Effects of Akebia and Dandelion on Growth Performance, Inflammation, and Gut Microbiota in Weaned Rabbits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animals and Feed
2.3. Sample Collection and Management
2.4. Cytokine Content Detection
2.5. RNA Extraction, cDNA Inversion Rate, and Real-Time PCR
2.6. Illumina MiSeq Double-Tailed Sequencing
2.7. Data Statistical Analysis
3. Results
3.1. Influence of Dandelion and Akebia Extract on Weaned Rabbits in Growth
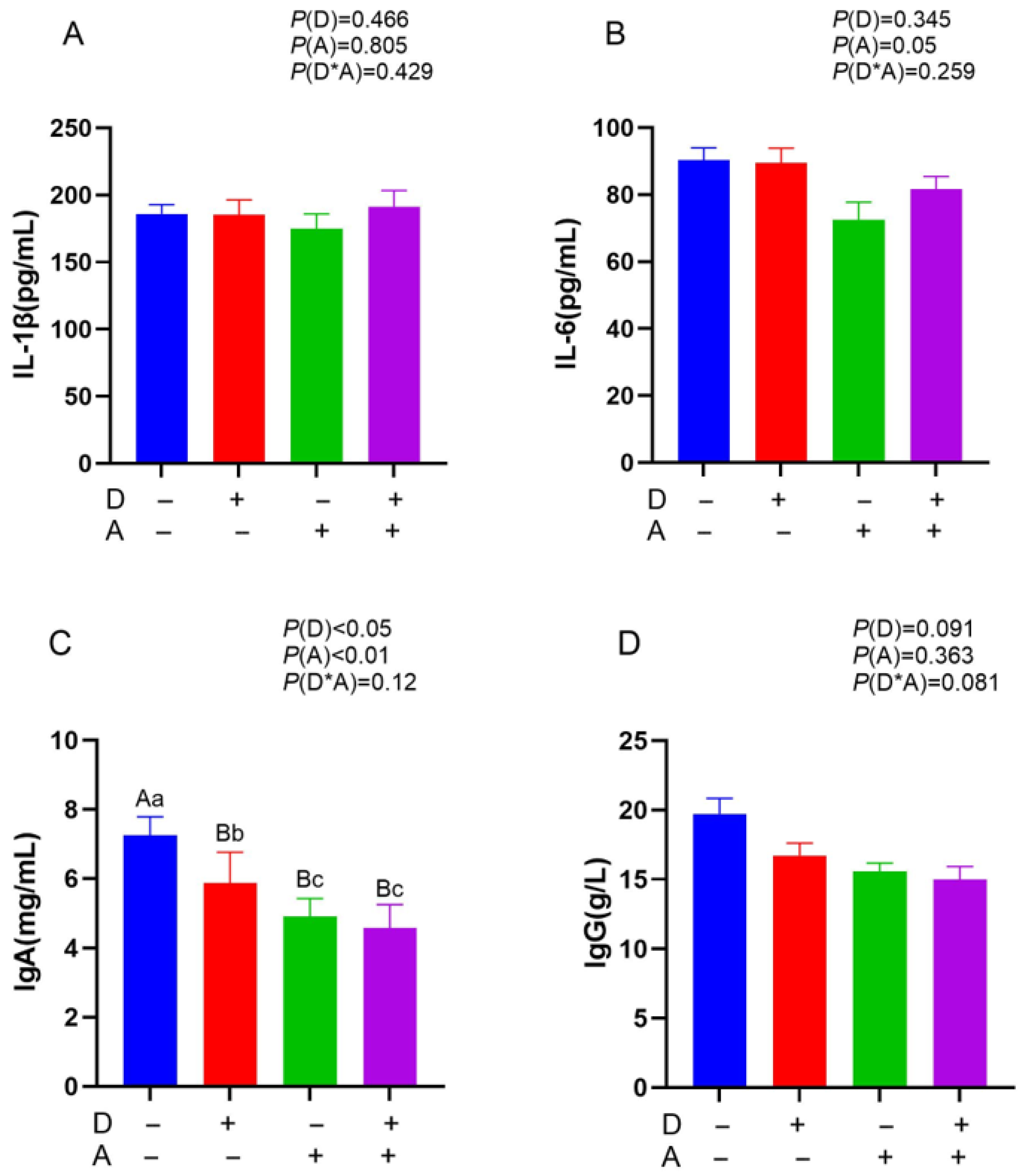
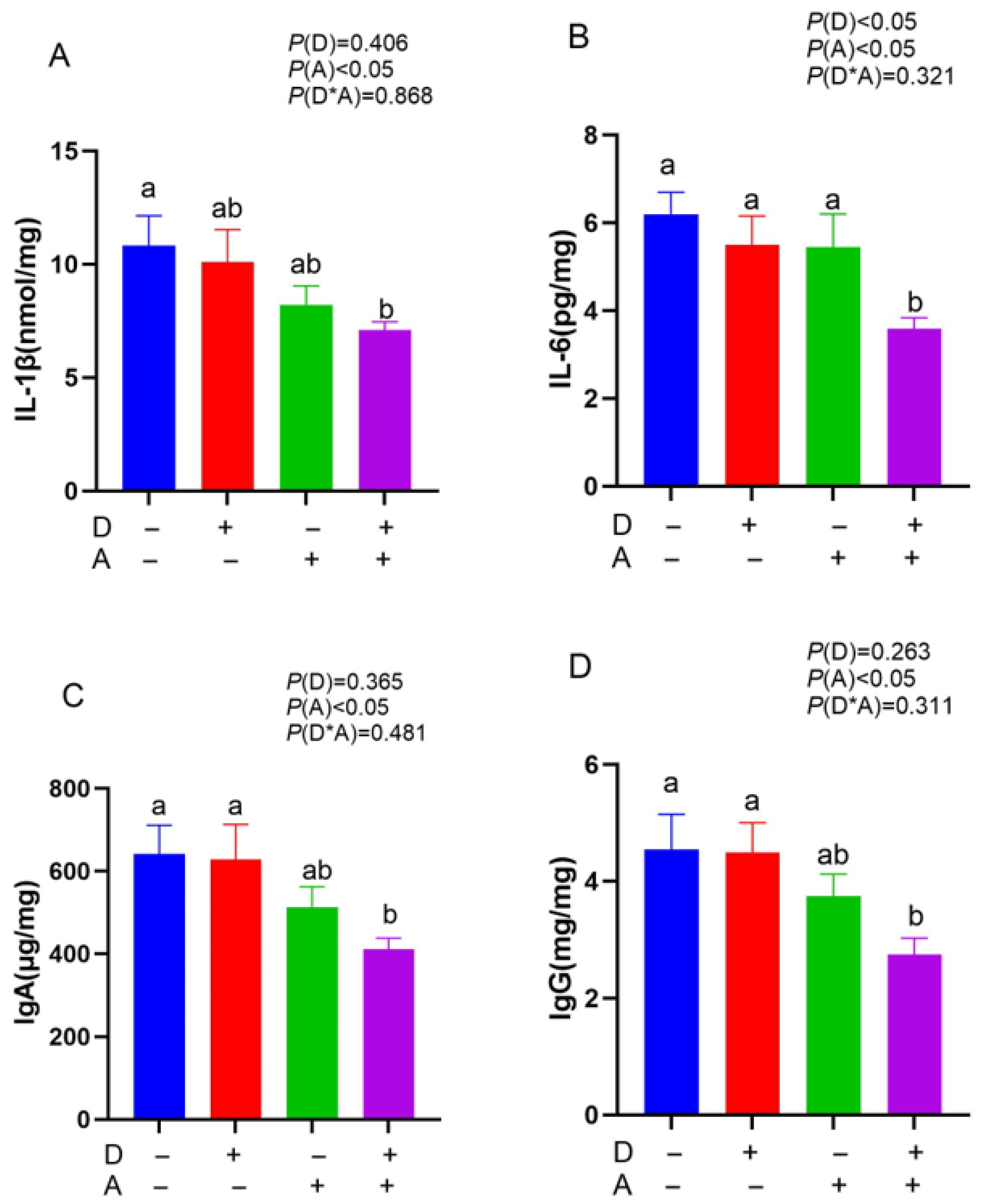
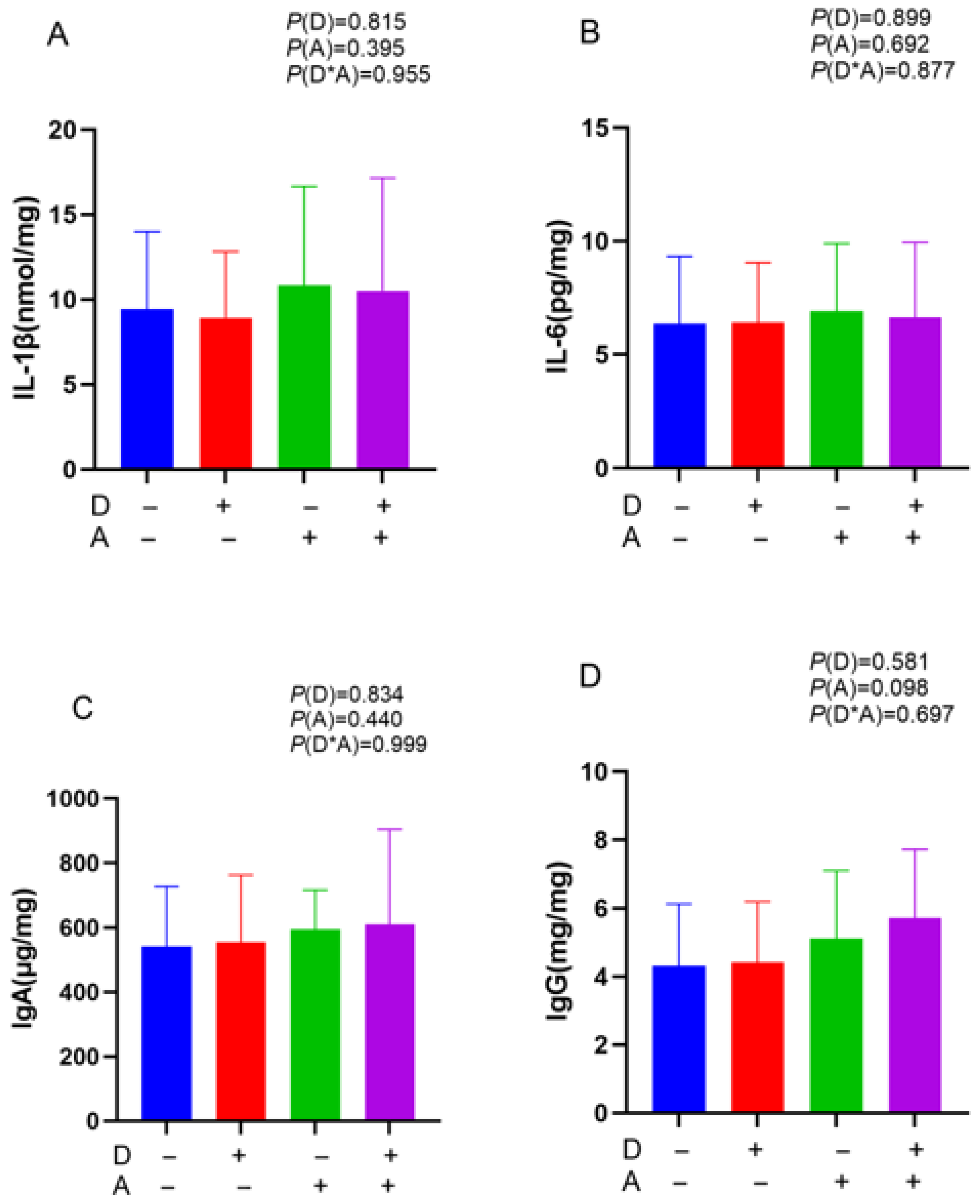
3.2. Influence of Dandelion and Akebia Extract Addition on IL-1β, IL-6, IgA, and IgG Concentration of Weaned Rabbits
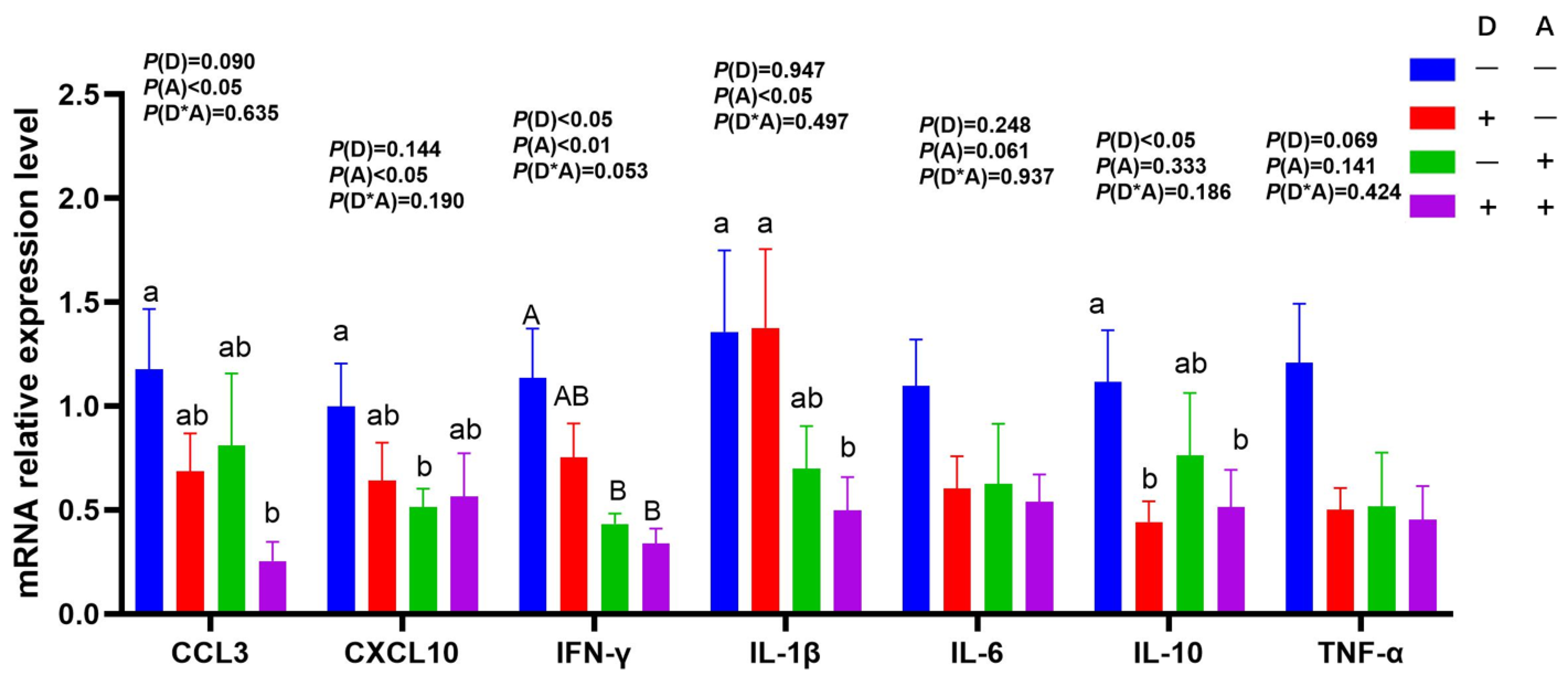
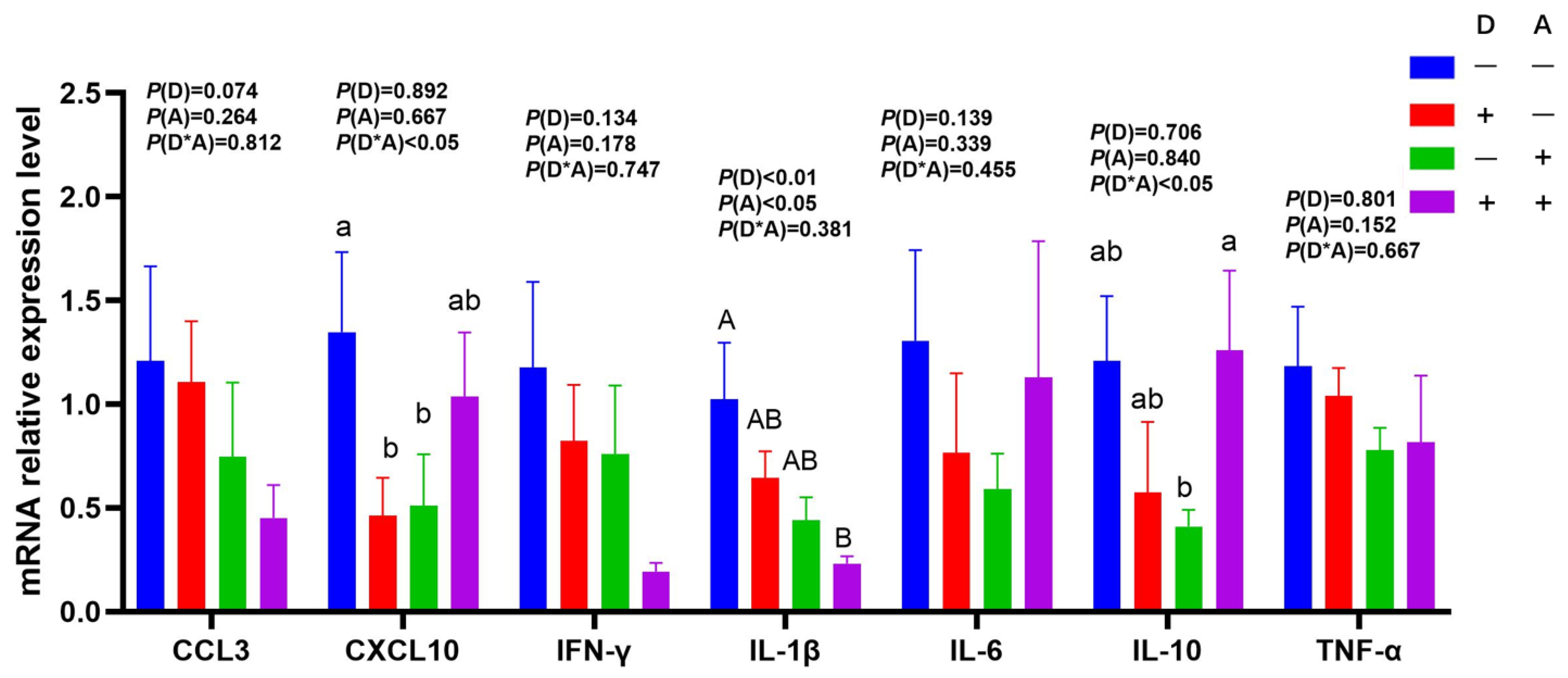
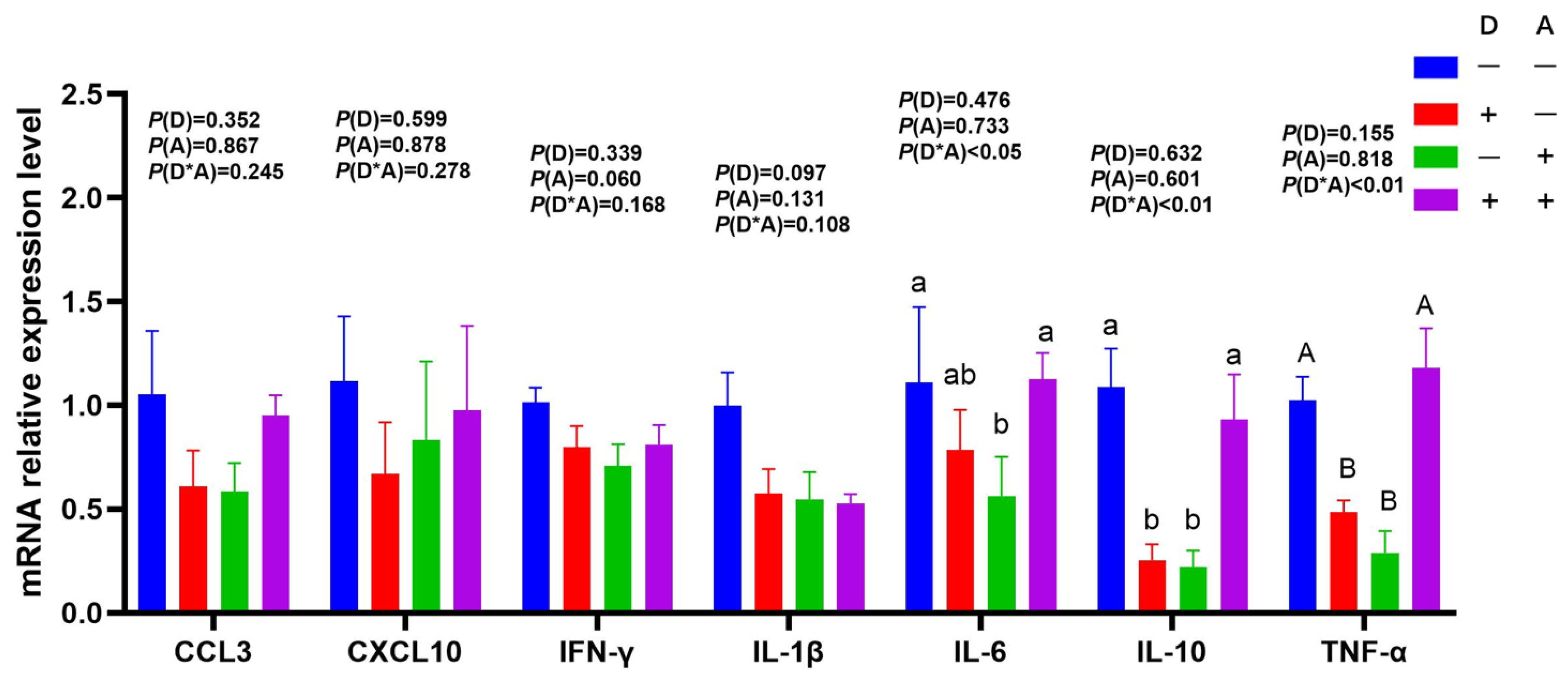
3.3. Influence of Dandelion and Akebia Extract on mRNA Expression Related to Genes of Inflammatory Response
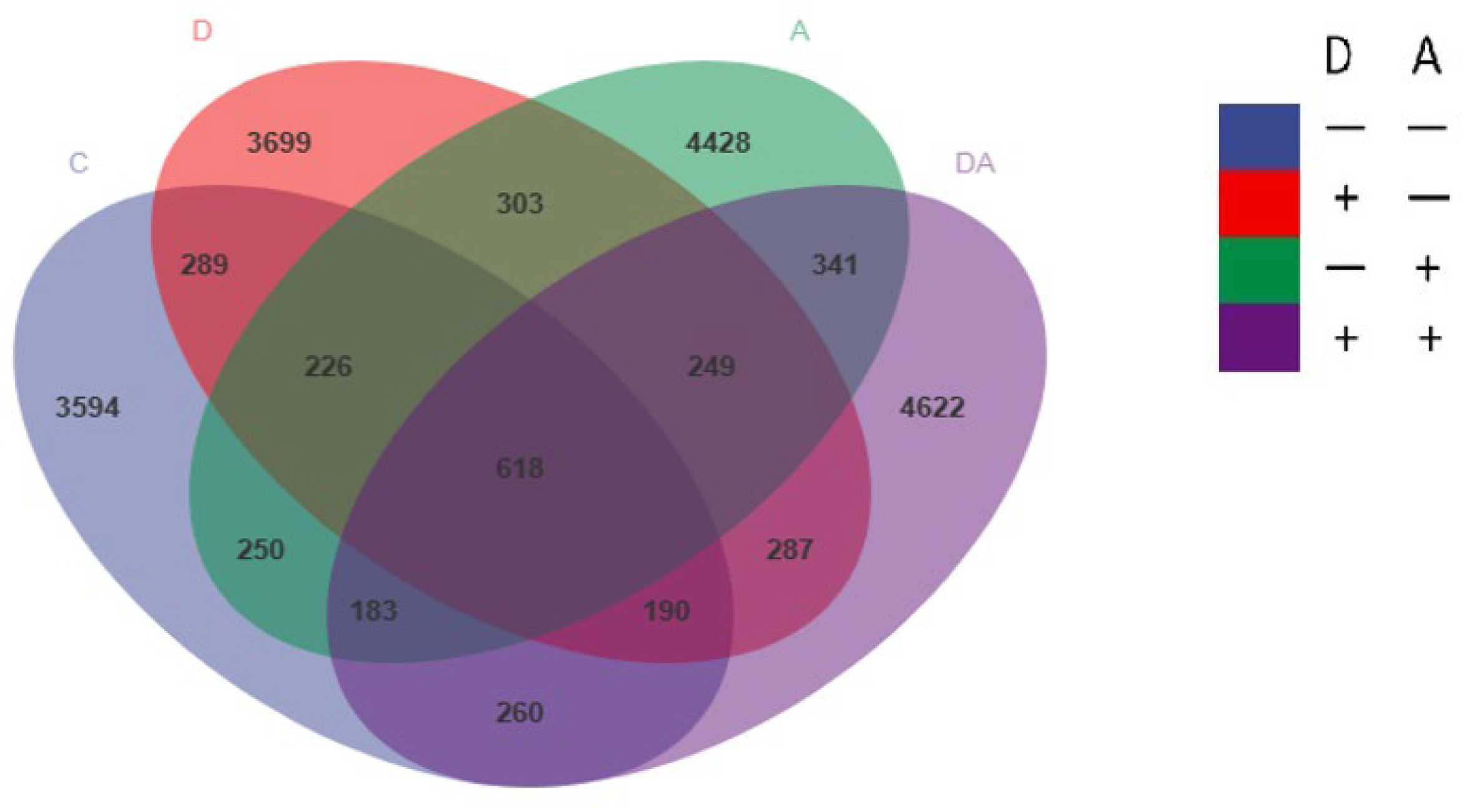
3.4. Influence of Dandelion or Akebia Extract Addition on Weaned Rabbits’ Intestinal Microbial Flora
3.5. Microbial Flora Composition of Samples
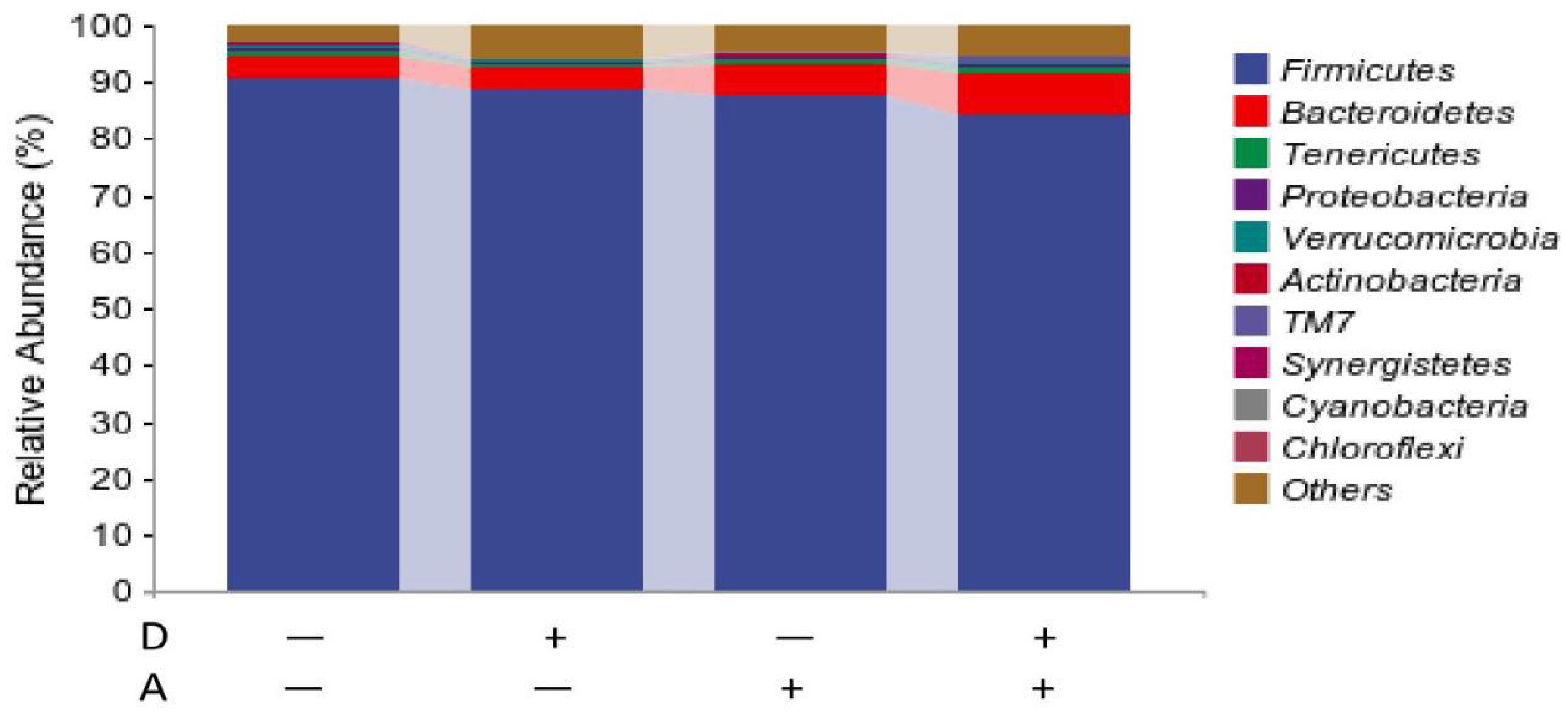
3.5.1. Microbiota Composition of Weaned Rabbits in the Level of Phylum
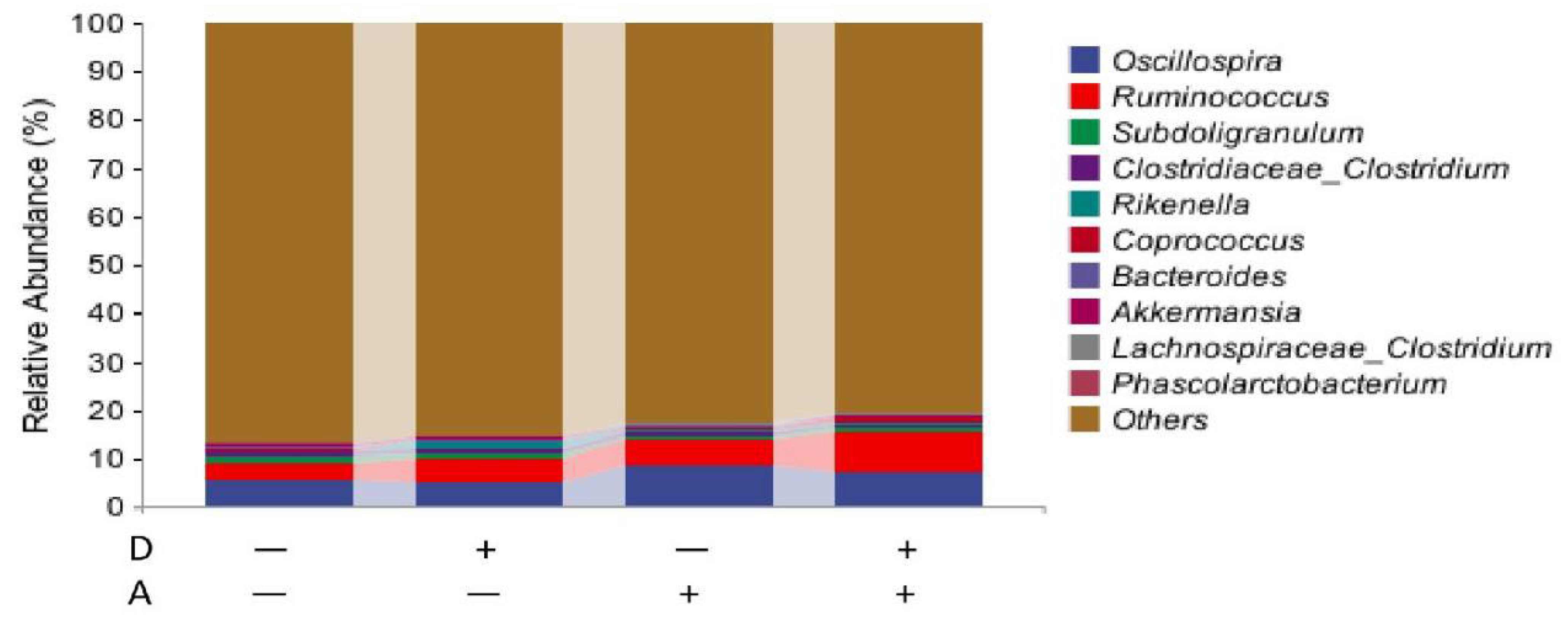
3.5.2. Microbiota Composition of Weaned Rabbits in the Level of Genus
3.6. Species Differences and Marker Species Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, S.M.; Chen, X.; Pan, J.H.; Chen, Q.H.; Zhou, L.W.; Wang, C.C.; Xiao, T.F.; Gan, Q.F. Dynamic distribution of gut microbiota in meat rabbits at different growth stages and relationship with average daily gain (ADG). BMC Microbiol. 2020, 20, 116. [Google Scholar] [CrossRef]
- Van Der Sluis, M.; Van Zeeland, Y.R.A.; De Greef, K.H. Digestive problems in rabbit production: Moving in the wrong direction? Front. Vet. Sci. 2024, 11, 1354651. [Google Scholar] [CrossRef]
- Oglesbee, B.L.; Lord, B. Gastrointestinal Diseases of Rabbits; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 174–187. [Google Scholar]
