Inhibitory Effect of Transfer Factor on Avian Reticuloendotheliosis Virus Infection in Chicks
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Reagents
2.2. Experimental Design for Preventive Effect of TF on REV Infection
2.3. Inhibitory Effect of TF on REV Horizontal Propagation
2.4. Suppressing the Effect of TF on REV Congenital Infection via Embryonic Yolk Sac Inoculation
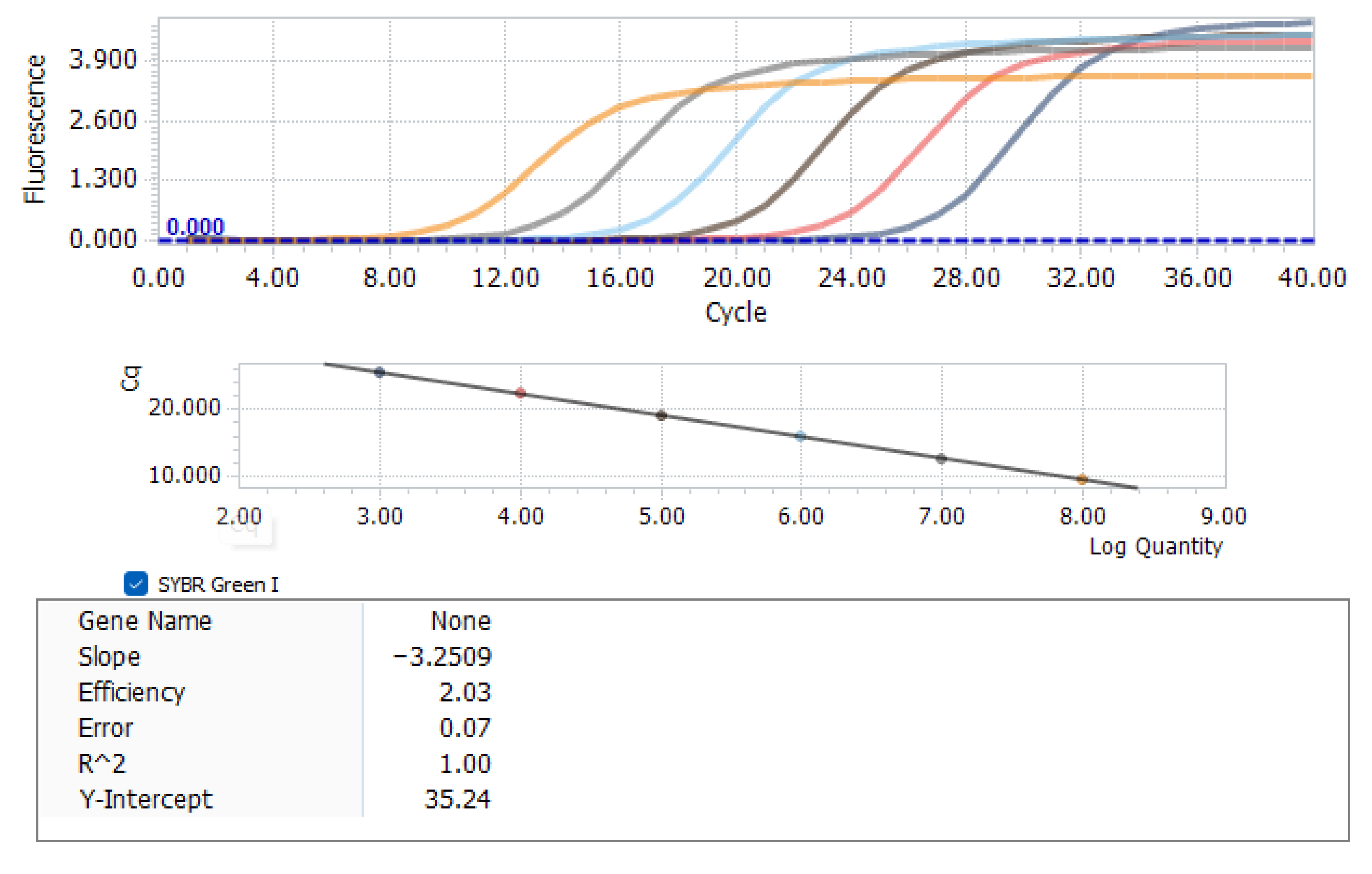
2.5. Detection of Various Pathogenic Indicators
2.6. Statistical Analysis
3. Results
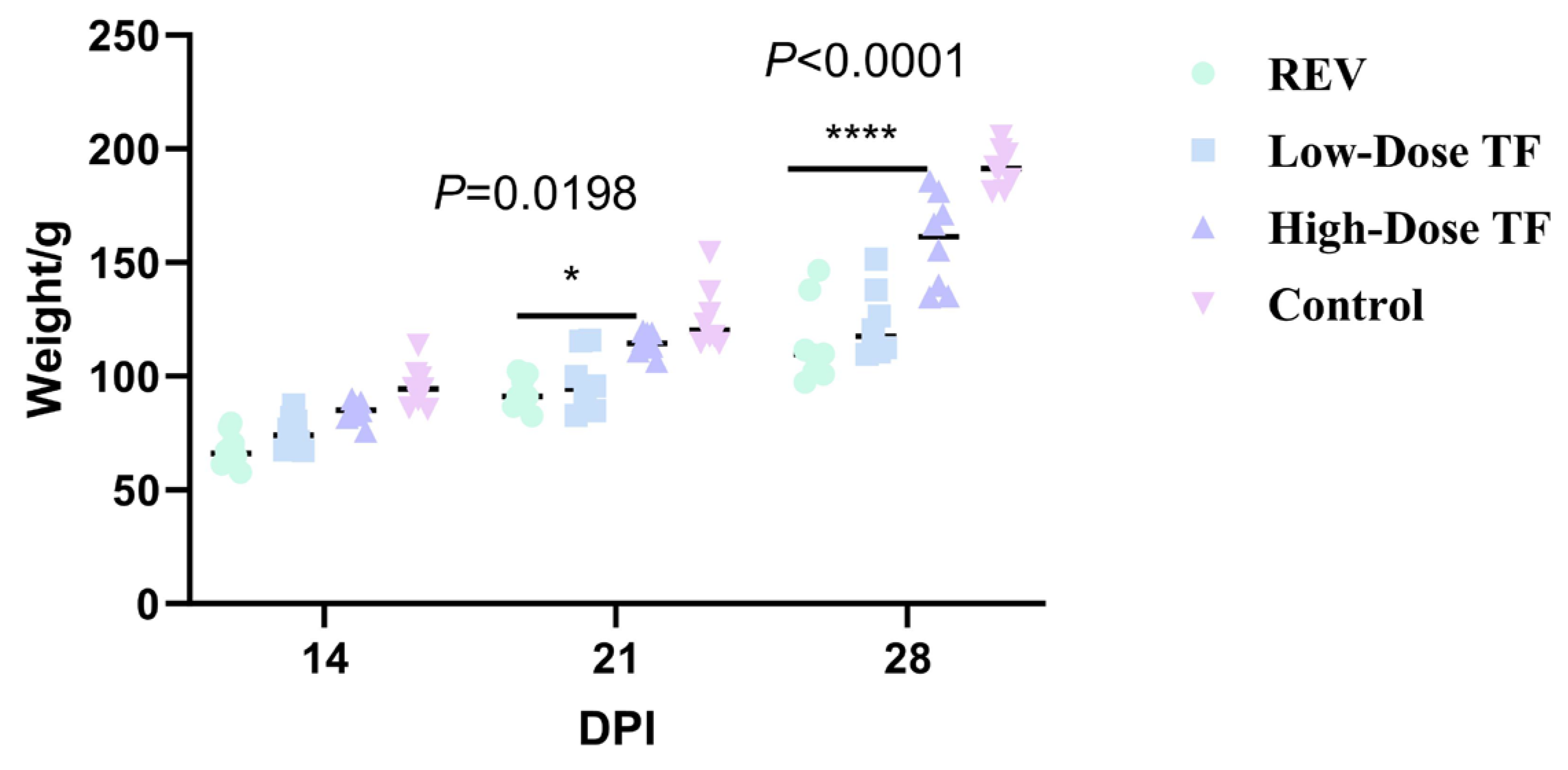
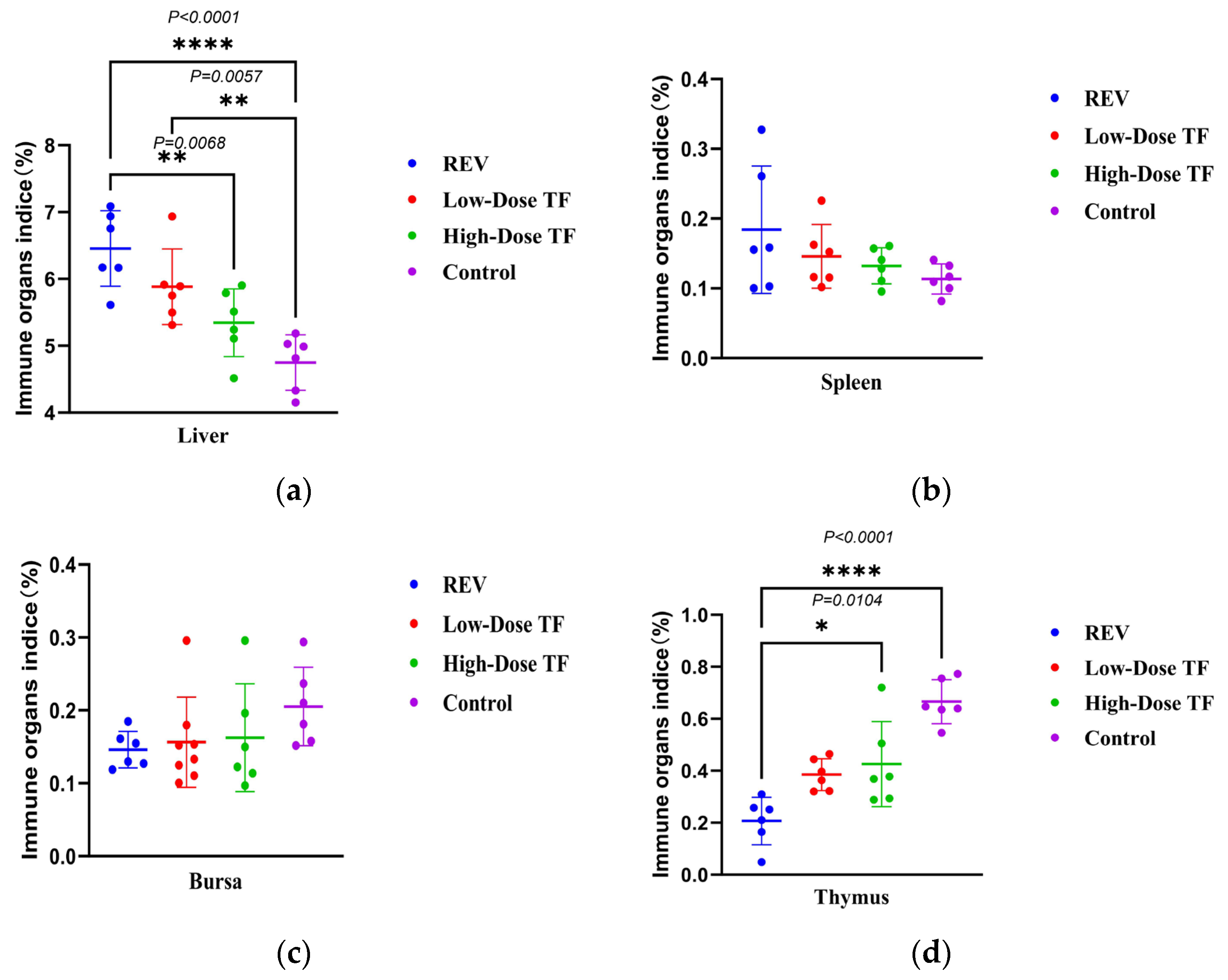
