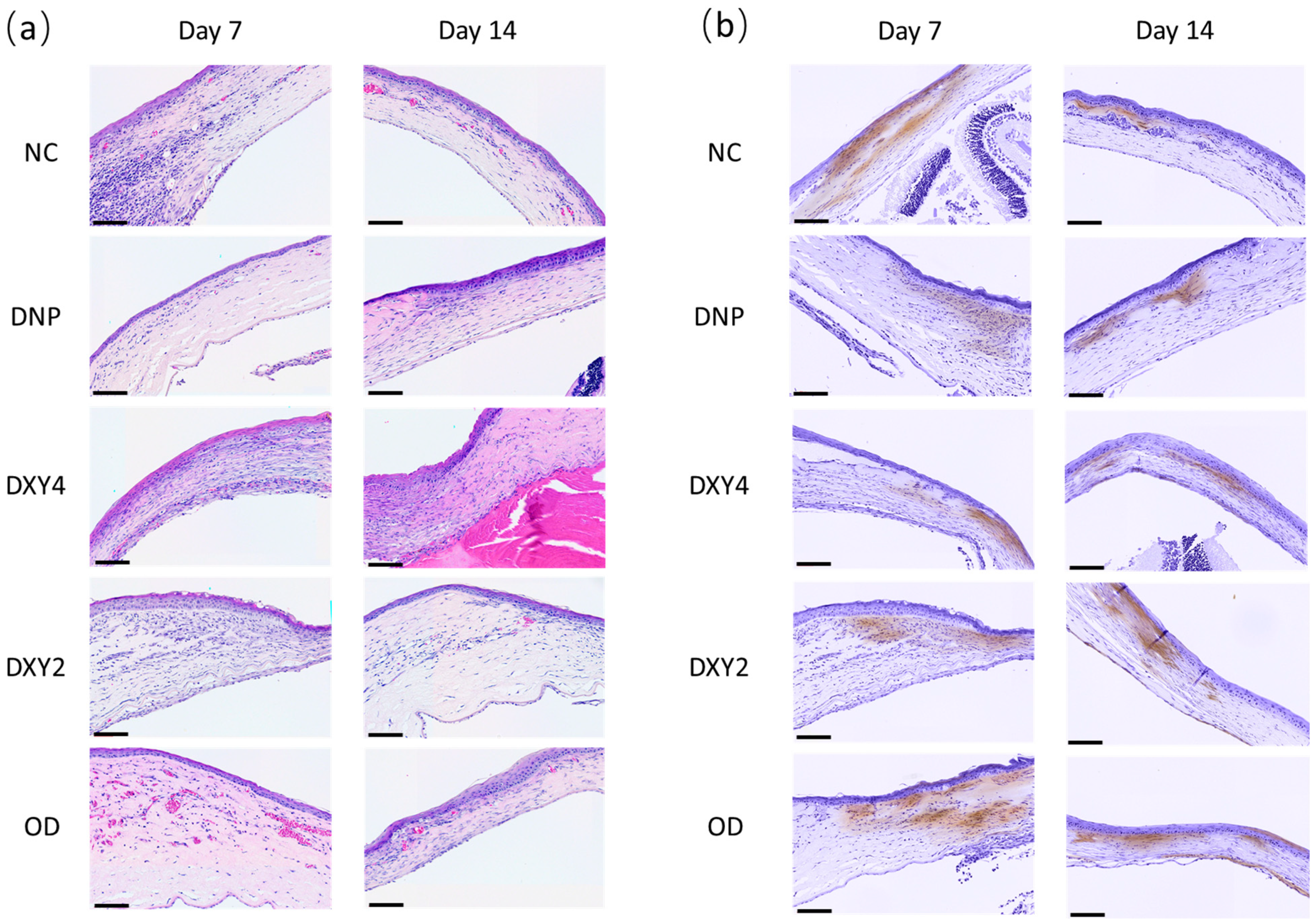
| Groups | NC | OD | DXY2 | DXY4 | DNP |
|---|---|---|---|---|---|
| Dxy concentration | / | 10 mg/kg | 0.1% | 0.1% | 0.01% |
| Dosing times (day) | / | 2 | 2 | 4 | 2 |
| Size (nm) | Zeta (mV) | PdI | E.E. (%) | |
|---|---|---|---|---|
| GNP | 98.83 ± 0.68 | 21.5 ± 0.30 | 0.117 ± 0.033 | / |
| DNP | 132.0 ± 0.20 | 22.4 ± 1.01 | 0.143 ± 0.007 | 53.80% ± 4.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, S.-M.; Tseng, C.-L.; Huang, W.-H.; Lin, C.-T. Therapeutic Assessment of Diverse Doxycycline-Based Formulations in Promoting Deep Corneal Wound Healing: Evidence from a Rat Model. Vet. Sci. 2025, 12, 143. https://doi.org/10.3390/vetsci12020143
Chan S-M, Tseng C-L, Huang W-H, Lin C-T. Therapeutic Assessment of Diverse Doxycycline-Based Formulations in Promoting Deep Corneal Wound Healing: Evidence from a Rat Model. Veterinary Sciences. 2025; 12(2):143. https://doi.org/10.3390/vetsci12020143
Chicago/Turabian StyleChan, Sze-Min, Ching-Li Tseng, Wei-Hsiang Huang, and Chung-Tien Lin. 2025. "Therapeutic Assessment of Diverse Doxycycline-Based Formulations in Promoting Deep Corneal Wound Healing: Evidence from a Rat Model" Veterinary Sciences 12, no. 2: 143. https://doi.org/10.3390/vetsci12020143
APA StyleChan, S.-M., Tseng, C.-L., Huang, W.-H., & Lin, C.-T. (2025). Therapeutic Assessment of Diverse Doxycycline-Based Formulations in Promoting Deep Corneal Wound Healing: Evidence from a Rat Model. Veterinary Sciences, 12(2), 143. https://doi.org/10.3390/vetsci12020143