- Li, M.; Zhang, H.N.; Hu, X.Y.; Liu, Y.M.; Liu, Y.F.; Song, M.J.; Wu, R.A.; Wu, J.R. Isolation of a New Polysaccharide from Dandelion Leaves and Evaluation of Its Antioxidant, Antibacterial, and Anticancer Activities. Molecules 2022, 27, 7641. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wang, Y.; Duan, T.; Yin, N.; Dong, C.; Ren, X.; Liu, N.; An, X.; Qi, J. Effect of fermented dandelion on productive performance, meat quality, immune function, and intestinal microbiota of broiler chickens. BMC Vet. Res. 2023, 19, 178. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, G.; Zhou, X.J.; Dong, W.X.; Wang, Q.Y.; Xiao, C.M.; Zhang, S. Effect of Dandelion root extract on growth performance, immune function and bacterial community in weaned pigs. Food Agric. Immunol. 2019, 30, 95–111. [Google Scholar] [CrossRef]
- Harrison, T.M.; Churgin, S.M. Acupuncture and Traditional Chinese Veterinary Medicine in Zoological and Exotic Animal Medicine: A Review and Introduction of Methods. Vet. Sci. 2022, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.-P.; Gao, X.C.; Zhang, L.Q.; Wei, S.Q.; Bi, S.; Yang, Z.C.; Cui, H. In vitro evaluation of enhancing effect of borneol on transcorneal permeation of compounds with different hydrophilicities and molecular sizes. Eur. J. Pharmacol. 2013, 705, 20–25. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wang, H.M.; Zeng, X.H. Research progress of active compounds and pharmacological effects in Akebia trifoliata (Thunb) koidz stems. IOP Conf. Ser. Earth Environ. Sci. 2018, 185, 012034. [Google Scholar] [CrossRef]
- Choi, J.N.; Choi, Y.H.; Lee, J.M.; Noh, I.C.; Park, J.W.; Choi, W.S.; Choi, J.H. Anti-inflammatory effects of β-sitosterol-βD-glucoside from Trachelospermum jasminoides (Apocynaceae) in lipopolysaccharide-stimulated RAW 264.7 murine macrophages. Nat. Prod. Res. 2012, 26, 2340–2343. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.D.; Liao, N.; Wang, Z.; Li, W.L.; Qin, X.J.; Hai, C.X. Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol. Cell. Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Sarkaki, A.; Rashno, M.; Farbood, Y. Memory deficits and hippocampal inflammation in cerebral hypoperfusion and reperfusion in male rats: Neuroprotective role of vanillic acid. Life Sci. 2018, 211, 126–132. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef]