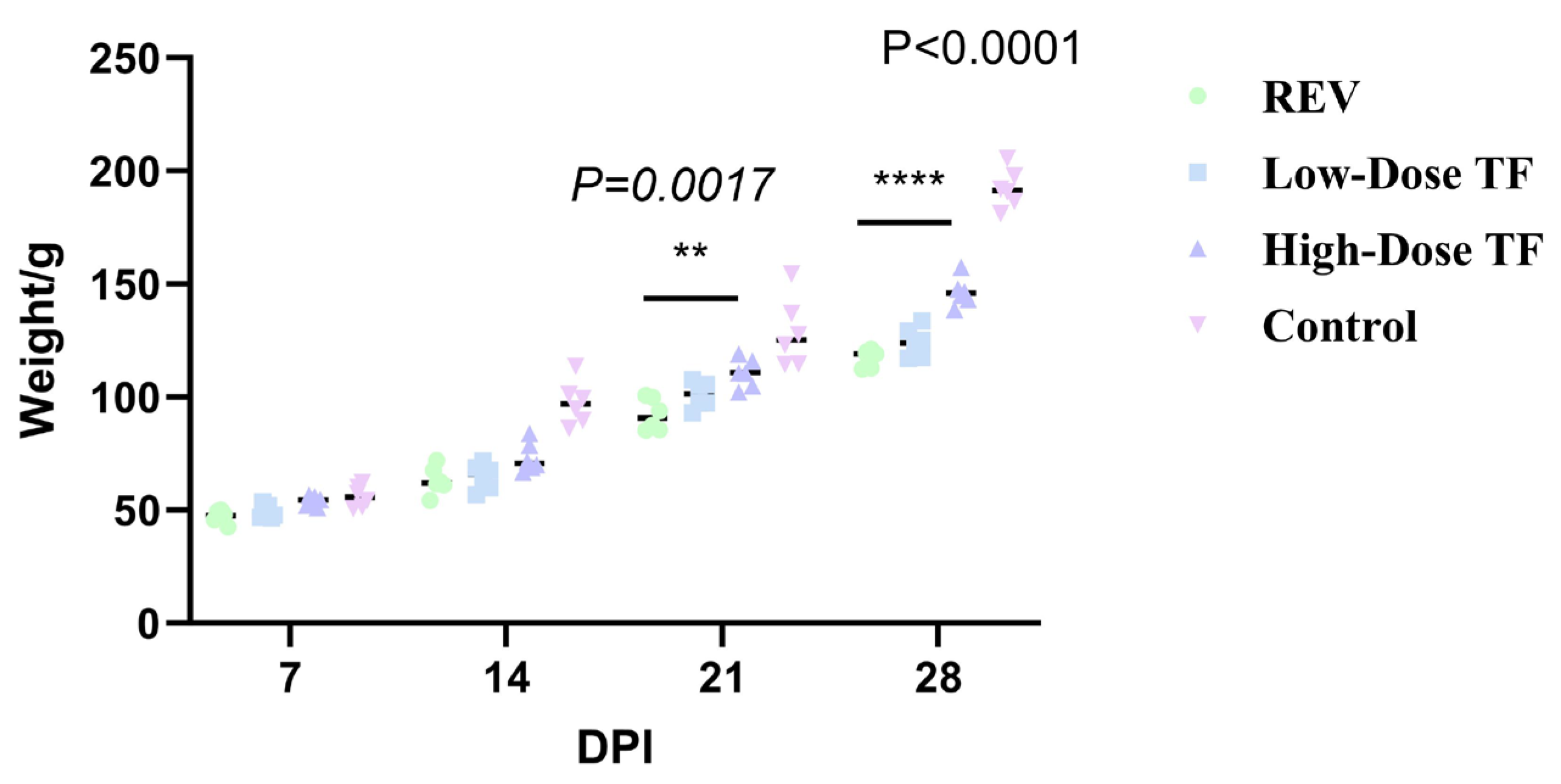
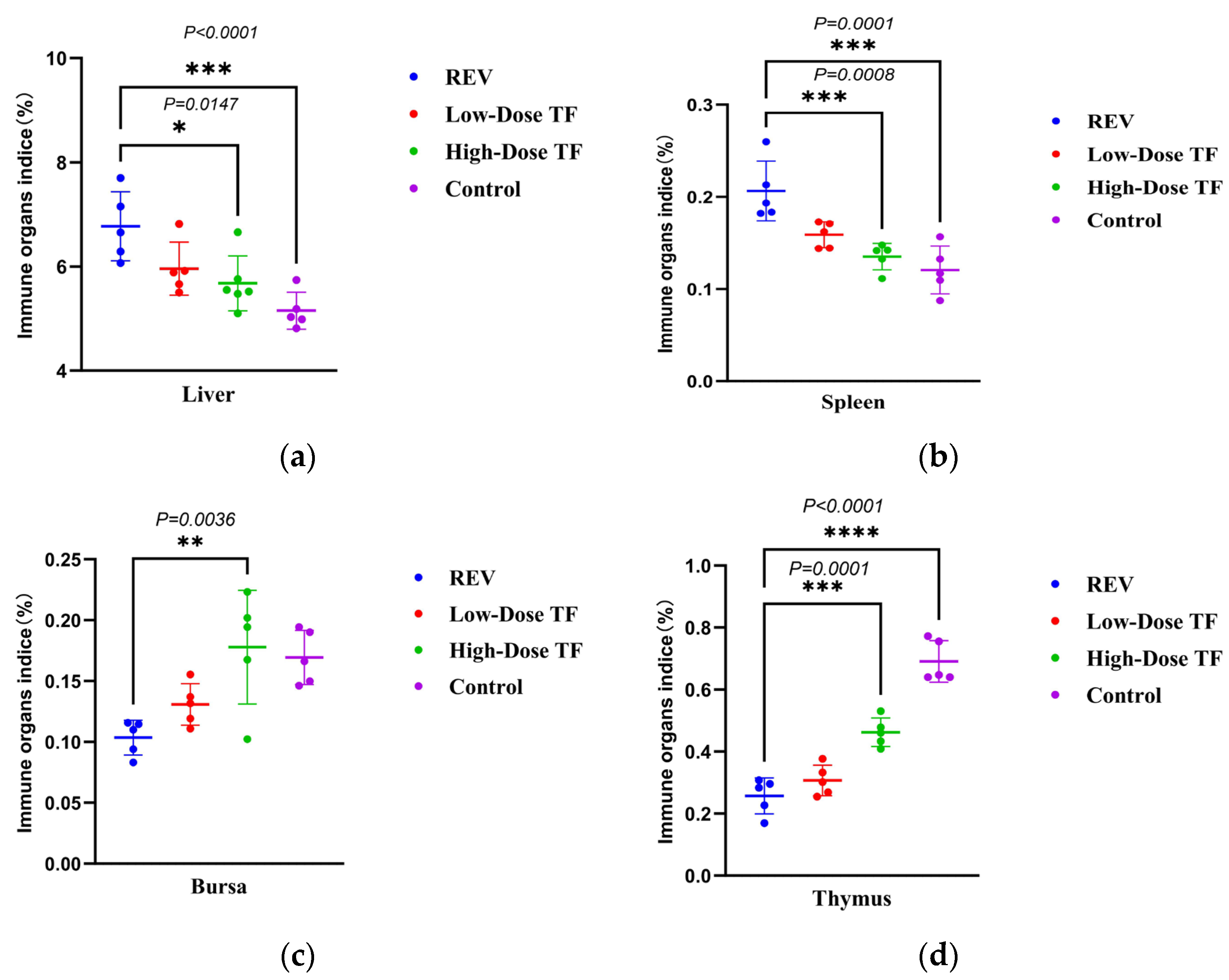
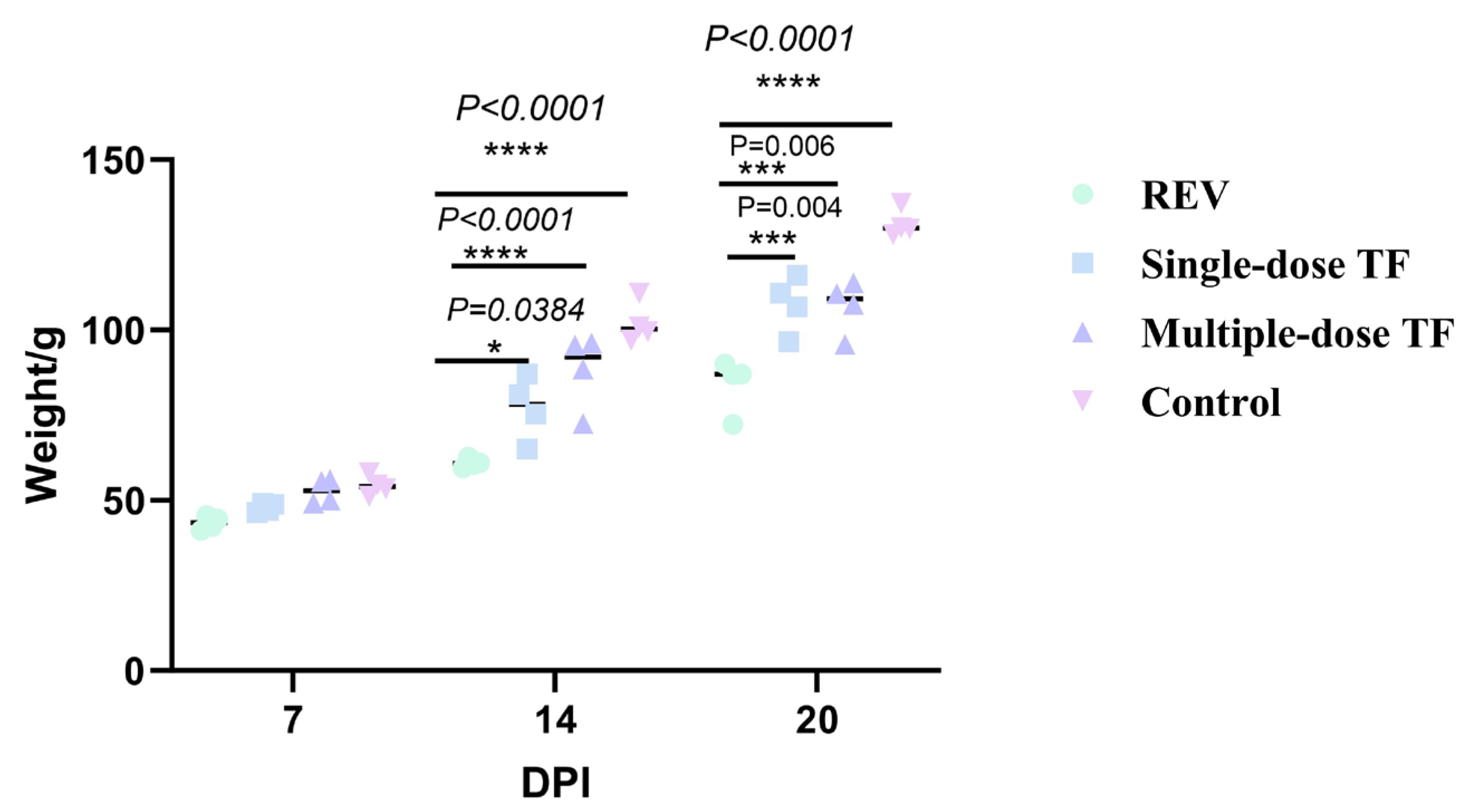
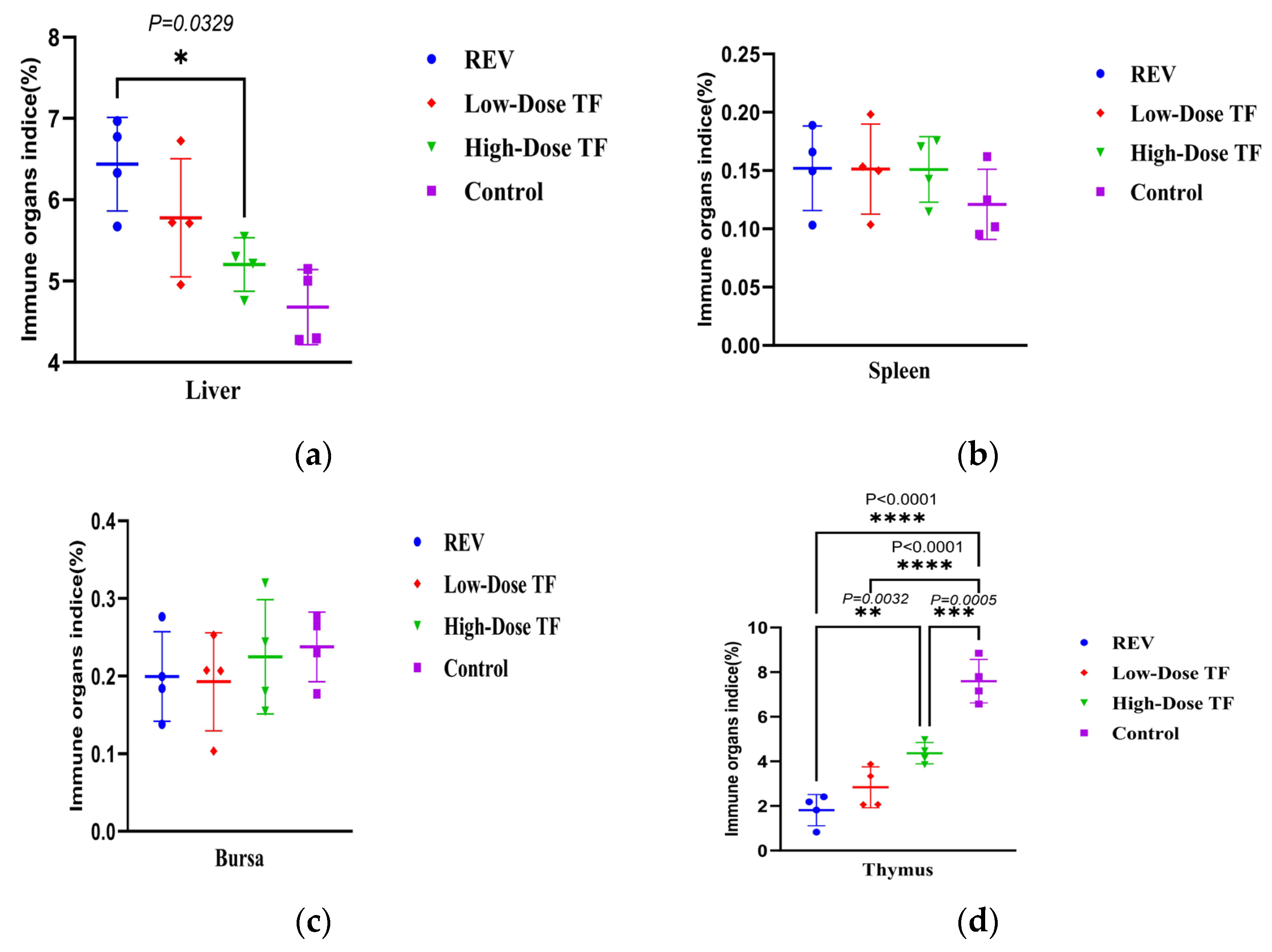
3.1. Preventive Effect of TF on REV Infection
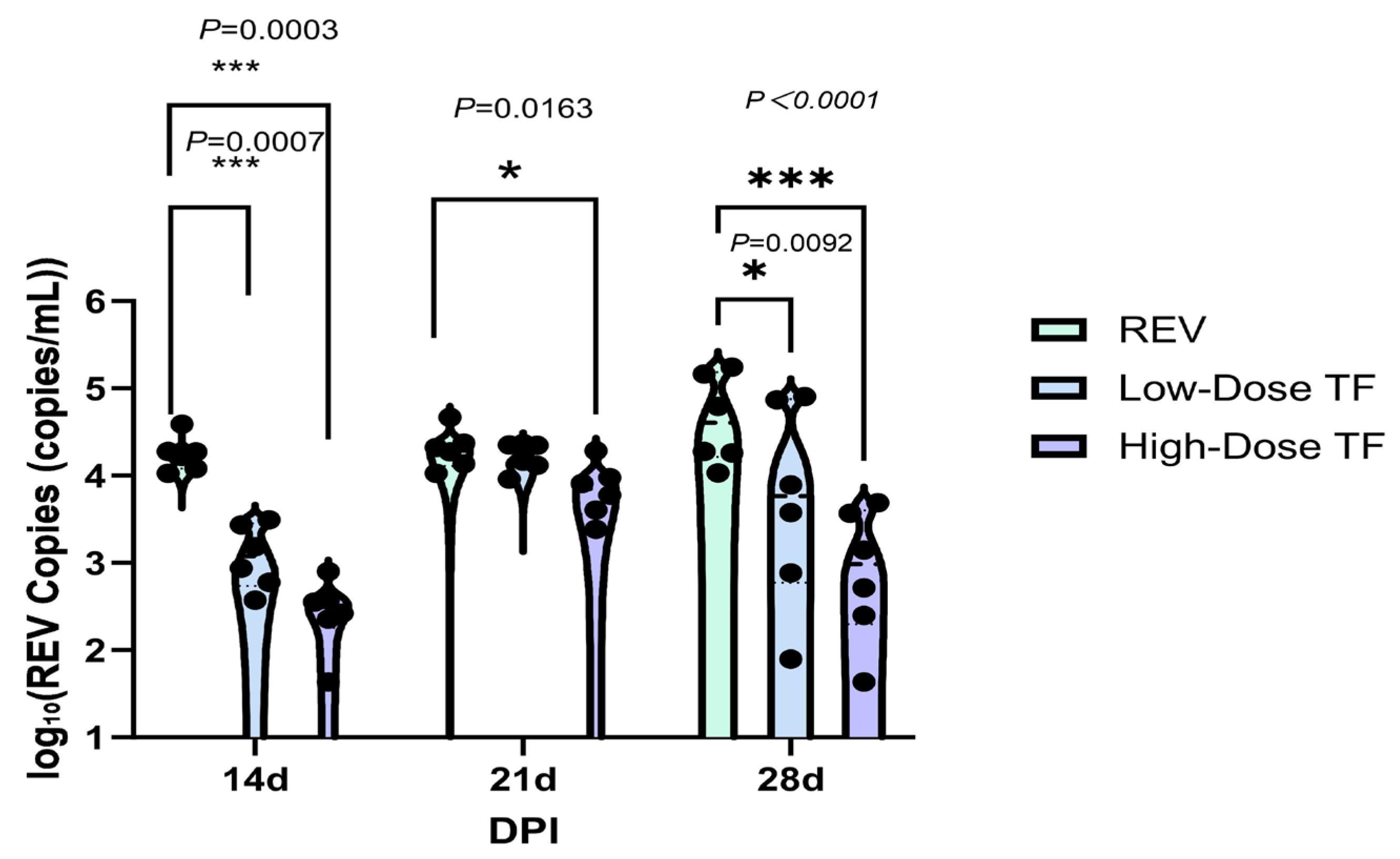
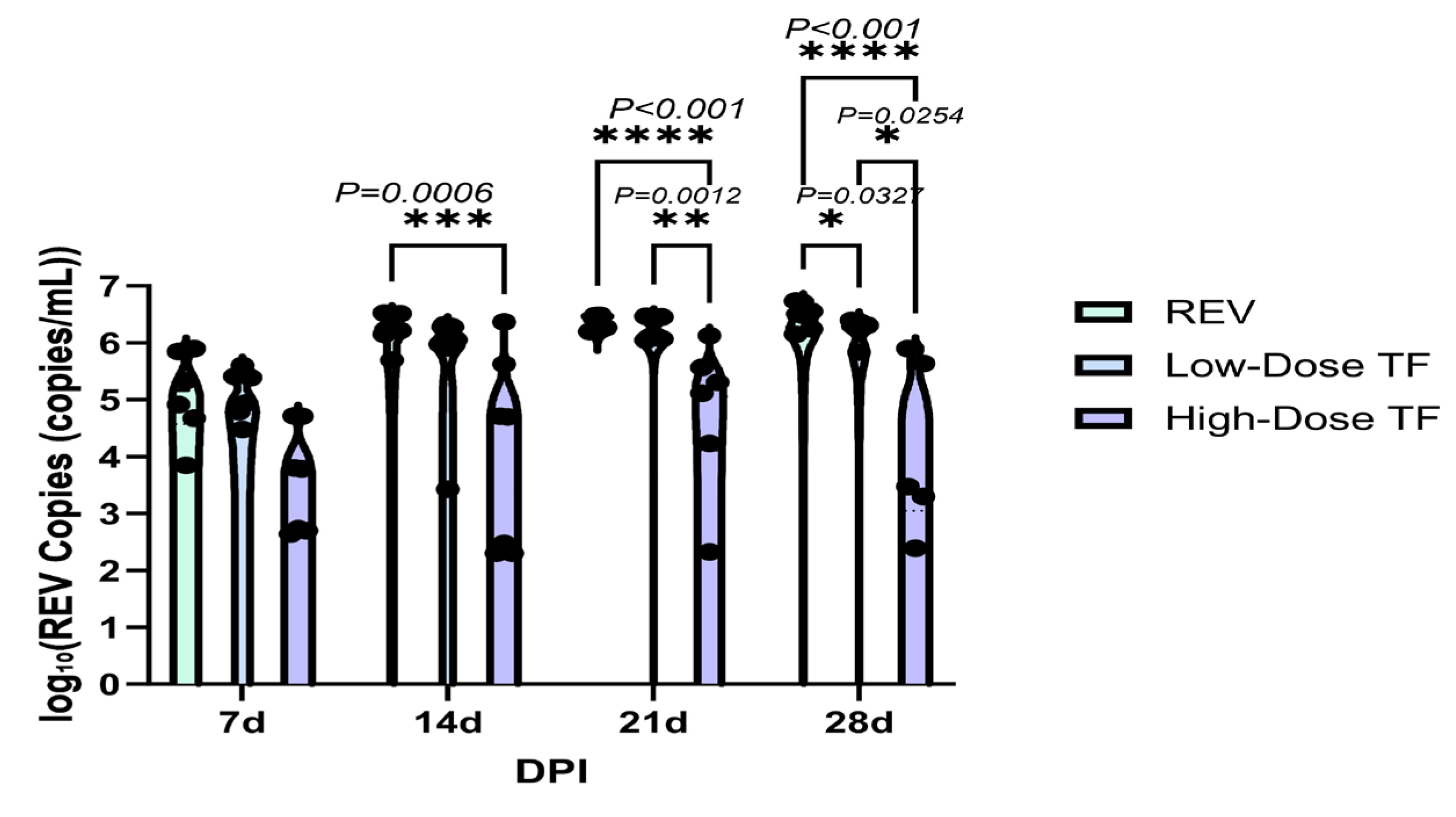
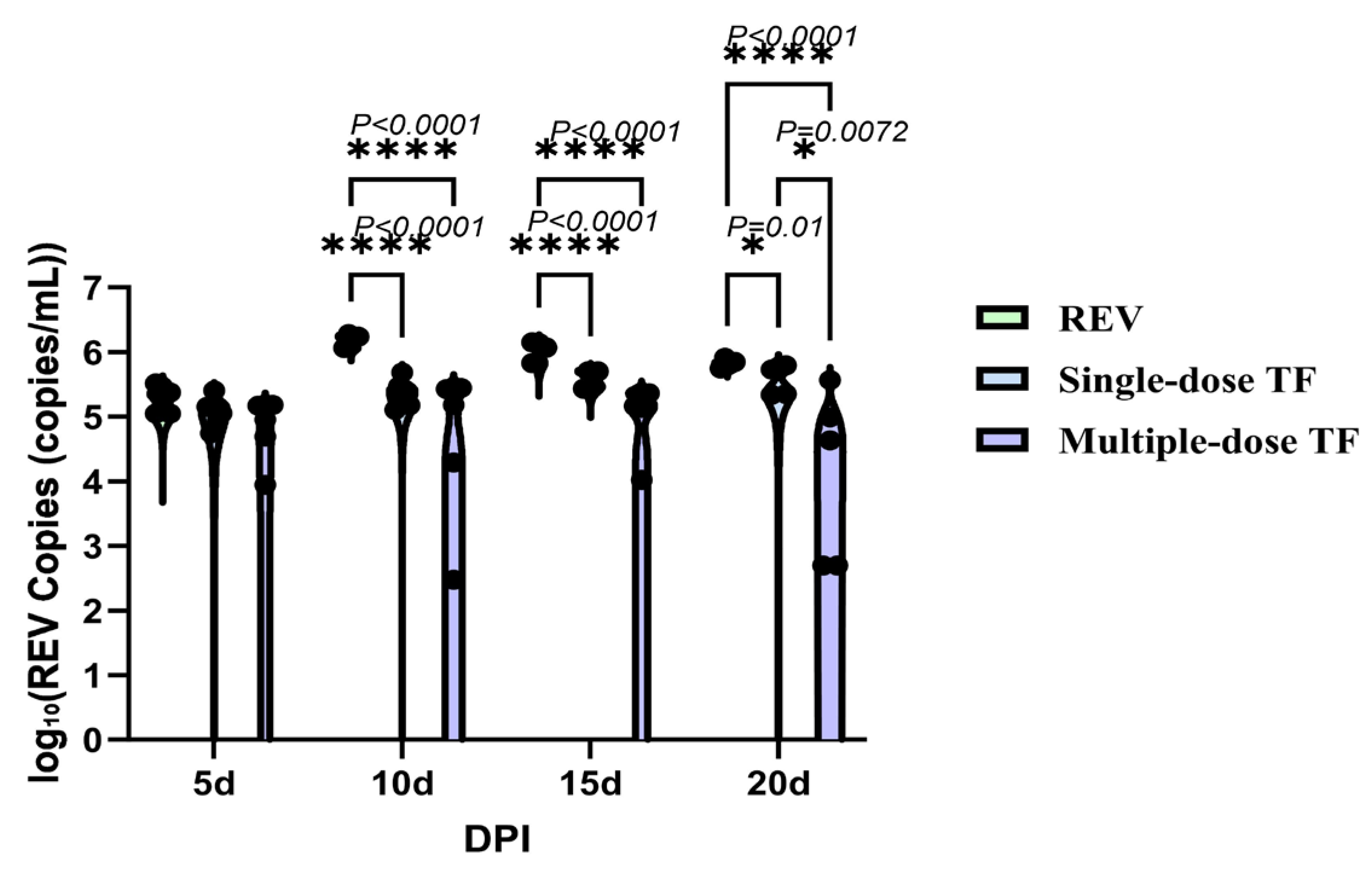
3.2. Inhibitory Effect of TF on REV Horizontal Transmission
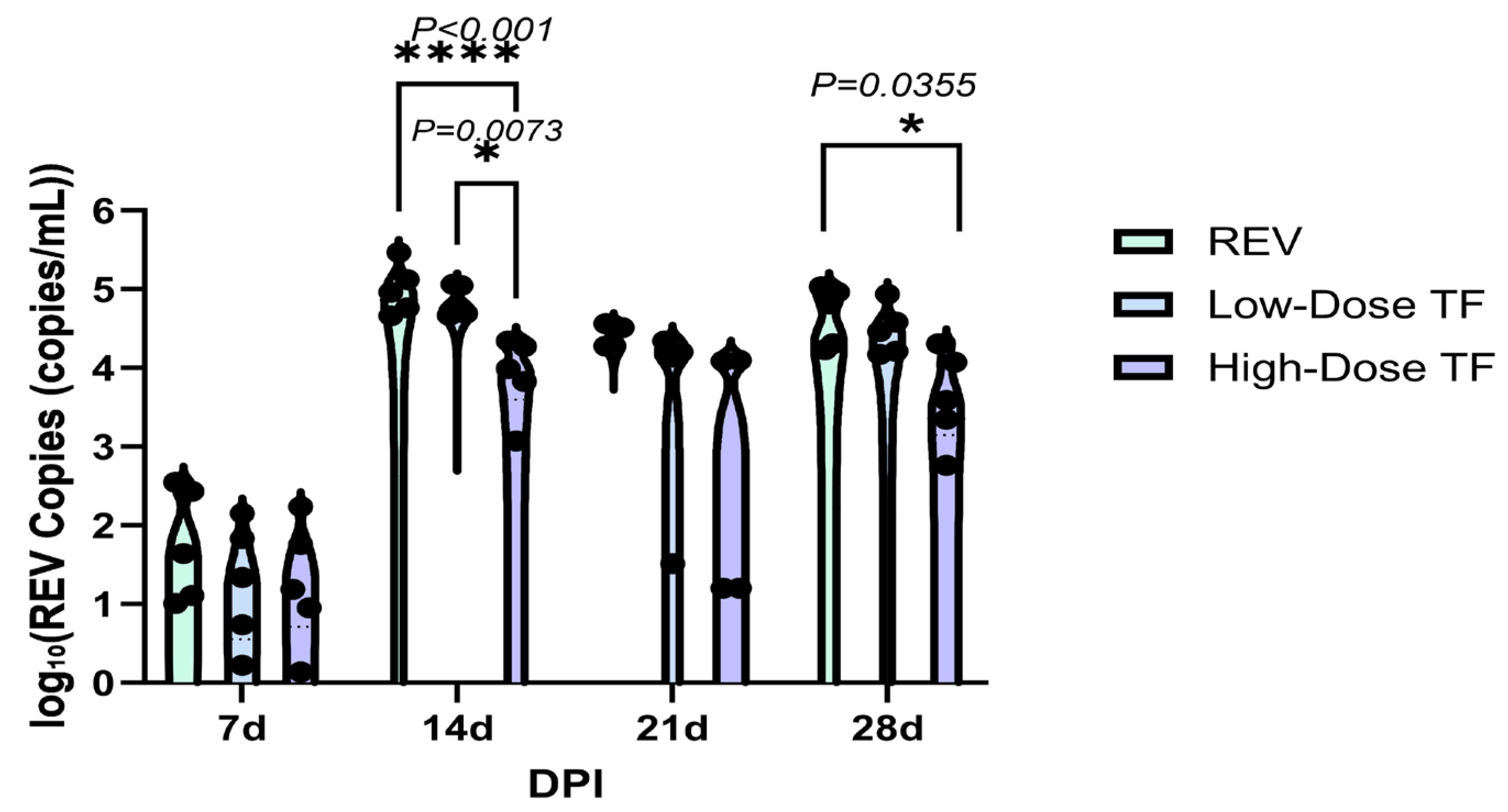
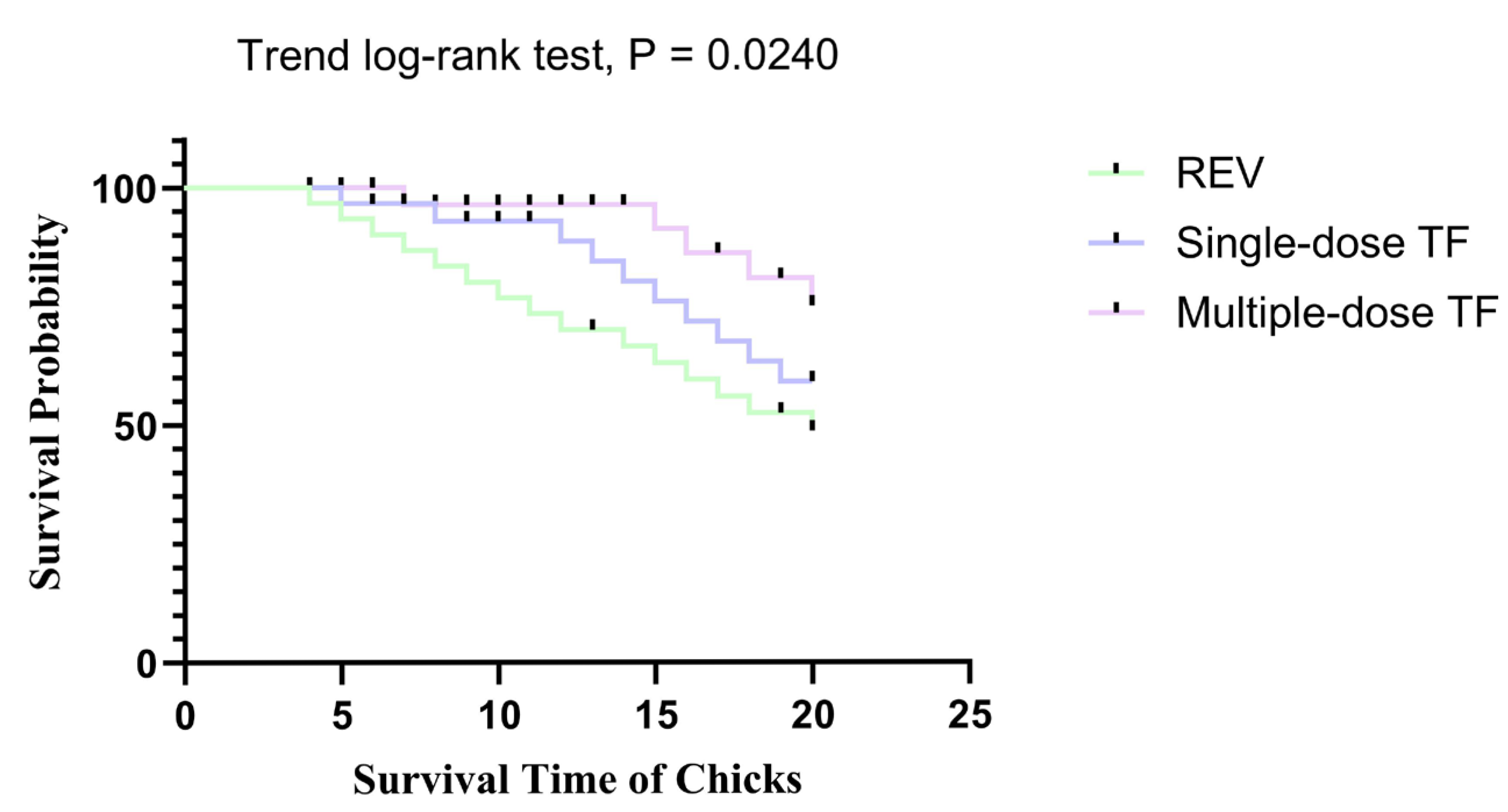
3.3. Inhibitory Effect of TF on REV Congenital Infection via Embryonic Yolk Sac Inoculation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| REV | Reticuloendotheliosis Virus |
