Performance Comparison of the Prediction Models for Enteric Methane Emissions from Dairy Cattle
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Development of the Database
2.2. Selecting the Existing Models for Predicting CH4 Emission of Dairy Cattle
2.3. Model Evaluation Method
2.3.1. Mean Square Prediction Error
2.3.2. Consistency Correlation Coefficient (CCC)
2.3.3. Coefficient of Determination (R2)
2.3.4. RMSPE to Standard Deviation of Observed Values Ratio (RSR)
3. Results
3.1. Variable Summary Statistics of the Database
3.2. Comparison of CH4 Prediction Model Performance
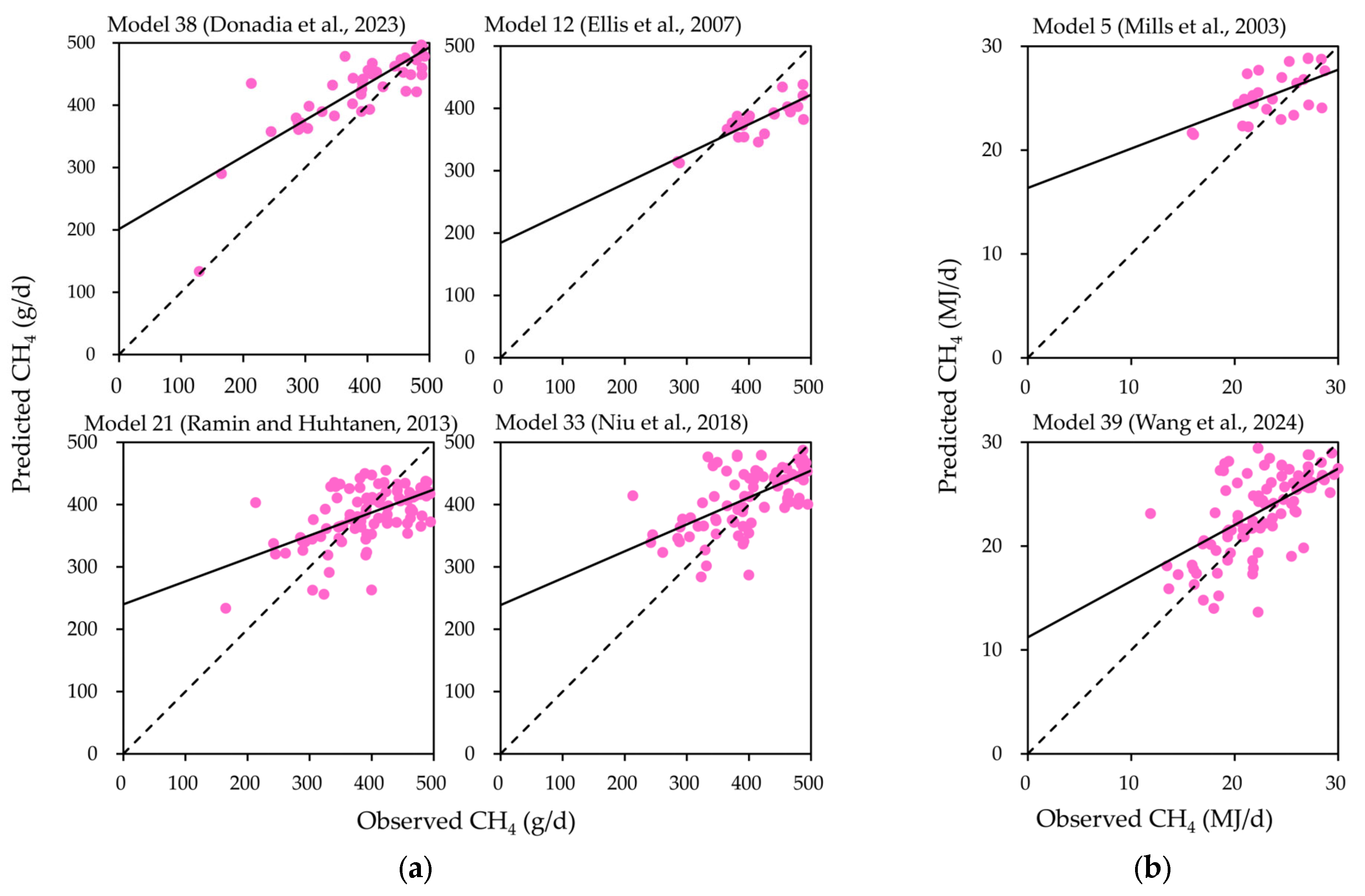
3.3. Model Regression and Residual Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Study | Author | Nation | Species | Method To Measure CH4 | Experimental Design | C:F |
|---|---|---|---|---|---|---|
| [57] | Dong et al. (2022) | China | Holstein dairy cows | SF6-T | a randomized complete block design | 48:52, 43:57, 38:62 |
| [63] | Olijhoek et al. (2022) | Denmark | Holstein and Jersey dairy cows | RC | allocated randomly | 49:51, 70:30, 91:9 |
| [66] | Giagnoni et al. (2024) | Denmark | Holstein cows | RC | a crossover design | 45:55 |
| [67] | van Gastelen et al. (2024) | Netherlands | Holstein-Friesian dairy cows | GFS | a randomized complete block design | 40:60 |
| [68] | van Gastelen et al. (2024) | Netherlands | Holstein-Friesian cows | GFS | a randomized complete block design | 39:61 |
| [69] | Räisänen et al. (2024) | Finland | Nordic Red cows | GFS | a switch-back experiment | 49:51, 48:52 |
| [70] | Martins et al. (2024) | USA | Holstein cows | GFS | a replicated 3 × 3 Latin square design | 39:61 |
| [71] | Thorsteinsson et al. (2024) | Denmark | Holstein dairy cows | RC | a complete 3 × 3 Latin square design | 40:60 |
| [72] | Redoy et al. (2025) | USA | Holstein cows | GFS | randomly assigned with a 2 × 2 factorial arrangement | 35:65, 48:52 |
| [73] | Akter et al. (2025) | USA | Holstein dairy cows | GFS | a 3 × 3 Latin square design | 61:39 |
| [74] | Colin et al. (2024) | USA | Jersey cows | RC | a replicated 3 × 3 Latin squares design | 80:20 |
| [75] | Ahvenjärvi et al. (2024) | Finland | Nordic Red dairy cows | RC | a replicated change-over study | 30:70, 50:50 |
| [76] | Molina-Botero et al. (2024) | Colombia | Jersey dairy | RC | a double change-over design | Not supplied |
| Jersey × Holstein cows | RC | a double change-over design | Not supplied | |||
| [77] | Zhou et al. (2024) | China | Holstein cows | SF6-T | a randomized complete block design | 55:45 |
| [78] | Dittmann et al. (2024) | Switzerland | Holstein dairy cows | RC | a cross-over experiment | 30:70 |
| [79] | Kabsuk et al. (2024) | Thailand | Holstein-Friesian Thai native crossbreeds | RC | a randomized complete block design | 90:10 |
| [80] | Ruchita et al. (2023) | Finland | Nordic Red cows | RC | a complete randomized block design | 50:50 |
| [81] | Starsmore et al. (2023) | Ireland | Holstein-Friesian and Holstein-Friesian × Jersey crossbred cows | SF6-T | a complete randomized block design | 0:100 |