- Grigoriadis, N.; Van Pesch, V. A basic overview of multiple sclerosis immunopathology. Eur. J. Neurol. 2015, 22, 3–13. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, M.J.; Ha, S.; Hwang, J.; Koyanagi, A.; Dragioti, E.; Radua, J.; Smith, L.; Jacob, L.; de Pablo, G.S.; et al. Association between autism spectrum disorder and inflammatory bowel disease: A systematic review and meta-analysis. Autism Res. 2022, 15, 340–352. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Y.Z.; Zhao, C.X.; Zhao, B.Y.; Wang, J.G. The Pharmacological Efficacy of Baicalin in Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 9317. [Google Scholar] [CrossRef]
- Sun, H.; Ni, X.; Song, X.; Wen, B.; Zhou, Y.; Zou, F.; Yang, M.; Peng, Z.; Zhu, H.; Zeng, Y.; et al. Fermented Yupingfeng polysaccharides enhance immunity by improving the foregut microflora and intestinal barrier in weaning rex rabbits. Appl. Microbiol. Biotechnol. 2016, 100, 8105–8120. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Y.J.; Wang, Z.L.; Chen, J.C.; Yang, Y.; Dong, G.Z. Dandelion Extract Alleviated Lipopolysaccharide-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells. Toxins 2020, 12, 496. [Google Scholar] [CrossRef]
- Yi, X.H.; Tang, X.Q.; Sui, Y.; Ren, Z.J.; Sun, X.Z.; Wang, S.H. Adding dandelion to rabbit diets: Enhancing the growth performance and meat quality by altering immune competence and gut microbiota. Microbiol. Spectr. 2025, 13, e00646-25. [Google Scholar] [CrossRef] [PubMed]
- Davaatseren, M.; Hur, H.J.; Yang, H.J.; Hwang, J.T.; Park, J.H.; Kim, H.J.; Kim, M.J.; Kwon, D.Y.; Sung, M.J. Taraxacum official (dandelion) leaf extract alleviates high-fat diet-induced nonalcoholic fatty liver. Food Chem. Toxicol. 2013, 58, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Jang, S.; Lee, S.W.; Park, S.D.; Sung, Y.Y.; Kim, H.K. Akebia quinata Decaisne aqueous extract acts as a novel anti-fatigue agent in mice exposed to chronic restraint stress. J. Ethnopharmacol. 2018, 222, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; van der Burgh, A.C.; Peeters, R.P.; van Hagen, P.M.; Dalm, V.A.S.H.; Chaker, L. Determinants of Serum Immunoglobulin Levels: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 664526. [Google Scholar] [CrossRef]
- Sun, W.; Chen, Z.; Huang, Z.Y.; Wan, A.F.; Zhou, M.; Gao, J. Effects of dietary traditional Chinese medicine residues on growth performance, intestinal health and gut microbiota compositions in weaned piglets. Front. Cell. Infect. Microbiol. 2023, 13, 1283789. [Google Scholar] [CrossRef]
