Differential Analysis of Fecal SCFAs and Their Contribution to Adipogenesis in UCP1 Knock-In Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Material and Sample Collection
2.2. The 16s rRNA Assay and Data Analysis
2.3. Determination of Short-Chain Fatty Acids
2.4. Isolation, Culture, and In Vitro Differentiation of Preadipocytes
2.5. Oil Red O Staining
2.6. Extraction and Reverse Transcription of RNA
2.7. Real-Time Fluorescence Quantification (qPCR)
2.8. Statistical Analysis
2.9. In Vitro Cell Experiment
3. Results
3.1. The Concentration of Short-Chain Fatty Acids (SCFAs) Is Markedly Diminished in the Feces of KI Pigs
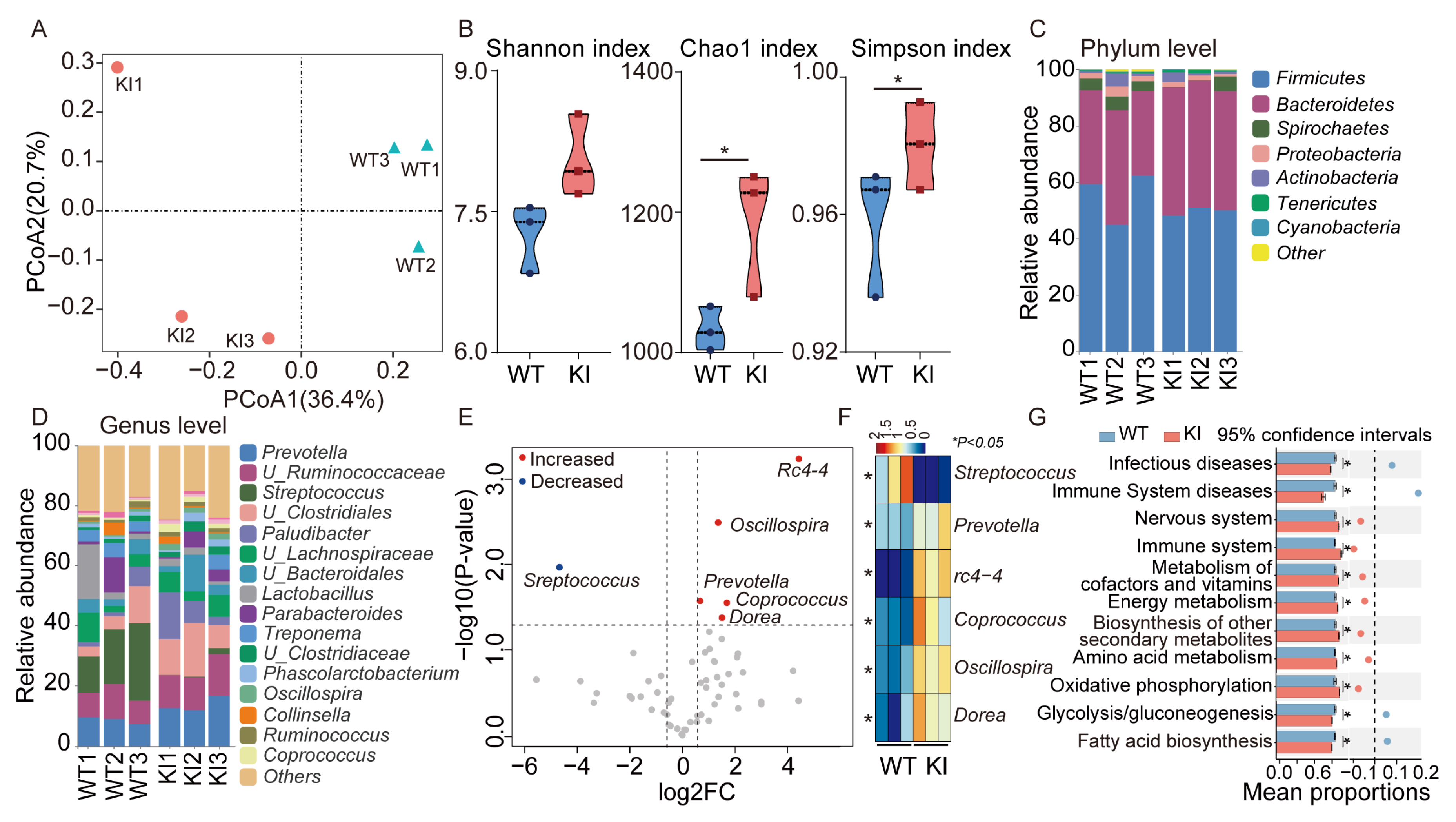
3.2. Distinctive Variations in the Fecal Microbiology of KI and WT Pigs
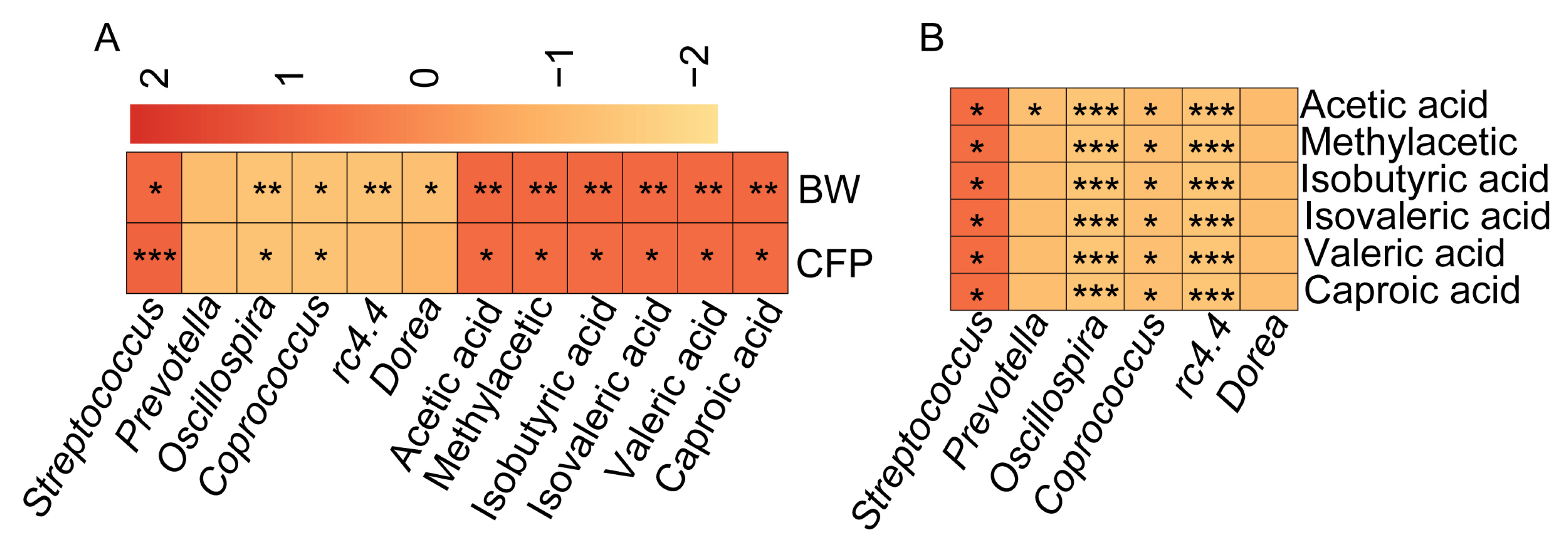
3.3. SCFA Content Is Significantly and Positively Correlated with Porcine Adiposity and Streptococcus spp. Abundance
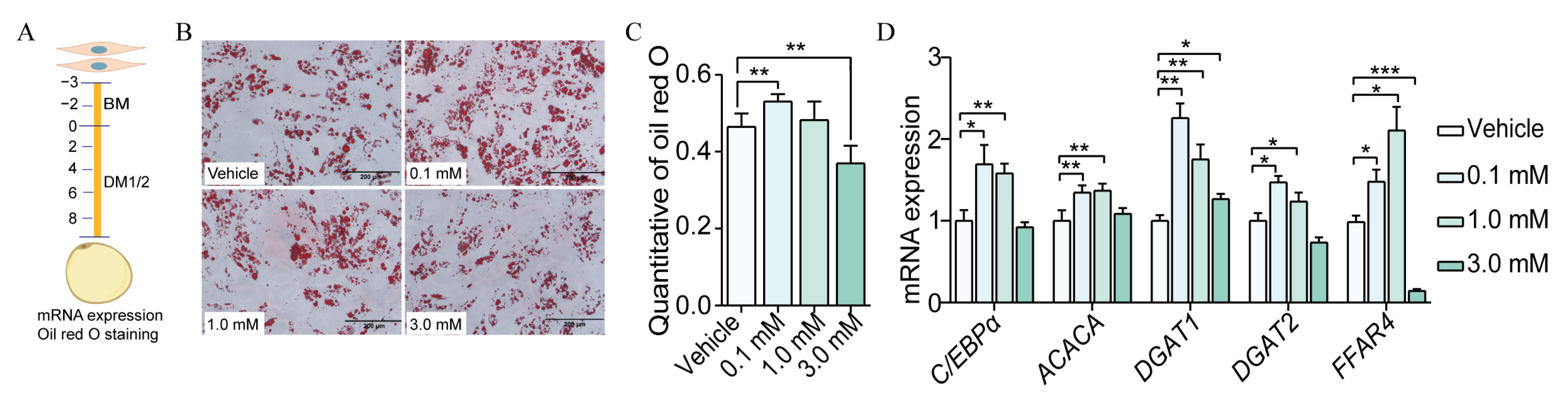
3.4. Caproic Acid Enhanced the Efficiency of SVF Cell Differentiation to Mature Adipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Q.T.; Lin, J.; Huang, J.J.; Zhang, H.Y.; Zhang, R.; Zhang, X.Y.; Cao, C.W.; Hambly, C.; Qin, G.S.; Yao, J.; et al. Reconstitution of UCP1 using CRISPR/Cas9 in the white adipose tissue of pigs decreases fat deposition and improves thermogenic capacity. Proc. Natl. Acad. Sci. USA 2017, 114, E9474–E9482. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Obeng, N.; Bansept, F.; Sieber, M.; Traulsen, A.; Schulenburg, H. Evolution of Microbiota–Host Associations: The Microbe’s Perspective. Trends Microbiol. 2021, 29, 779–787. [Google Scholar] [CrossRef]
