Effect of Alpha-Lipoic Acid on the Development, Oxidative Stress, and Cryotolerance of Bovine Embryos Produced In Vitro
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Production Media and Reagents
2.2. Collection, Transportation, and Processing of Ovaries
2.3. In Vitro Production of Bovine Embryos
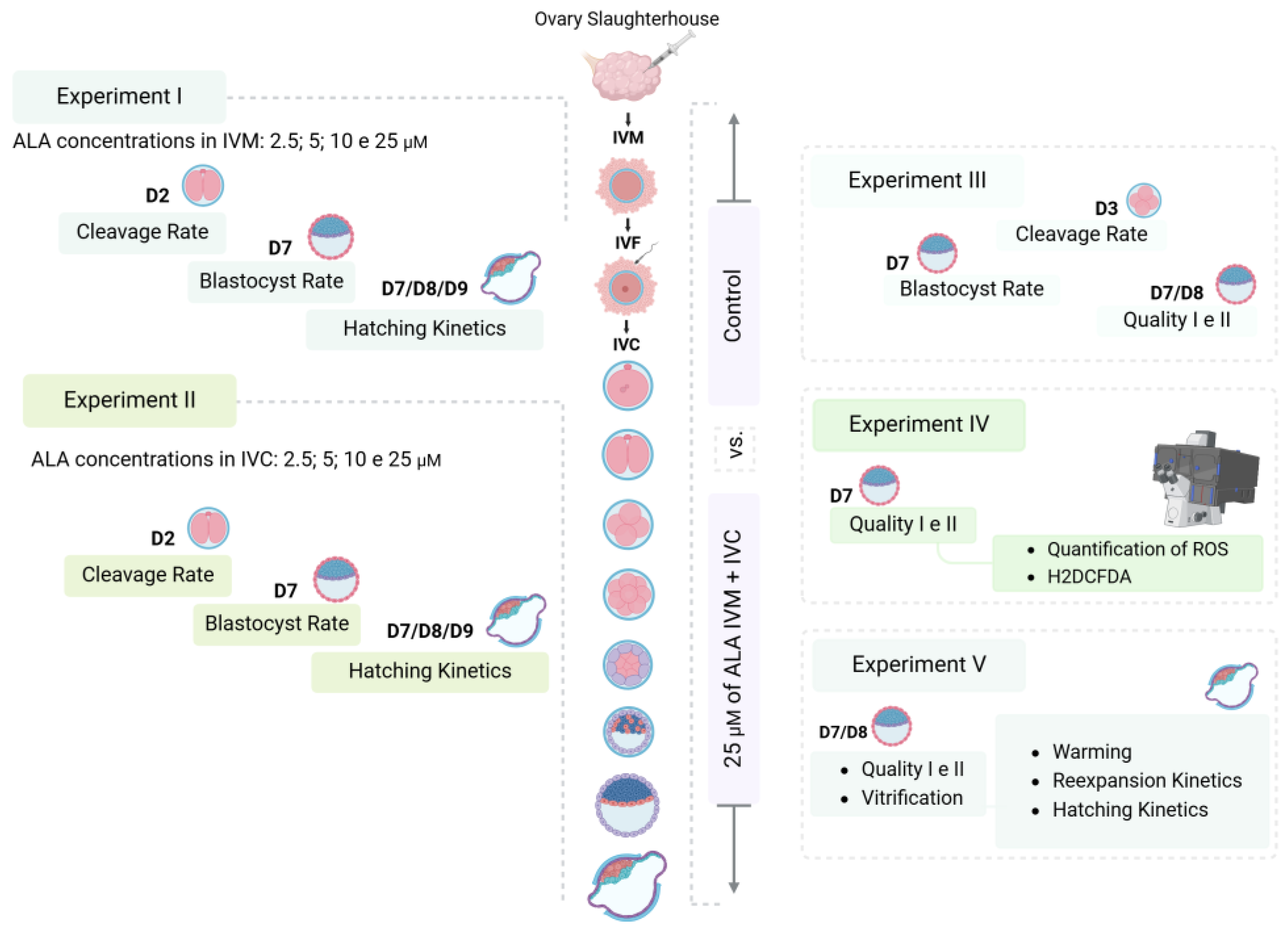
2.4. Experimental Design
2.4.1. Experiment I and II: Evaluation of the Effects of Including Different Concentrations of ALA in the Production Medium on Embryonic Development
2.4.2. Experiment III: Effects of Including ALA in the Maturation and Culture Medium on the Development and Quality of Embryos
2.4.3. Experiment IV: Measurement of Intracellular ROS Levels Using the Dichlorofluorescein Assay
2.4.4. Experiment V: Evaluation of Cryotolerance Through Vitrification