- Wang, W.; Wu, L.; Li, J.; Ji, J.; Chen, K.; Yu, Q.; Li, S.; Feng, J.; Liu, T.; Zhang, J.; et al. Alleviation of Hepatic Ischemia Reperfusion Injury by Oleanolic Acid Pretreating via Reducing HMGB1 Release and Inhibiting Apoptosis and Autophagy. Mediat. Inflamm. 2019, 2019, 3240713. [Google Scholar] [CrossRef] [PubMed]
- Bamias, G.; Cominelli, F. Cytokines and intestinal inflammation. Curr. Opin. Gastroen. 2016, 32, 437–442. [Google Scholar] [CrossRef]
- Sang, R.; Yu, Y.F.; Ge, B.J.; Xu, L.; Wang, Z.; Zhang, X.M. Taraxasterol from Taraxacum prevents concanavalin A-induced acute hepatic injury in mice via modulating TLRs/NF-κB and Bax/Bc1-2 signalling pathways. Artif. Cell Nanomed. B 2019, 47, 3929–3937. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Cao, H.; Zheng, Y.; Lu, Z.; Chen, Y.; Liu, D.; Yang, H.; Quan, J.; Huo, C.; Liu, J.; et al. Liang-Ge-San, a classic traditional Chinese medicine formula, attenuates acute inflammation in zebrafish and RAW 264.7 cells. J. Ethnopharmacol. 2020, 249, 112427. [Google Scholar] [CrossRef]
- Che, L.; Li, Y.; Song, R.F.; Qin, C.H.; Hao, W.W.; Wang, B.X.; Yang, L.; Peng, P.J.; Xu, F. Anti-inflammatory and anti-apoptosis activity of taraxasterol in ulcerative colitis in vitro and in vivo. Exp. Ther. Med. 2019, 18, 1745–1751. [Google Scholar] [CrossRef]
- Yao, X.Y.; Li, G.L.; Bai, Q.; Xu, H.; Lü, C.T. Taraxerol inhibits LPS-induced inflammatory responses through suppression of TAK1 and Akt activation. Int. Immunopharmacol. 2013, 15, 316–324. [Google Scholar] [CrossRef]
- Zhai, Y.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Liver Ischemia and Reperfusion Injury: New Insights into Mechanisms of Innate—Adaptive Immune-Mediated Tissue Inflammation. Am. J. Transplant. 2011, 11, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; Kirschner, D.E. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zou, X.B.; Chai, Y.F.; Yao, Y.M. Macrophage Polarization in Inflammatory Diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Elemam, N.; Talaat, I.; Maghazachi, A. CXCL10 Chemokine: A Critical Player in RNA and DNA Viral Infections. Viruses 2022, 14, 2445. [Google Scholar] [CrossRef] [PubMed]