| [82] | Peng et al. (2023) | China | Holstein cows | GFS | randomly assigned | 57:43 |
| [83] | Reyes et al. (2023) | USA | Holstein and Jersey cows | GFS | a randomized complete block design | 33:67 |
| [84] | Fresco et al. (2023) | France | Holstein cows | GFS | observational data collection under controlled management | 12:88 |
| [85] | Lifeng et al. (2023) | China | Jersey dairy cows | GFS | randomly assigned | 39:61 |
| [86] | Noe et al. (2023) | México | Bos taurus × Bos indicus cows | SNI | a 4 × 4 Latin Square design | 89:11 |
| [87] | Chaouki et al. (2023) | Canada | Holstein cow | RC | a replicated 3 ×3 Latin square design | 37:63 |
| Ayrshire cow | RC | a replicated 3 ×3 Latin square design | 42:58, 41:59 | |||
| [88] | Muizelaar et al. (2023) | Netherlands | Holstein-Friesian dairy cows | GFS | a randomized complete block design | 25:75 |
| [89] | Lazzari et al. (2023) | Switzerland | Swiss Holstein-Friesian cows | GFS | a 3 × 6 incomplete Latin square design | 21:79 |
| [90] | Rebelo et al. (2023) | Wooster | Holstein cows | GFS | a replicated 3 × 3 Latin square design | 47:53 |
| [91] | Niu et al. (2023) | Norway | Brown Swiss dairy cows | RC | a randomized cyclic change-over design | 9:91 |
| [92] | Thorsteinsson et al. (2023) | Denmark | Holstein dairy cows | RC | a 4 × 4 Latin square design | 39:61 |
| [93] | Mirka et al. (2023) | Denmark | Holstein dairy cows | RC | a 4 × 4 Latin square design | 47:53 |
| [94] | Silvestre et al. (2023) | USA | Holstein cows | GFS | a randomized complete block design | 42:58 |
| [95] | Almeida et al. (2023) | USA | Jersey cows | SF6-T | a replicated 4 × 4 Latin square design | 37:63 |
| [96] | Bach et al. (2023) | Spain | Holstein cows | SF6-T | a complete randomized design | 60:40 |
| [97] | Williams et al. (2023) | Australia | Holstein-Friesian cows | SF6-T | randomly distributed | 28:72 |
| [98] | Khan et al. (2022) | Norway | Holstein cows | RC | a 3 × 3 Latin square design | 57:43 |
| [99] | Della Rosa et al. (2022) | New Zealand | Holstein × Jersey dairy cows | RC | randomly assigned | 0:100 |
| [100] | Silvestre et al. (2022) | USA | Holstein cows | GFS | a replicated 4 × 4 Latin square design | 42:58 |
| [101] | Florencia et al. (2022) | Argentina | Holstein Friesian cows | SF6-T | randomly distributed | 53:47, 52:48 |
| [102] | Peng et al. (2022) | China | Holstein cows | GFS | randomly distributed | 57:43 |
| [103] | Daniel et al. (2022) | Kenya | Friesian × Boran cows | RC | a 3 × 3 Latin square design | 13:87 |
| [104] | Li et al. (2021) | China | Holstein dairy cows | SF6-T | a randomized complete design | 58:42 |
| [105] | Bayat et al. (2021) | Finland | Nordic Red dairy cows | RC | a replicated 4 × 4 Latin square design | 54:45 |
| [106] | Civiero et al. (2021) | Brazil | Holstein and Jersey × Holstein cows | SF6-T | a replicated 3 × 3 Latin square design | 40:60 |
| [107] | Stefenoni et al. (2021) | USA | Holstein cows | GFS | a replicated 4 × 4 Latin square design | 40:60 |
| [108] | Hassanat and Benchaar (2021) | Canada | Holstein cows | RC | a replicated 4 × 4 Latin square design | 39:61 |