2.5. Statistical Analysis
3. Results
3.1. Effects of Supplementing the Production Medium with Different ALA Concentrations on Embryonic Development
3.2. Effect of Including ALA in the Maturation and Culture Media on the Development and Quality of Embryos
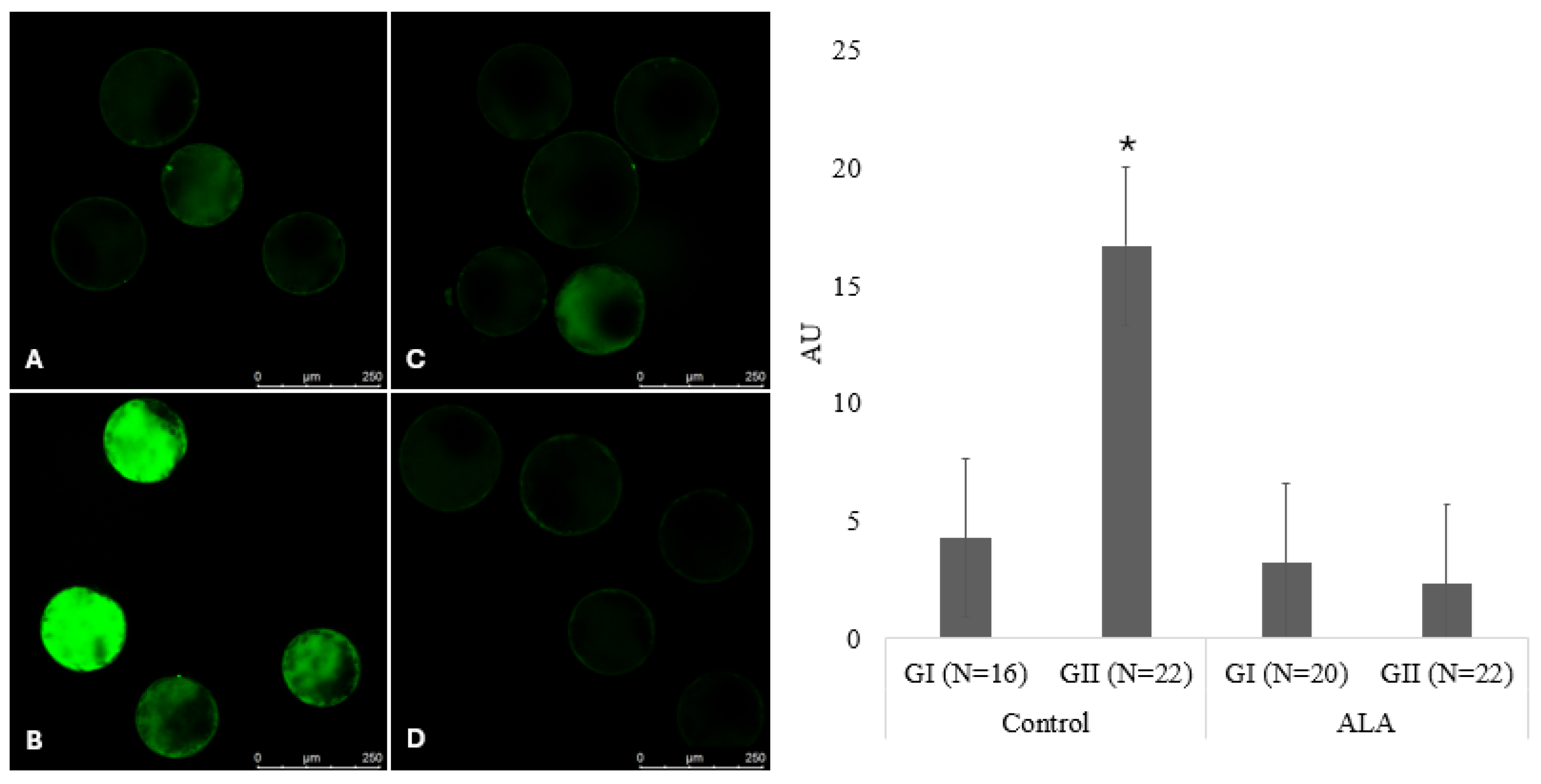
3.3. Measurement of Intracellular ROS Levels Using Dichlorofluorescein Assay
3.4. Evaluation of Cryotolerability Through Vitrification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Embryo Transfer Society—IETS. 2022 Statistics of Embryo Collection and Transfer in Domestic Farm Animals. Embryo Transf. News Letter. 2023, 39, 39–43. Available online: https://www.iets.org/Portals/0/Documents/Public/Committees/DRC/IETS_Data_Retrieval_Report_2022.pdf (accessed on 5 September 2024).