- Saiman, Y.; Friedman, S.L. The Role of Chemokines in Acute Liver Injury. Front. Physiol. 2012, 3, 213. [Google Scholar] [CrossRef]
- Song, X.; Gao, X.; Wang, Y.D.; Raja, R.; Zhang, Y.Y.; Yang, S.L.; Li, M.; Yao, Z.Y.; Wei, L. HCV Core Protein Induces Chemokine CCL2 and CXCL10 Expression Through NF-κB Signaling Pathway in Macrophages. Front. Immunol. 2021, 12, 654998. [Google Scholar] [CrossRef]
- Brass, A.; Brenndörfer, E. The Role of Chemokines in Hepatitis C Virus-Mediated Liver Disease. Int. J. Mol. Sci. 2014, 15, 4747–4779. [Google Scholar] [CrossRef]
- Karthika, C.; Rahman, M.H.; Sureshkumar, R.; Akter, R.; Khan, A.A.; Alanazi, A.M.; Azad, A.; Barai, P.; Barai, H.R. 5-Fluorouracil and Curcumin Combination Coated with Pectin and Its Strategy towards Titanium Dioxide, Dimethylhydrazine Colorectal Cancer Model with the Evaluation of the Blood Parameters. Polymers 2022, 14, 2868. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, W.M.; Li, P.H.; Zheng, Z.Q.; Tu, Y.Y.; Zhang, Y.; You, T. Curcumin Enhances the Effects of 5-Fluorouracil and Oxaliplatin in Inducing Gastric Cancer Cell Apoptosis Both In Vitro and In Vivo. Oncol. Res. 2015, 23, 29–34. [Google Scholar] [CrossRef]
- Hildebrand, F.; Nguyen, T.L.A.; Brinkman, B.; Yunta, R.G.; Cauwe, B.; Vandenabeele, P.; Liston, A.; Rase, J. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biol. 2013, 14, R4. [Google Scholar] [CrossRef]
- De Blas, J.C. Nutritional impact on health and performance in intensively reared rabbits. Animal 2013, 7, 102–111. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome–A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Saier, M.H. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef] [PubMed]
- Konikoff, T.; Gophna, U. Oscillospira: A Central, Enigmatic Component of the Human Gut Microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef] [PubMed]


| Ingredient | Content (%) | Nutrient Level | Content |
|---|---|---|---|
| Alfalfa powder | 36 | Digestible Energy/(MJ·kg−1) | 12.02 |
| Corn | 25 | Crude protein (%) | 16.78 |
| Soybean meal | 12 | Ether extract (%) | 3.11 |
| Wheat bran | 23.5 | Crude fiber (%) | 12.92 |
| Fish meal | 1.5 | Calcium (%) | 0.79 |
| CaHCO3 | 1 | Phosphorus (%) | 0.64 |
| NaCl | 0.5 | ||
| Premix 1 | 0.5 | ||
| Total | 100 |
| Gene | Primer Sequence (5′–3′) | Product Fragment Length/bp | Gene Bank Number |