- Quan, L.H.; Zhang, C.; Dong, M.; Jiang, J.; Xu, H.; Yan, C.; Liu, X.; Zhou, H.; Zhang, H.; Chen, L.; et al. Myristoleic acid produced by enterococci reduces obesity through brown adipose tissue activation. Gut 2020, 69, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liang, X.; Wang, K.; Lin, J.; Wang, X.; Wang, P.; Zhang, Y.; Nie, Q.; Liu, H.; Zhang, Z.; et al. Intestinal hypoxia-inducible factor 2α regulates lactate levels to shape the gut microbiome and alter thermogenesis. Cell Metab. 2021, 33, 1988–2003.e7. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tao, C.; Cao, C.; Zheng, Q.; Lam, S.M.; Shui, G.; Liu, X.; Li, K.; Zhao, J.; Wang, Y. Adipose lipidomics and RNA-Seq analysis revealed the enhanced mitochondrial function in UCP1 knock-in pigs. Biochim. Et Biophys. Acta BBA Mol. Cell Biol. Lipids 2019, 1864, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chui, L.; Liu, T.; Zheng, Q.; Liu, X.; Liu, L.; Zhao, Y.; Zhang, L.; Song, M.; Han, J.; et al. Fecal Microbiota Was Reshaped in UCP1 Knock-In Pigs via the Adipose-Liver-Gut Axis and Contributed to Less Fat Deposition. Microbiol. Spectr. 2023, 11, e03540-22. [Google Scholar] [CrossRef] [PubMed]
- Hood, L. Tackling the Microbiome. Science 2012, 336, 1209. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef]
- McFall-Ngai, M. Are biologists in “future shock”? Symbiosis integrates biology across domains. Nat. Rev. Microbiol. 2008, 6, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Immune regulation by microbiome metabolites. Immunology 2018, 154, 220–229. [Google Scholar] [CrossRef]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.C.; Wang, Y.; Wang, Z.B.; Liu, W.Y.; Sun, S.; Li, L.; Su, D.F.; Zhang, L.C. Propionate Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress. Front. Pharmacol. 