- Marsico, T.V.; Silva, M.V.; Valente, R.S.; Annes, K.; Rissi, V.B.; Glanzner, W.G.; Sudano, M.J. Unraveling the consequences of oxygen imbalance on early embryo development: Exploring mitigation strategies. Animals 2023, 13, 2171. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heras, S.; Paramio, M.-T. Impact of oxidative stress on oocyte competence for in vitro embryo production programs. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Effects of oxidative stress on embryonic development. Birth Defects Res. C Embryo Today 2007, 81, 155–162. [Google Scholar] [CrossRef]
- Dumollard, R.; Ward, Z.; Carroll, J.; Duchen, M.R. Regulation of redox metabolism in the mouse oocyte and embryo. Development 2007, 134, 455–465. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Richter, C. Biophysical consequences of lipid peroxidation in membranes. Chem. Phys. Lipids 1987, 44, 175–189. [Google Scholar] [CrossRef]
- Park, S.-Y.; Yoon, S.-J.; Hakomori, S.I.; Kim, J.M.; Kim, J.-Y.; Bernert, B.; Ullman, T.; Itzkowitz, S.H.; Kim, J.H. Dimeric Le(a) (Le(a)-on-Le(a)) status of beta-haptoglobin in sera of colon cancer, chronic inflammatory disease and normal subjects. Int. J. Oncol. 2010, 36, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Leoni, G.G.; Rosati, I.; Succu, S.; Bogliolo, L.; Bebbere, D.; Berlinguer, F.; Ledda, S.; Naitana, S. A low oxygen atmosphere during IVF accelerates the kinetic of formation of in vitro produced ovine blastocysts. Reprod. Domest. Anim. 2007, 42, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.G.E.; Simpson, A.C.; Pugh, P.A.; Donnelly, P.E.; Tervit, H.R. Effect of oxygen concentration on in vitro development of preimplantation sheep and cattle embryos. J. Reprod. Fertil. 1990, 89, 573–578. [Google Scholar] [CrossRef]
- Yuan, Y.Q.; Van Soom, A.; Coopman, F.O.J.; Mintiens, K.; Boerjan, M.L.; Van Zeveren, A.; de Kruif, A.; Peelman, L.J. Influence of oxygen tension on apoptosis and hatching in bovine embryos cultured in vitro. Theriogenology 2003, 59, 1585–1596. [Google Scholar] [CrossRef]
- Leite, R.F.; Annes, K.; Ispada, J.; de Lima, C.B.; dos Santos, É.C.; Fontes, P.K.; Nogueira, M.F.G.; Milazzotto, M.P. Oxidative stress alters the profile of transcription factors related to early development on in vitro produced embryos. Med. Oxid. Longev. Cell. 2017, 15, 14. [Google Scholar] [CrossRef]
- de Andrade Melo-Sterza, F.; Poehland, R. Lipid metabolism in bovine oocytes and early embryos under in vivo, in vitro, and stress conditions. Int. J. Mol. Sci. 2021, 22, 3421. [Google Scholar] [CrossRef]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta. 2009, 1790, 1149–1160. [Google Scholar] [CrossRef]
- Kagan, V.E.; Shvedova, A.; Serbinova, E.; Khan, S.; Swanson, C.; Powell, R.; Packer, L. Dihydrolipoic acid—A universal antioxidant both in the membrane and in the aqueous phase. Reduction of peroxyl, ascorbyl and chromanoxyl radicals. Biochem. Pharmacol. 1992, 44, 1637–1649. [Google Scholar] [CrossRef]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Yamasaki, M.; Kawabe, A.; Nishimoto, K.; Madhyastha, H.; Sakakibara, Y.; Suiko, M.; Okamoto, T.; Suda, T.; Uehira, K.; Nishiyama, K. Dihydro-alpha-lipoic acid has more potent cytotoxicity than alpha-lipoic acid. In Vitro Cell. Dev. Biol. Anim. 2009, 45, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.; Zavareh, S.; Kashani, M.H.; Lashgarbluki, I.; Karimi, I. The effect of alpha lipoic acid on the developmental competence of mouse isolated preantral follicles. J. Assist. Reprod. Genet. 2012, 29, 175–183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palaniappan, A.R.; Dai, A. Mitochondrial ageing and the beneficial role of α-lipoic acid. Neurochem. Res. 2007, 32, 1552–1558. [Google Scholar] [CrossRef]
- Xu, D.P.; Wells, W.W. α-Lipoic acid dependent regeneration of ascorbic acid from dehydroascorbic acid in rat liver mitochondria. J. Bioenerg. Biomembr. 1996, 28, 77–85. [Google Scholar] [CrossRef]
- Brufani, M. Acido α-lipoico: Farmaco o integratore? Una panoramica sulla farmacocinetica, le formulazioni disponibili e le evidenze cliniche nelle complicanze del diabete. Prog. Nutr. 2014, 16, 62–74. [Google Scholar]