| TF | Transfer Factor |
| RE | Reticuloendotheliosis |
| SPF | Specific Pathogen-Free |
| TCID50 | Tissue Culture Infectious Dose 50 |
| PCR | Polymerase Chain Reaction |
| LOD | Limit of Detection |
| LOQ | Limit of Quantitation |
References
- Zhai, S.-L.; Chen, S.-N.; Lin, T.; Wen, X.-H.; Wei, W.-K.; Lv, D.-H.; Chen, R.-A. Emergence of reticuloendotheliosis virus in pigeons in Guangdong Province, Southern China. Arch. Virol. 2016, 161, 2007–2011. [Google Scholar] [CrossRef]
- Al-Sarraf, M.; Baker, L.H. Transfer factor. Cancer Treat. Rev. 1979, 6, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, H.S.; Borkowsky, W. Transfer factor—Current status and future prospects. Biotherapy 1996, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Viza, D.; Fudenberg, H.H.; Palareti, A.; Pizza, G. Transfer factor: An overlooked potential for the prevention and treatment of infectious diseases. Folia Biol. 2013, 59, 53–67. [Google Scholar] [CrossRef]
- Macias, A.E.; Guaní-Guerra, E. Transfer Factor: Myths and Facts. Arch. Med. Res. 2020, 51, 613–622. [Google Scholar] [CrossRef]