2016, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate Enhances Intestinal Epithelial Barrier Function via Up-Regulation of Tight Junction Protein Claudin-1 Transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Mansuy-Aubert, V.; Ravussin, Y. Short chain fatty acids: The messengers from down below. Front. Neurosci. 2023, 17, 1197759. [Google Scholar] [CrossRef]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 2018, 8, 74. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pan, J.; Cao, C.; Liang, X.; Yang, S.; Liu, L.; Tao, C.; Zhao, J.; Wang, Y. RNF20 affects porcine adipocyte differentiation via regulation of mitotic clonal expansion. Cell Prolif. 2021, 54, e13131. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Cao, C.; Liu, J.; Zhao, Y.; Pan, J.; Tao, C.; Wang, Y. Distinct Transcriptional Responses of Skeletal Muscle to Short-Term Cold Exposure in Tibetan Pigs and Bama Pigs. Int. J. Mol. Sci. 2023, 24, 7431. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Tao, C.; Pan, J.; Zhang, L.; Liu, L.; Zhao, Y.; Fan, Y.; Cao, C.; Liu, J.; Zhang, J.; et al. Rnf20 deficiency in adipocyte impairs adipose tissue development and thermogenesis. Protein Cell 2021, 12, 475–492. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Huang, X.; Yan, F.; Yin, J.; Xiao, Y. The Role of Gut Microbiota in the Skeletal Muscle Development and Fat Deposition in Pigs. Antibiotics 2022, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Bauer, P.V.; Hamr, S.C.; Duca, F.A. Regulation of energy balance by a gut–brain axis and involvement of the gut microbiota. Cell. Mol. Life Sci. 2016, 73, 737–755. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Santiago, J.P.; Iablokov, S.N.; Chopra, D.; Rodionov, D.A.; Peterson, S.N. Short-Chain Fatty Acids Modulate Healthy Gut Microbiota Composition and Functional Potential. Curr. Microbiol. 2022, 79, 128. [Google Scholar] [CrossRef]