- Packer, L.; Cadenas, E. Lipoic acid: Energy metabolism and redox regulation of transcription and cell signaling. J. Clin. Biochem. Nutr. 2011, 48, 26–32. [Google Scholar] [CrossRef]
- Konrad, D.; Somwar, R.; Sweeney, G.; Yaworsky, K.; Hayashi, M.; Ramlal, T.; Klip, A. The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: Potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes 2001, 50, 1464–1471. [Google Scholar] [CrossRef]
- Chen, W.-L.; Kang, C.-H.; Wang, S.-G.; Lee, H.-M. α-Lipoic acid regulates lipid metabolism through induction of sirtuin 1 (SIRT1) and activation of AMP-activated protein kinase. Diabetologia 2012, 55, 1824–1835. [Google Scholar] [CrossRef]
- Salehi, B.; Yılmaz, Y.B.; Antika, G.; Tumer, T.B.; Mahomoodally, M.F.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef]
- Gomes, R.G.; Silva, C.B.; González, S.M.; Oliveira, R.L.; Max, M.C.; Lisboa, L.A.; Barreiros, T.R.R.; Santos, M.M.; Sarapião, F.D.; Gastal, E.L.; et al. Alpha lipoic acid (ALA) effects on developmental competence of equine preantral follicles in short-term culture. Theriogenology 2018, 105, 169–173. [Google Scholar] [CrossRef]
- Makvandi, A.; Kowsar, R.; Hajian, M.; Mahdavi, A.H.; Tanhaei Vash, N.; Nasr-Esfahani, M.H. Alpha lipoic acid reverses the negative effect of LPS on mouse spermatozoa and developmental competence of resultant embryos in vitro. Andrology 2019, 7, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, L.Z.; Bonato, D.V.; Bizarro-Silva, C.; Bonato, F.G.C.; González, S.M.; Rossaneis, A.C.; Verri, W.A.; Morotti, F.; Seneda, M.M. Culture of preantral ovarian follicles of Bos taurus indicus with alpha-lipoic acid. Zygote 2022, 30, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, B.; Liu, H.; Qiu, M.; Liu, J.; Zhang, Y.; Quan, F. Improving development of cloned goat embryos by supplementing α-lipoic acid to oocyte in vitro maturation medium. Theriogenology 2013, 80, 228–233. [Google Scholar] [CrossRef]
- Hassan, B.M.S.; Fang, X.; Roy, P.K.; Shin, S.T.; Cho, J.K. Effect of Alpha lipoic acid as an antioxidant supplement during in vitro maturation medium on bovine embryonic development. J. Emb. Trans. 2017, 32, 123–130. [Google Scholar] [CrossRef]
- Hassan, M.A.E.; Khalil, W.A.; Abdelnour, S.A.; Aman, R.M. Supplementation of alpha-lipoic acid-loaded nanoliposomes in semen extender improves freezability of buffalo spermatozoa. Sci. Rep. 2022, 12, 22464. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Zhang, H.; Zhang, Y.; Quan, F. Alpha-lipoic acid improves the maturation and the developmental potential of goat oocytes in vitro. Reprod. Domest. Anim. Zuchthygiene. 2021, 56, 545–554. [Google Scholar] [CrossRef]
- Fabra, M.C.; Anchordoquy, J.P.; Carranza-Martín, A.C.; Farnetano, N.; Anchordoquy, J.M.; Furnus, C.C.; Nikoloff, N. Alpha-lipoic acid improves bovine preimplantation blastocyst quality and cryotolerance. Theriogenology 2023, 198, 61–68. [Google Scholar] [CrossRef]
- Moghimi Khorasgani, A.; Moradi, R.; Jafarpour, F.; Ghazvinizadehgan, F.; Ostadhosseini, S.; Heydarnezhad, A.; Fouladi-Nashta, A.A.; Nasr-Esfahani, M.H. Alpha-lipoic acid can overcome the reduced developmental competency induced by alcohol toxicity during ovine oocyte maturation. Cell J. 2021, 23, 164–173. [Google Scholar] [CrossRef]
- Mokhtari, S.; Mahdavi, A.H.; Hajian, M.; Kowsar, R.; Rouhollahi Varnosfaderani, S.; Nasr-Esfahani, M.H. The attenuation of the toxic effects of LPS on mouse pre-implantation development by alpha-lipoic acid. Theriogenology 2020, 143, 139–147. [Google Scholar] [CrossRef]
- Lee, S.-H.; Sun, M.-H.; Jiang, W.-J.; Li, X.-H.; Heo, G.; Zhou, D.; Chen, Z.; Cui, X.-S. Alpha-lipoic acid attenuates heat stress-induced apoptosis via upregulating the heat shock response in porcine parthenotes. Sci. Rep. 2023, 13, 8427. [Google Scholar] [CrossRef]