|---|---|---|---|
| CCL3 | F:GCTCTCAGCACCCAGGTCTT | 142 | 100348776 |
| R:GAGCACTGGCTGCTGGTCTC | |||
| CXCL10 | F:CCTCCAGTCTCAGCACCATGAATC | 138 | 100353112 |
| R:GATGCAGGTACAGCGTACAGTTCTA | |||
| IFN-γ | F:TTCCCAAGGATAGCAGTGGT | 160 | AB010386 |
| R:TGAAGCCAGAAGTCCTCAAAA | |||
| IL-1β | F:GCTGGAGAGTGTAGAC | 136 | M26295 |
| R:CTGAGAGGTGCTGATG | |||
| IL-6 | F:TTCGGGGCTGATGGAGTT | 83 | AF169176 |
| R:TGCTGACCCTGGTGTTTTCTT | |||
| IL-10 | F:GCCCTGTGGGATTTGAGTGT | 100 | KT279661 |
| R:CAGGCACAGAAGGAATGGAA | |||
| TNF-α | F:CTCCTACCCGAACAAGGTCA | 138 | M12845 |
| R:CGGTCACCCTTCTCCAACT |
| ITEMS | A− | A− | A+ | A+ | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| D− | D+ | D− | D+ | D | A | D*A | ||
| Body weight(kg) | ||||||||
| Initial | 1.2 | 1.2 | 1.2 | 1.2 | 0.051 | 0.919 | 0.888 | 0.956 |
| Week 1 | 1.4 | 1.4 | 1.5 | 1.4 | 0.045 | 0.613 | 0.377 | 0.337 |
| Week 2 | 1.7 | 1.8 | 1.8 | 1.8 | 0.058 | 0.714 | 0.463 | 0.365 |
| Week 3 | 2.0 | 2.1 | 2.1 | 2.1 | 0.102 | 0.863 | 0.763 | 0.687 |
| Week 4 | 2.1 | 2.2 | 2.2 | 2.2 | 0.222 | 0.626 | 0.730 | 0.918 |
| Average daily gain(g/d) | ||||||||
| Week 1 | 27.9 b | 32.0 a | 33.1 a | 30.5 ab | 2.287 | 0.527 | 0.136 | 0.009 |
| Week 2 | 46.2 | 45.9 | 46.0 | 45.5 | 2.870 | 0.850 | 0.879 | 0.965 |
| Week 3 | 44.0 | 42.7 | 42.1 | 42.7 | 5.312 | 0.945 | 0.848 | 0.839 |
| Week 4 | 20.8 | 21.1 | 19.0 | 23.8 | 4.183 | 0.677 | 0.94 | 0.717 |
| Average feed intake(g/d) | ||||||||
| Week 1 | 115.6 | 115.8 | 115.8 | 115.7 | 0.242 | 0.255 | 0.791 | 0.099 |
| Week 2 | 129.0 | 130.0 | 129.4 | 130.0 | 1.197 | 0.196 | 0.784 | 0.784 |
| Week 3 | 138.6 | 140.0 | 138.0 | 137.8 | 3.265 | 0.710 | 0.401 | 0.612 |
| Week 4 | 147.8 | 148.0 | 147.9 | 147.9 | 0.162 | 0.415 | 0.900 | 0.280 |
| Feed/gain ratio | ||||||||
| Week 1 | 4.2 a | 3.7 b | 3.6 b | 3.9 ab | 0.285 | 0.446 | 0.131 | 0.006 |
| Week 2 | 2.9 | 2.9 | 2.8 | 2.9 | 0.230 | 0.950 | 0.897 | 0.785 |
| Week 3 | 3.5 | 3.9 | 3.4 | 3.5 | 0.880 | 0.473 | 0.581 | 0.734 |
| Week 4 | 7.0 | 7.0 | 7.3 | 6.2 | 1.348 | 0.522 | 0.291 | 0.534 |
| Diarrhea rate | 0.2 | 0.2 | 0.2 | 0.1 | 0.144 | 0.412 | 0.864 | 0.926 |
| Alpha Diversity Index | A− | A− | A+ | A+ | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| D− | D+ | D− | D+ | D | A | D*A | ||