- Wang, J.W.; Jia, S.Z.; Wang, S.C.; Sun, Y.P.; Xu, K. Effect of chicken spleen-specific transfer factor on immune function of chicks. J. Laiyang Agric. Coll. 1995, 2, 148–151. [Google Scholar]
- Qiao, H.-X.; Zhang, X.-G.; Ren, M.; Ning, Y.-C.; Bai, J. Preparation of chicken spleen-derived transfer factor and its effect on immune response in chicks. J. Gansu Agric. Univ. 2012, 47, 13–16. (In Chinese) [Google Scholar]
- Guo, X.L.; Meng, Q.J.; Li, K.Q.; Ma, Y.J.; Chen, X.Y. Preparation and Study on Some Properties of Transfer Factor from Normal Pigs. Chin. J. Vet. Sci. Technol. 1986, 16, 15–18. (In Chinese) [Google Scholar]
- Ma, J.; Ma, B.; Wang, Z.; Li, X.; Zhang, H. Effect of splenic transfer factor on the development of intestinal mucosal barrier in laying hens. J. Sci. Food Agric. 2023, 103, 1342–1354. [Google Scholar] [CrossRef]
- Xu, L.; Yu, X.X.; Liao, H.Z.; Liu, Y.F.; Zhong, Y.Q.; Jiang, L.; Xie, Z.J.; Yan, L.P.; Zeng, L.M.; Du, J.; et al. Protective effect of transfer factor combined with La Sota strain immunization against repeated infection of NDV F48E9 virulent strain in chickens. Chin. J. Vet. Sci. 2022, 42, 2374–2384. (In Chinese) [Google Scholar]
- Wu, W.J.; Lv, X.P.; Wang, X.Y.; Li, X.; Liu, X. Effects of Reticuloendotheliosis virus on TLR-3/IFN-Β pathway in specific pathogen-free chickens. Res. Vet. Sci. 2023, 156, 36–44. [Google Scholar] [CrossRef]
- Fu, L.; Wang, X.; Zhai, J.; Li, X.; Liu, X. Changes in apoptosis, proliferation and T lymphocyte subtype on thymic cells of SPF chickens infected with reticuloendotheliosis virus. Mol. Immunol. 2019, 111, 87–94. [Google Scholar] [CrossRef] [PubMed]
- GB/T 14233.2-2005; Test Methods for Medical Infusion, Transfusion, and Injection Devices—Part 2: Biological Test Methods. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of China, China Standards Press: Beijing, China, 2005.