- Jamil, M.; Debbarh, H.; Aboulmaouahib, S.; Aniq Filali, O.; Muonaji, K.; Zarqaoui, M.; Saadani, M.; Louanjli, N.; Cadi, R. Reactive oxygen species in reproduction: Harmful, essential or both? Zygote 2020, 28, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Fabra, M.C.; Izquierdo, I.; Anchordoquy, J.M.; Anchordoquy, J.P.; Carranza-Martín, A.C.; Nikoloff, N.; Furnus, C.C. Effect of alpha-lipoic acid during preimplantation development of cattle embryos when there were different in vitro culture conditions. Anim. Reprod. Sci. 2020, 221, 106550. [Google Scholar] [CrossRef] [PubMed]
- Seneda, M.M.; Esper, C.R.; Garcia, J.M.; Oliveira, J.A.; Vantini, R. Relationship between follicle size and ultrasound-guided transvaginal oocyte recovery. Anim. Reprod. Sci. 2001, 67, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bó, G.A.; Mapletoft, R.J. Evaluation and classification of bovine embryos. Anim. Reprod. 2013, 10, 344–348. [Google Scholar]
- Bain, N.T.; Madan, P.; Betts, D.H. The early embryo to intracellular reactive oxygen species is developmentally regulated. Reprod. Fertil. Dev. 2011, 23, 561–575. [Google Scholar] [CrossRef]
- Vajta, G.; Holm, P.; Greve, T.; Callesen, H. Vitrification of porcine embryos using the Open Pulled Straw (OPS) method. Acta Vet. Scand. 1997, 38, 349–352. [Google Scholar] [CrossRef]
- Truong, T.; Gardner, D.K. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum. Reprod. 2017, 32, 2404–2413. [Google Scholar] [CrossRef]
- Truong, T.T.; Gardner, D.K. Antioxidants increase blastocyst cryosurvival and viability post-vitrification. Hum. Reprod. 2020, 35, 12–23. [Google Scholar] [CrossRef]
- Truong, T.T.; Soh, Y.M.; Gardner, D.K. Antioxidants improve mouse preimplantation embryo development and viability. Hum. Reprod. 2016, 31, 1445–1454. [Google Scholar] [CrossRef]
- Blanco, M.R.; Demyda, S.; Moreno Millán, M.; Genero, E. Developmental competence of in vivo and in vitro matured oocytes: A review. Biotechnol. Mol. Biol. Rev. 2011, 6, 155–165. [Google Scholar]
- Urrego, R.; Rodriguez-Osorio, N.; Niemann, H. Epigenetic disorders and altered gene expression after use of assisted reproductive technologies in domestic cattle. Epigenetics 2014, 9, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Remião, M.H.; Lucas, C.G.; Domingues, W.B.; Silveira, T.; Barther, N.N.; Komninou, E.R.; Basso, A.C.; Jornada, D.S.; Beck, C.R.; Pohlmann, A.R.; et al. Melatonin delivery by nanocapsules during in vitro bovine oocyte maturation decreased the reactive oxygen species of oocytes and embryos. Reprod. Toxicol. 2016, 63, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.; Abu Hamed, S.; Khalifa, M.; Amin, A.; El-Sayed, A.; Swiefy, S.A.; El-Assal, S. Retinoic acid improves maturation rate and upregulates the expression of antioxidant-related genes in in vitro matured buffalo (Bubalus bubalis) oocytes. Int. J. Vet. Sci. Med. 2018, 6, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Yan, K.; Sui, L.; Zhang, H.; Zhang, H.; Yang, X.; Lu, S.; Lu, K.; Liang, X. Mogroside V improves porcine oocyte in vitro maturation and subsequent embryonic development. Theriogenology 2020, 141, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Zoheir, K.M.; Harisa, G.I.; Allam, A.A.; Yang, L.; Li, X.; Liang, A.; Abd-Rabou, A.A.; Harrath, A.H. Effect of alpha lipoic acid on in vitro development of bovine secondary preantral follicles. Theriogenology 2017, 88, 124–130. [Google Scholar] [CrossRef]
- Ferreira, E.M.; Vireque, A.A.; Adona, P.R.; Meirelles, F.V.; Ferriani, R.A.; Navarro, P.A.A.S. Cytoplasmic maturation of bovine oocytes: Structural and biochemical modifications and acquisition of developmental competence. Theriogenology 2009, 71, 836–848. [Google Scholar] [CrossRef]
- Gilchrist, R.B. Recent insights into oocyte–follicle cell interactions provide opportunities for the development of new approaches to in vitro maturation. Reprod. Fertil. Dev. 2011, 23, 23–31. [Google Scholar] [CrossRef]
- Winata, C.L.; Korzh, V. The translational regulation of maternal mRNAs in time and space. FEBS Lett. 2018, 592, 3007–3023. [Google Scholar] [CrossRef]
- Alves, G.P.; Cordeiro, F.B.; de Lima, C.B.; Annes, K.; dos Santos, É.C.; Ispada, J.; Fontes, P.K.; Nogueira, M.F.G.; Nichi, M.; Milazzotto, M.P. Follicular environment as a predictive tool for embryo development and kinetics in cattle. Reprod. Fertil. Dev. 2019, 31, 451–461. [Google Scholar] [CrossRef]