- Rahat-Rozenbloom, S.; Fernandes, J.; Gloor, G.B.; Wolever, T.M.S. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int. J. Obes. 2014, 38, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.P.; Shanahan, F. Gut microbiota and obesity: Role in aetiology and potential therapeutic target. Best Pract. 2014, 28, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G Protein-coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Al Mahri, S.; Malik, S.S.; Al Ibrahim, M.; Haji, E.; Dairi, G.; Mohammad, S. Free Fatty Acid Receptors (FFARs) in Adipose: Physiological Role and Therapeutic Outlook. Cells 2022, 11, 750. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, A.; Hirasawa, A.; Poulain-Godefroy, O.; Bonnefond, A.; Hara, T.; Yengo, L.; Kimura, I.; Leloire, A.; Liu, N.; Iida, K.; et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 2012, 483, 350–354. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signal Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Jocken, J.W.; González Hernández, M.A.; Hoebers, N.T.; Van der Beek, C.M.; Essers, Y.P.; Blaak, E.E.; Canfora, E.E. Short-Chain Fatty Acids Differentially Affect Intracellular Lipolysis in a Human White Adipocyte Model. Front. Endocrinol. 2018, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Z.; Ye, B.; Ma, J.H.; Ji, S.; Sheng, W.; Ye, S.; Ou, Y.; Peng, Y.; Yang, X.; et al. Sodium butyrate ameliorates diabetic retinopathy in mice via the regulation of gut microbiota and related short-chain fatty acids. J. Transl. Med. 2023, 21, 451. [Google Scholar] [CrossRef] [PubMed]