| Chao1 | 1258.502 b | 1345.605 ab | 1556.967 a | 1515.186 ab | 278.423 | 0.837 | 0.044 | 0.561 |
| Observed species | 1135.117 | 1190.867 | 1346.000 | 1334.033 | 242.477 | 0.826 | 0.086 | 0.734 |
| Shannon | 7.965 | 7.955 | 8.407 | 8.250 | 0.697 | 0.778 | 0.222 | 0.805 |
| Simpson | 0.981 | 0.976 | 0.988 | 0.985 | 0.016 | 0.580 | 0.225 | 0.880 |
| Faith_pd | 64.882 | 68.342 | 74.509 | 74.097 | 9.657 | 0.694 | 0.058 | 0.618 |
| Pielou_e | 0.786 | 0.779 | 0.809 | 0.796 | 0.050 | 0.641 | 0.357 | 0.862 |
| Goods coverage | 0.988 a | 0.984 ab | 0.980 b | 0.981 ab | 0.005 | 0.621 | 0.009 | 0.253 |
| Items % | A− | A− | A+ | A+ | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| D− | D+ | D− | D+ | D | A | D*A | ||
| Firmicutes | 83.345 a | 85.987 ab | 90.213 ab | 90.751 b | 5.257 | 0.547 | 0.037 | 0.690 |
| Bacteroidetes | 8.381 | 5.262 | 4.314 | 3.887 | 4.167 | 0.417 | 0.218 | 0.536 |
| Tenericutes | 0.651 | 0.712 | 0.493 | 0.733 | 0.470 | 0.552 | 0.787 | 0.725 |
| Proteobacteria | 0.613 | 0.547 | 0.444 | 0.557 | 0.227 | 0.849 | 0.516 | 0.468 |
| Verrucomicrobiota | 0.431 | 0.165 | 0.325 | 0.448 | 0.198 | 0.487 | 0.390 | 0.069 |
| Actinobacteria | 0.268 | 0.359 | 0.178 | 0.292 | 0.140 | 0.152 | 0.267 | 0.872 |
| TM7 | 0.597 | 0.125 | 0.138 | 0.086 | 0.368 | 0.198 | 0.220 | 0.299 |
| Synergistetes | 0.208 | 0.074 | 0.032 | 0.071 | 0.088 | 0.439 | 0.160 | 0.172 |
| Cyanobacteria | 0.073 | 0.062 | 0.065 | 0.062 | 0.043 | 0.467 | 0.689 | 0.787 |
| Chloroflexi | 0.000 | 0.004 | 0.000 | 0.000 | 0.003 | 0.210 | 0.210 | 0.210 |
| Others | 5.459 | 6.737 | 3.818 | 3.13 | 2.915 | 0.839 | 0.086 | 0.507 |
| Items % | A− | A− | A+ | A+ | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| D− | D+ | D− | D+ | D | A | D*A | ||
| Oscillospira | 5.798 | 5.141 | 8.740 | 6.739 | 3.336 | 0.436 | 0.190 | 0.692 |
| Ruminococcus | 3.427 | 5.115 | 5.236 | 6.551 | 1.762 | 0.105 | 0.081 | 0.835 |
| Subdoligranulum | 1.292 | 0.992 | 0.608 | 1.072 | 0.812 | 0.842 | 0.466 | 0.359 |
| Clostridiaceae_Clostridium | 0.839 | 0.802 | 0.955 | 0.499 | 0.380 | 0.210 | 0.628 | 0.284 |
| Rikenella | 0.175 | 1.295 | 0.361 | 0.300 | 1.407 | 0.461 | 0.572 | 0.412 |
| Coprococcus | 0.421 | 0.150 | 0.699 | 0.747 | 0.543 | 0.686 | 0.123 | 0.563 |
| Bacteroides | 0.450 | 0.563 | 0.255 | 0.257 | 0.431 | 0.792 | 0.259 | 0.801 |