- Cagnone, G.L.M.; Dufort, I.; Vigneault, C.; Sirard, M.-A. Differential gene expression in bovine blastocysts resulting from exposure to hyperglycemia during early cleavage stages. Biol. Reprod. 2012, 86, 50. [Google Scholar] [CrossRef]
- Rizos, D.; Lonergan, P.; Boland, M.P.; Arroyo-García, R.; Pintado, B.; de la Fuente, J.; Gutiérrez-Adán, A. Analysis of differential messenger RNA expression between bovine blastocysts produced in different culture systems: Implications for blastocyst quality. Biol. Reprod. 2002, 66, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Gad, A.; Salilew-Wondim, D.; Prastowo, S.; Held, E.; Hoelker, M.; Rings, F.; Tholen, E.; Neuhoff, C.; Looft, C.; et al. Bovine embryo survival under oxidative-stress conditions is associated with activity of the NRF2-mediated oxidative-stress-response pathway: NRF2-mediated oxidative stress response activity in embryos. Mol. Reprod. Dev. 2014, 81, 497–513. [Google Scholar] [CrossRef] [PubMed]
- Pioltine, E.M.; Costa, C.B.; Barbosa Latorraca, L.; Franchi, F.F.; Dos Santos, P.H.; Mingoti, G.Z.; de Paula-Lopes, F.F.; Nogueira, M.F.G. Treatment of in vitro-matured bovine oocytes with tauroursodeoxycholic acid modulates the oxidative stress signaling pathway. Front. Cell Dev. Biol. 2021, 9, 623852. [Google Scholar] [CrossRef]
- de Lima, C.B.; do Amaral, D.T.; Ispada, J.; dos Santos, É.C.; Fontes, P.K.; Nogueira, M.F.G.; Milazzotto, M.P. Dynamics of transcription is affected by oxygen tension and developmental speed during in vitro production of bovine embryos. Reprod. Domest. Anim. 2024, 59, e14620. [Google Scholar] [CrossRef] [PubMed]
- Betts, D.H.; Madan, P. Permanent embryo arrest: Molecular and cellular concepts. Mol. Hum. Reprod. 2008, 14, 445–453. [Google Scholar] [CrossRef]
- Nasr-Esfahani, M.M.; Johnson, M.H. The origin of reactive oxygen species in mouse embryo cultured in vitro. Development 1991, 113, 551–560. [Google Scholar] [CrossRef]
- Niemann, H.; Wrenzycki, C. Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: Implications for subsequent development. Theriogenology 2000, 53, 21–34. [Google Scholar] [CrossRef]
- Gardner, D.K. Changes in the requirements and utilization of nutrients during mammalian preimplantation embryo development and their significance in embryo culture. Theriogenology 1998, 49, 83–102. [Google Scholar] [CrossRef]
- Rieger, D. Relationship between energy metabolism and development of early mammalian embryos. Theriogenology 1992, 37, 75–93. [Google Scholar] [CrossRef]
- Attia, M.; Essa, E.A.; Zaki, R.M.; Elkordy, A.A. An overview of the antioxidant effects of ascorbic acid and alpha lipoic acid (in liposomal forms) as adjuvant in cancer treatment. Antioxidants 2020, 9, 359. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Çakatay, U. Pro-oxidant actions of alpha-lipoic acid and dihydrolipoic acid. Med. Hypotheses 2006, 66, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Liemburg-Apers, D.C.; Willems, P.H.G.M.; Koopman, W.J.H.; Grefte, S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch. Toxicol. 2015, 89, 1209–1226. [Google Scholar] [CrossRef]
- Linck, D.W.; Larman, M.G.; Gardner, D.K. Lipoic acid: An antioxidant that improves embryo development and protects against oxidative stress. Fertil. Steril. 2007, 88, S36–S37. [Google Scholar] [CrossRef]
- Luberda, Z. The role of glutathione in mammalian gametes. Reprod. Biol. 2005, 5, 5–17. [Google Scholar]
- Stringfellow, D.; Seidel, S. Manual da Sociedade Internacional de Transferência de Embrioes: Um guia de Procedimentos e Informações Gerais para uso em Tecnologia de Transferência de Embriões Enfatizando Procedimentos Sanitários; Savoy: London, UK, 1998; Volume 3, p. 180. [Google Scholar]
- International Embryo Transfer Society—IETS. 2016 statistics of embryo collection and transfer in domestic farm animals. Embryo Transf. Newletter 2017, 18. Available online: https://www.iets.org/Portals/0/Documents/Public/Committees/DRC/IETS_Data_Retrieval_Report_2017.pdf (accessed on 8 September 2024).