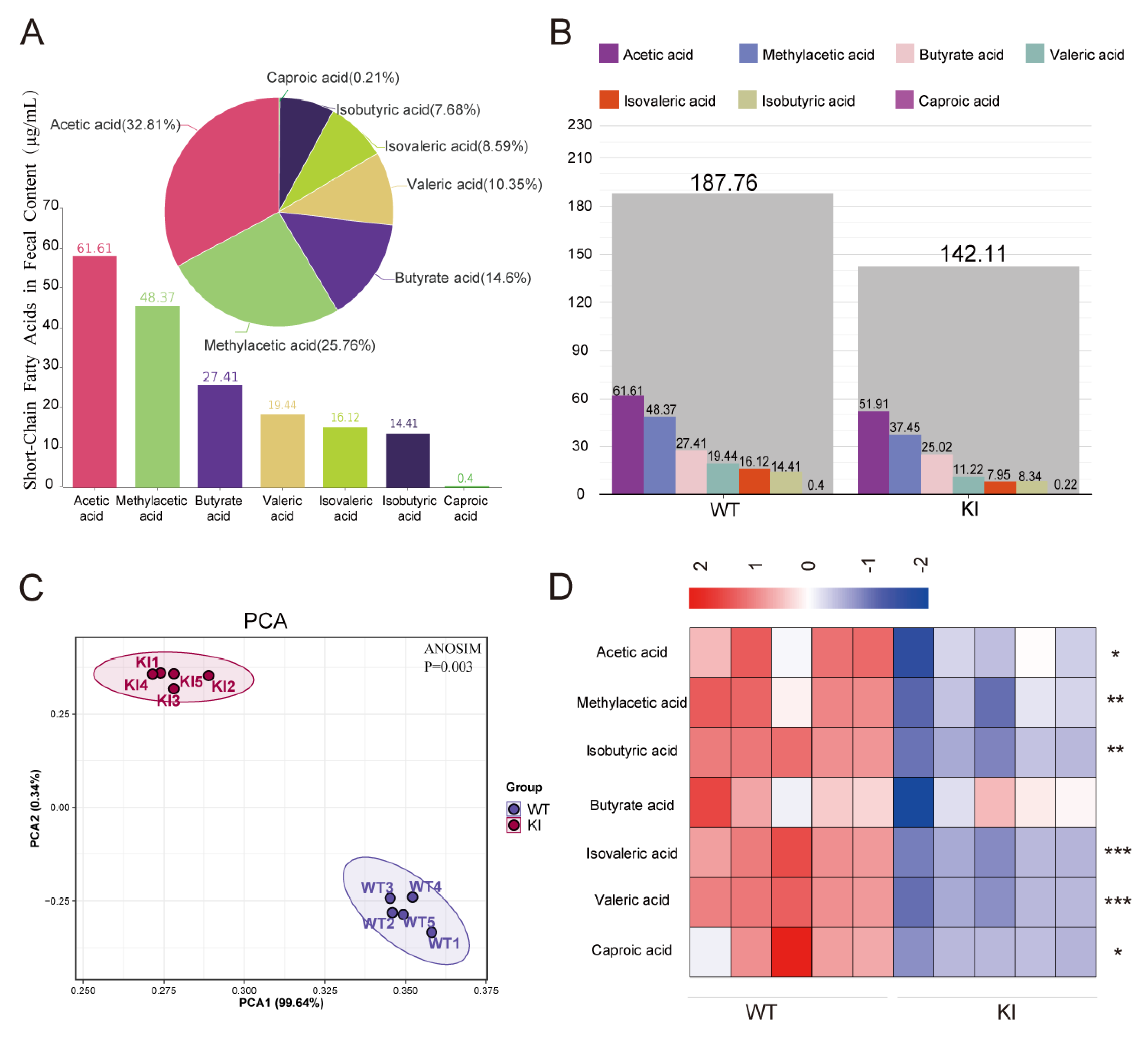
| Species | WT (μg/mL) | KI (μg/mL) | p-Value | Fold Change |
|---|---|---|---|---|
| Acetic acid | 61.61 ± 0.65 | 51.91 ± 1.43 | 4.37 × 10−4 | 1.19 |
| Methylacetic acid | 48.37 ± 1.37 | 37.45 ± 1.03 | 3.89 × 10−4 | 1.29 |
| Isobutyric acid | 14.41 ± 0.57 | 8.34 ± 0.40 | 1.12 × 10−4 | 1.73 |
| Butyrate acid | 27.41 ± 0.99 | 25.02 ± 0.98 | 4.09 × 10−2 | 1.10 |
| Isovaleric acid | 16.12 ± 0.66 | 7.95 ± 0.35 | 4.53 × 10−5 | 2.03 |
| Valeric acid | 19.44 ± 0.76 | 11.22 ± 0.47 | 9.09 × 10−5 | 1.73 |
| Caproic acid | 0.40 ± 0.02 | 0.22 ± 0.01 | 4.09 × 10−5 | 1.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Pan, J.; Wang, Y.; Zhao, J.; Huang, J. Differential Analysis of Fecal SCFAs and Their Contribution to Adipogenesis in UCP1 Knock-In Pigs. Vet. Sci. 2025, 12, 102. https://doi.org/10.3390/vetsci12020102
Zhao C, Pan J, Wang Y, Zhao J, Huang J. Differential Analysis of Fecal SCFAs and Their Contribution to Adipogenesis in UCP1 Knock-In Pigs. Veterinary Sciences. 2025; 12(2):102. https://doi.org/10.3390/vetsci12020102
Chicago/Turabian StyleZhao, Chengyu, Jianfei Pan, Yanfang Wang, Jianguo Zhao, and Jiaojiao Huang. 2025. "Differential Analysis of Fecal SCFAs and Their Contribution to Adipogenesis in UCP1 Knock-In Pigs" Veterinary Sciences 12, no. 2: 102. https://doi.org/10.3390/vetsci12020102
APA StyleZhao, C., Pan, J., Wang, Y., Zhao, J., & Huang, J. (2025). Differential Analysis of Fecal SCFAs and Their Contribution to Adipogenesis in UCP1 Knock-In Pigs. Veterinary Sciences, 12(2), 102. https://doi.org/10.3390/vetsci12020102