| Akkermansia | 0.448 | 0.259 | 0.200 | 0.430 | 0.204 | 0.841 | 0.710 | 0.054 |
| Lachnospiraceae_Clostridium | 0.288 | 0.100 | 0.201 | 0.236 | 0.179 | 0.404 | 0.791 | 0.230 |
| Phascolarctobacterium | 0.202 | 0.143 | 0.177 | 0.208 | 0.186 | 0.881 | 0.834 | 0.633 |
| Parabacteroides | 0.036 | 0.131 | 0.174 | 0.167 | 0.125 | 0.485 | 0.182 | 0.423 |
| Blautia | 0.215 a | 0.106 ab | 0.092 ab | 0.041 b | 0.077 | 0.051 | 0.024 | 0.466 |
| Oxalobacter | 0.053 ab | 0.031 b | 0.110 a | 0.069 ab | 0.036 | 0.092 | 0.015 | 0.597 |
| Dorea | 0.051 | 0.045 | 0.014 | 0.163 | 0.094 | 0.143 | 0.397 | 0.114 |
| [Ruminococcus] | 0.023 | 0.082 | 0.097 | 0.045 | 0.051 | 0.891 | 0.478 | 0.044 |
| Sutterella | 0.037 b | 0.030 b | 0.101 a | 0.056 ab | 0.036 | 0.165 | 0.022 | 0.300 |
| Synergistes | 0.059 | 0.032 | 0.044 | 0.063 | 0.066 | 0.898 | 0.819 | 0.505 |
| Butyricimonas | 0.031 | 0.072 | 0.032 | 0.048 | 0.038 | 0.154 | 0.562 | 0.519 |
| Alistipes | 0.044 | 0.030 | 0.048 | 0.039 | 0.045 | 0.617 | 0.754 | 0.915 |
| Lactobacillus | 0.004 b | 0.135 a | 0.006 b | 0.033 b | 0.055 | 0.033 | 0.031 | 0.025 |
| Others | 86.106 | 84.744 | 81.849 | 82.267 | 3.791 | 0.806 | 0.092 | 0.644 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, Y.; Mgeni, M.S.; Jiang, X.; Chen, Y.; Zhang, J.; Lv, J. Effects of Akebia and Dandelion on Growth Performance, Inflammation, and Gut Microbiota in Weaned Rabbits. Vet. Sci. 2025, 12, 1048. https://doi.org/10.3390/vetsci12111048
Sun Y, Zhang Y, Mgeni MS, Jiang X, Chen Y, Zhang J, Lv J. Effects of Akebia and Dandelion on Growth Performance, Inflammation, and Gut Microbiota in Weaned Rabbits. Veterinary Sciences. 2025; 12(11):1048. https://doi.org/10.3390/vetsci12111048
Chicago/Turabian StyleSun, Yawang, Yan Zhang, Mussa Suleiman Mgeni, Xiaoyu Jiang, Yu Chen, Junqiu Zhang, and Jingzhi Lv. 2025. "Effects of Akebia and Dandelion on Growth Performance, Inflammation, and Gut Microbiota in Weaned Rabbits" Veterinary Sciences 12, no. 11: 1048. https://doi.org/10.3390/vetsci12111048
APA StyleSun, Y., Zhang, Y., Mgeni, M. S., Jiang, X., Chen, Y., Zhang, J., & Lv, J. (2025). Effects of Akebia and Dandelion on Growth Performance, Inflammation, and Gut Microbiota in Weaned Rabbits. Veterinary Sciences, 12(11), 1048. https://doi.org/10.3390/vetsci12111048