- Marsico, T.V.; de Camargo, J.; Valente, R.S.; Sudano, M.J. Embryo competence and cryosurvival: Molecular and cellular features. Anim. Reprod. 2019, 16, 423–439. [Google Scholar] [CrossRef]
- Seidel, G.E., Jr. Modifying oocytes and embryos to improve their cryopreservation. Theriogenology 2006, 65, 228–235. [Google Scholar] [CrossRef]
- Valente, R.S.; de Almeida, T.G.; Alves, M.F.; Paschoal, D.M.; Basso, A.C.; Sudano, M.J. Cellular and apoptotic status monitoring according to the ability and speed to resume post-cryopreservation embryonic development. Theriogenology 2020, 158, 290–296. [Google Scholar] [CrossRef]
- Ferré, L.B.; Kjelland, M.E.; Taiyeb, A.M.; Campos-Chillon, F.; Ross, P.J. Recent progress in bovine in vitro-derived embryo cryotolerance: Impact of in vitro culture systems, advances in cryopreservation and future considerations. Reprod. Domest. Anim. 2020, 55, 659–676. [Google Scholar] [CrossRef] [PubMed]
| Group | COCs | Cleavage | Blastocyst | Hatching Day 7 | Hatching Day 8 | Hatching Day 9 | Total Hatching |
|---|---|---|---|---|---|---|---|
| Control | 609 | 441 (74.4 ± 4.8) | 182 (32.3 ± 5.8) | 13 (7.2 ± 3.8) | 58 (32.4 ± 6.4) | 35 (19.2 ± 2.8) | 106 (58.8 ± 8.7) |
| 2.5 µM ALA | 560 | 407 (72.6 ± 4.8) | 174 (32.1 ± 4.9) | 19 (10.7 ± 3.9) | 60 (34.2 ± 5.9) | 36 (18.2 ± 4.4) | 115 (63.1 ± 7.9) |
| 5 µM ALA | 583 | 440 (74.8 ± 6.0) | 177 (31.9 ± 6.3) | 10 (4.3 ± 2.0) | 60 (27.3 ± 6.4) | 37 (23.1 ± 6.8) | 107 (54.7 ± 9.3) |
| 10 µM ALA | 583 | 419 (72.7 ± 4.5) | 197 (36.1 ± 5.4) | 13 (6.3 ± 2.0) | 69 (31.9 ± 6.4) | 31 (17.0 ± 4.6) | 113 (55.2 ± 5.9) |
| 25 µM ALA | 600 | 451 (76.8 ± 3.6) | 201 (34.5 ± 1.9) | 8 (4.1 ± 1.3) | 77 (39.0 ± 3.5) | 43 (21.4 ± 1.7) | 128 (64.5 ± 3.8) |
| p-value | 0.91 | 0.90 | 0.47 | 0.34 | 0.47 | 0.49 |
| Group | Possible Zygotes | Cleavage | Blastocyst | Hatching Day 7 | Hatching Day 8 | Hatching Day 9 | Total Hatching |
|---|---|---|---|---|---|---|---|
| Control | 443 | 340 (77.1 ± 2.2) | 200 (46.2 ± 2.8) | 17 (8.4 ± 1.6) | 83 (42.3 ± 4.7) | 51 (25.1 ± 3.5) | 151 (75.8 ± 5.2) |
| 2.5 µM ALA | 443 | 326 (73.8 ± 2.6) | 135 (30.9 ± 3.9) | 7 (4.1 ± 1.7) | 46 (35.7 ± 4.7) | 35 (25.1 ± 4.1) | 88 (64.8 ± 2.7) |
| 5 µM ALA | 440 | 339 (77.2 ± 1.9) | 146 (34.2 ± 3.4) | 12 (7.9 ± 2.1) | 57 (39.5 ± 5.8) | 29 (19.5 ± 3.2) | 98 (66.9 ± 3.3) |
| 10 µM ALA | 435 | 334 (77.5 ± 2.6) | 159 (37.3 ± 4.6) | 14 (9.5 ± 3.0) | 55 (34.1 ± 2.6) | 43 (27.7 ± 2.2) | 112 (71.4 ± 3.4) |
| 25 µM ALA | 440 | 320 (72.9 ± 4.9) | 163 (34.3 ± 6.2) | 15 (8.1 ± 2.8) | 56 (34.4 ± 3.1) | 50 (28.6 ± 3.7) | 121 (71.0 ± 4.9) |
| p-value | 0.60 | 0.15 | 0.54 | 0.61 | 0.38 | 0.36 |
| Group | COCs | Cleavage | Blastocyst | D7GI | D7G2 | D8G1 | D8G2 | Vitrified Blastocysts |
|---|---|---|---|---|---|---|---|---|
| Control | 1514 | 1163 (76.9 ± 1.0) | 355 (23.8 ± 1.6) | 55 (15.6 ± 1.9) | 42 (11.9 ± 1.1) | 24 (6.7 ± 0.6) | 38 (11.4 ± 1.6) | 159 (45.7 ± 2.4) |
| 25 µM ALA | 1522 | 1183 (77.5 ± 1.0) | 359 (23.7 ± 1.0) | 65 (18.3 ± 1.5) | 59 (16.1 ± 2.8) | 22 (6.7 ± 1.7) | 31 (8.5 ± 1.3) | 177 (49.6 ± 3.6) |
| p-value | 0.69 | 0.96 | 0.11 | 0.13 | 0.97 | 0.10 | 0.22 |
| Expansion | Hatching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | Total | 12 h | 24 h | 48 h | 72 h | Total | ||