- Fadly, A.M. Avian retroviruses. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.N.; Venugopal, K. Neoplastic diseases: Marek’s disease, avian leukosis and reticuloendotheliosis. Rev. Sci. Tech. 2000, 19, 544–564. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Sun, S.; Zhang, Z.; Li, X.; Liu, X. Simultaneous endemic infections with subgroup J avian leukosis virus and reticuloendotheliosis virus in commercial and local breeds of chickens. Avian Pathol. 2009, 38, 443–448. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, P.; Chang, S.; Ju, S.; Wang, Y. Synergistic pathogenic effects of co-infection of subgroup J avian leukosis virus and reticuloendotheliosis virus in broiler chickens. Avian Pathol. 2015, 44, 43–49. [Google Scholar] [CrossRef]
- Meng, F.; Li, Q.; Zhang, Y.; Wang, X.; Li, J. Characterization of subgroup J avian Leukosis virus isolated from Chinese indigenous chickens. Virol. J. 2018, 15, 33. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Duan, Y.; Wang, X.; Li, X. Current knowledge on the epidemiology and prevention of Avian leukosis virus in China. Poult. Sci. 2024, 103, 104009. [Google Scholar] [CrossRef]
- Xu, F.X. Pathogenicity of Reticuloendotheliosis Virus (REV) via Different Infection Routes in Specific Pathogen-Free (SPF) Chickens and the Inhibitory Effect of Type I Interferon on REV In Vivo and In Vitro. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2023. [Google Scholar]
- Viza, D.; Pizza, G.; De Vinci, C.; Fudenberg, H.H. Transfer Factor as an Option for Managing the COVID-19 Pandemic. Folia Biol. 2020, 66, 86–90. [Google Scholar] [CrossRef]
- Habar, T.R.; Islam, A.A.; Hatta, M.; Setiawan, A. Transfer factor treatment in management of peritonitis condition: An experimental study in rat. Ann. Med. Surg. 2021, 69, 102755. [Google Scholar] [CrossRef]
- Bi, Y.; Xu, L.; Qiu, L.; Zhang, Y.; Li, J. Reticuloendotheliosis Virus Inhibits the Immune Response Acting on Lymphocytes from Peripheral Blood of Chicken. Front. Physiol. 2018, 9, 4. [Google Scholar] [CrossRef]
- Zhou, L.; Zheng, S.J. The Roles of MicroRNAs (miRNAs) in Avian Response to Viral Infection and Pathogenesis of Avian Immunosuppressive Diseases. Int. J. Mol. Sci. 2019, 20, 54–57. [Google Scholar] [CrossRef]
- Thapa, P.; Farber, D.L. The Role of the Thymus in the Immune Response. Thorac. Surg. Clin. 2019, 29, 123–131. [Google Scholar] [CrossRef]
- Shichkin, V.P.; Antica, M. Key Factors for Thymic Function and Development. Front. Immunol. 2022, 13, 926516. [Google Scholar] [CrossRef]
- Li, Y.R.; Zúñiga-Pflücker, J.C. Thymus aging and immune reconstitution, progresses and challenges. Semin. Immunol. 2023, 70, 101837. [Google Scholar] [CrossRef]
- Miao, Y.T.; Sun, Y.G.; Wang, Y.X.; Chang, S.; Ren, Z.H.; Zhao, P. Study on the effect of transfer factor in reducing the pathogenicity of chicken infectious anemia virus infection to SPF chickens. Chin. J. Poult. 2025, 47, 48–54. (In Chinese) [Google Scholar]


| Day of Age | REV-Infected Control Group | Low-Dose TF Group | High-Dose TF Group | Blank Control Group | ||||
|---|---|---|---|---|---|---|---|---|
| Plasma | Cloacal Cavity | Plasma | Cloacal Cavity | Plasma | Cloacal Cavity | Plasma | Cloacal Cavity | |
| 14 d | 8/8 (100%) | 6/8 (75%) | 8/8 (100%) | 6/8 (75%) | 8/8 (100%) | 4/8 (50%) | 0/8 (0%) | 0/8 (0%) |
| 21 d | 7/7 (100%) | 7/7 (100%) | 5/6 (83%) | 7/7 (100%) | 7/7 (100%) | 6/7 (85%) | 0/8 (0%) | 0/8 (0%) |
| 28 d | 6/6 (100%) | 7/7 (100%) | 6/6 (100%) | 6/7 (85%) | 3/6 (50%) | 4/7 (57%) | 0/8 (0%) | 0/8 (0%) |
| Day of Age | REV-Infected Control Group | Low-Dose TF Group | High-Dose TF Group | Blank Control Group | ||||
|---|---|---|---|---|---|---|---|---|
| Plasma | Cloacal Cavity | Plasma | Cloacal Cavity | Plasma | Cloacal Cavity | Plasma | Cloacal Cavity | |
| 7 d | 8/8 (100%) | 2/8 (25%) | 7/8 (88%) | 0/8 (0%) | 6/8 (75%) | 0/8 (0%) | 0/6 (0%) | 0/8 (0%) |
| 14 d | 8/8 (100%) | 8/8 (100%) | 7/8 (88%) | 8/8 (100%) | 4/8 (50%) | 8/8 (100%) | 0/6 (0%) | 0/8 (0%) |
| 21 d | 8/8 (100%) | 8/8 (100%) | 8/8 (100%) | 7/8 (88%) | 6/8 (75%) | 7/8 (88%) | 0/6 (0%) | 0/8 (0%) |
| 28 d | 8/8 (100%) | 8/8 (100%) | 8/8 (100%) | 8/8 (100%) | 7/8 (88%) | 6/8 (75%) | 0/6 (0%) | 0/8 (0%) |
| Dayof Age | REV-Infected Control Group | Single-Dose TF Group | Multiple-Dose TF Group | Blank Control Group | ||||
|---|---|---|---|---|---|---|---|---|
| Plasma | Cloacal Cavity | Plasma | Cloacal Cavity | Plasma | Cloacal Cavity | Plasma | Cloacal Cavity | |
| 5 d | 6/6 (100%) | 5/6 (83%) | 6/6 (100%) | 3/6 (50%) | 6/6 (100%) | 2/6 (33%) | 0/6 (0%) | 0/6 (0%) |
| 10 d | 4/4 (100%) | 4/4 (100%) | 6/6 (100%) | 4/6 (100%) | 5/6 (83%) | 2/6 (33%) | 0/6 (0%) | 0/6 (0%) |
| 15 d | 3/3 (100%) | 3/3 (100%) | 4/4 (100%) | 4/4 (100%) | 5/5 (100%) | 5/5 (100%) | 0/6 (0%) | 0/6 (0%) |
| 20 d | 3/3 (100%) | 3/3 (100%) | 4/4 (100%) | 4/4 (100%) | 3/5 (60%) | 3/5 (60%) | 0/6 (0%) | 0/6 (0%) |
| Group | Median Survival Time (Days) | 95% CI | Average Survival Period (Days) | 95% CI |
|---|---|---|---|---|
| REV | 12 | 6.9–17.1 | 12.3 | 9.8–14.9 |
| Single-dose TF | 15 | 11.9–18.1 | 14.7 | 12.5–16.9 |
| Multiple-dose TF | 18 | 14.0–22.0 | 17.3 | 14.9–19.6 |
| Group | B | SE | Wald | df | Sig. | Exp(B) |
|---|---|---|---|---|---|---|
| REV | 4.640 | 2 | 0.098 | |||
| Single-dose TF | 1.099 | 0.516 | 4.526 | 1 | 0.033 | 3.000 |
| Multiple-dose TF | 0.693 | 0.548 | 1.602 | 1 | 0.206 | 2.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Song, M.; Wu, Q.; Ren, Z.; Cui, W.; Wang, Y.; Chang, S.; Zhao, P. Inhibitory Effect of Transfer Factor on Avian Reticuloendotheliosis Virus Infection in Chicks. Vet. Sci. 2025, 12, 1041. https://doi.org/10.3390/vetsci12111041
Wang X, Song M, Wu Q, Ren Z, Cui W, Wang Y, Chang S, Zhao P. Inhibitory Effect of Transfer Factor on Avian Reticuloendotheliosis Virus Infection in Chicks. Veterinary Sciences. 2025; 12(11):1041. https://doi.org/10.3390/vetsci12111041
Chicago/Turabian StyleWang, Xinli, Mengyu Song, Qingyue Wu, Zhihao Ren, Wenping Cui, Yixin Wang, Shuang Chang, and Peng Zhao. 2025. "Inhibitory Effect of Transfer Factor on Avian Reticuloendotheliosis Virus Infection in Chicks" Veterinary Sciences 12, no. 11: 1041. https://doi.org/10.3390/vetsci12111041
APA StyleWang, X., Song, M., Wu, Q., Ren, Z., Cui, W., Wang, Y., Chang, S., & Zhao, P. (2025). Inhibitory Effect of Transfer Factor on Avian Reticuloendotheliosis Virus Infection in Chicks. Veterinary Sciences, 12(11), 1041. https://doi.org/10.3390/vetsci12111041