| D7GI | Control (N = 54) | 0 | 47 (89.5 ± 4.6) | 4 (7.0 ± 4.0) | 51 (96.5 ± 2.4) | 8 (12.2 ± 5.4)b | 14 (30.6 ± 8.8) | 20 (33.5 ± 8.1) | 2 (4.0 ± 2.9) | 44 (80.3 ± 4.4) |
| ALA (N = 63) | 2 (2.7 ± 1.9) | 55 (84.4 ± 6.9) | 5 (11.0 ± 5.6) | 62 (98.2 ± 1.8) | 27 (37.3 ± 10.4)a | 14 (24.7 ± 6.6) | 10 (17.1 ± 6.1) | 6 (9.8 ± 3.2) | 57 (88.9 ± 3.2) | |
| p-value | 0.182 | 0.549 | 0.571 | 0.604 | 0.047 | 0.600 | 0.127 | 0.201 | 0.131 | |
| D7GII | Control (N = 40) | 0 | 33 (87.6 ± 7.9) | 3 (5.4 ± 3.6) | 36 (93.0 ± 5.0) | 5 (10.2 ± 7.6) | 11 (32.2 ± 8.3) | 12 (30.3 ± 8.1) | 4 (11.6 ± 6.6) | 32 (84.3 ± 5.5) |
| ALA (N = 56) | 0 | 49 (85.0 ± 8.1) | 1 (2.2 ± 2.2) | 50 (87.2 ± 6.7) | 10 (19.4 ± 8.6) | 11 (22.9 ± 7.5) | 14 (20.9 ± 6.7) | 0 (0.0 ± 0.0) | 35 (63.3 ± 11.6) | |
| p-value | 0.820 | 0.467 | 0.499 | 0.430 | 0.421 | 0.383 | 0.096 | 0.122 | ||
| Expansion | Hatching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | Total | 12 h | 24 h | 48 h | 72 h | Total | ||
| D8GI | Control (N = 24) | 0 | 21 (90.7 ± 6.3) | 1 (3.7 ± 3.7) | 22 (94.4 ± 5.6) | 4 (21.3 ± 11.0) | 2 (11.1 ± 11.1) | 6 (26.9 ± 11.1) | 2 (8.3 ± 5.9) | 14 (67.6 ± 12.5) |
| ALA (N = 22) | 0 | 16 (75.9 ± 7.9) | 4 (16.7 ± 7.4) | 20 (92.6 ± 5.63) | 6 (29.6 ± 12.0) | 8 (35.2 ± 10.9) | 2 (7.4 ± 5.6) | 1 (3.7 ± 3.7) | 17 (75.9 ± 11.8) | |
| p-value | 0.162 | 0.135 | 0.818 | 0.617 | 0.142 | 0.138 | 0.515 | 0.634 | ||
| D8GII | Control (N = 35) | 0 | 31 (89.6 ± 4.3) | 3 (6.7 ± 3.3) | 34 (96.3 ± 3.7) | 2 (5.3 ± 3.8)b | 4 (10.9 ± 6.1) | 8 (22.5 ± 7.3) | 2 (14.8 ± 11.3) | 16 (53.6 ± 10.6) |
| ALA (N = 31) | 0 | 30 (94.4 ± 5.6) | 1 (5.6 ± 5.6) | 31 (100.0 ± 0.0) | 8 (24.3 ± 8.0)a | 8 (32.1 ± 12.7) | 5 (16.1 ± 7.2) | 4 (9.9 ± 5.8) | 25 (82.5 ± 11.1) | |
| p-value | 0.504 | 0.866 | 0.332 | 0.048 | 0.150 | 0.541 | 0.705 | 0.077 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anjos, M.M.d.; de Paula, G.R.; Yokomizo, D.N.; Costa, C.B.; Bertozzi, M.M.; Verri, W.A., Jr.; Alfieri, A.A.; Morotti, F.; Seneda, M.M. Effect of Alpha-Lipoic Acid on the Development, Oxidative Stress, and Cryotolerance of Bovine Embryos Produced In Vitro. Vet. Sci. 2025, 12, 120. https://doi.org/10.3390/vetsci12020120
Anjos MMd, de Paula GR, Yokomizo DN, Costa CB, Bertozzi MM, Verri WA Jr., Alfieri AA, Morotti F, Seneda MM. Effect of Alpha-Lipoic Acid on the Development, Oxidative Stress, and Cryotolerance of Bovine Embryos Produced In Vitro. Veterinary Sciences. 2025; 12(2):120. https://doi.org/10.3390/vetsci12020120
Chicago/Turabian StyleAnjos, Mariana Moreira dos, Gabriela Rodrigues de Paula, Deborah Nakayama Yokomizo, Camila Bortoliero Costa, Mariana Marques Bertozzi, Waldiceu Aparecido Verri, Jr., Amauri Alcindo Alfieri, Fábio Morotti, and Marcelo Marcondes Seneda. 2025. "Effect of Alpha-Lipoic Acid on the Development, Oxidative Stress, and Cryotolerance of Bovine Embryos Produced In Vitro" Veterinary Sciences 12, no. 2: 120. https://doi.org/10.3390/vetsci12020120
APA StyleAnjos, M. M. d., de Paula, G. R., Yokomizo, D. N., Costa, C. B., Bertozzi, M. M., Verri, W. A., Jr., Alfieri, A. A., Morotti, F., & Seneda, M. M. (2025). Effect of Alpha-Lipoic Acid on the Development, Oxidative Stress, and Cryotolerance of Bovine Embryos Produced In Vitro. Veterinary Sciences, 12(2), 120. https://doi.org/10.3390/vetsci12020120