| [109] | Ramin et al. (2021) | Sweden | Nordic Red dairy cows | GFS | a replicated 4 × 4 Latin square design | 42:58 |
| [110] | Benchaar et al. (2021) | Canada | Holstein cows | RC | a replicated 4 × 4 Latin square design | 48:52 |
| [111] | Cueva et al. (2021) | USA | Holstein cows | GFS | a randomized complete block design | 41:59 |
| [112] | Fant et al. (2021) | Sweden | Nordic Red dairy cows | GFS | a replicated 4 × 4 Latin square design | 40:60 |
| [113] | Darabighane et al. (2021) | Finland | Nordic Red cows | SF6-T | a 4 × 4 Latin square design | 45:55 |
| [114] | Schilde et al. (2021) | Germany | German Holstein cows | GFS | randomly assigned with a 2 × 2 factorial design | 15:85, 40:60 |
| [115] | Melgar et al. (2021) | USA | Holstein cows | GFS | a randomized complete block design | 40:60 |
| [116] | Børsting et al. (2020) | Denmark | Holstein dairy cows | RC | a 4 × 4 Latin square design | 51:49 |
| [117] | Moate et al. (2020) | Australia | Holstein-Friesian cows | SF6-T | randomly assigned | 11:89 |
| [118] | Melgar et al. (2020) | USA | Holstein dairy cows | GFS | a randomized complete block design | 40:60 |
| [119] | van Gastelen et al. (2020) | Netherlands | Holstein-Friesian cows | RC | a completely randomized block design | 40:60 |
| [120] | Williams et al. (2020) | Australia | Holstein-Friesian cows | RC | a double Latin square crossover design | 24:76 |
| [121] | Moate et al. (2020) | Australia | Holstein Friesian cows | SF6-T | randomly assigned | 28:72 |
| [122] | Mekuriaw et al. (2020) | Japan | Fogera dairy cows | DMI-Est | a replicated 4 ×4 Latin square design | 30:70 |
| [123] | Boland et al. (2020) | Ireland | Holstein × Friesian cows | SF6-T | a randomized block design | Not supplied |
| [124] | Enriquez-Hidalgo et al. (2020) | UK | Holstein-Friesian and Montbeliard | SF6-T | randomly allocated | 54:46 |
| [125] | Benchaar (2020) | Canada | cows | SF6-T | a replicated 4 × 4 Latin square design | 40:60 |
| [126] | Bougouin et al. (2019) | France | Holstein cows | RC | a 4 × 4 Latin square design | 40:60 |
| [127] | Van Wesemael et al. (2019) | Belgium | Holstein Friesian cows | GFS | randomly assigned | 34:66 |
| [128] | Focant et al. (2019) | Belgium | Holstein cows | Milk-MIR | a 3 × 3 duplicated Latin square design | 36:64, 35:65 |
| [129] | Sun et al. (2019) | Madison | Holstein dairy cows | GFS | randomly assigned | 39:61 |
| [130] | Kliem et al. (2019) | UK | Holstein-Friesian cows | RC | a 4 × 4 Latin square design | 49:51 |
| [131] | Judy et al. (2018) | Lincoln | Jersey cows | RC | a crossover design | 46:54 |
| [132] | Cherif et al. (2018) | Canada | Holstein cows | RC | a replicated 3 × 3 Latin square design | 41:59 |
| [133] | van Wyngaard et al. (2018) | South Africa | Jersey cows | SF6-T | a 3 × 3 Latin square design | 0:100 |
| [134] | Kidane et al. (2018) | Norway | Norwegian Red dairy cows | SF6-T | a 4 × 4 Latin square design | 51:49 |
| [135] | Kolling et al. (2018) | Brazil | Holstein cows and crossbred Holstein-Gir | RC | randomly assigned | 40:60 |
| [136] | Williams et al. (2018) | Australia | Holstein-Friesian cows | SF6-T | randomly assigned | 36:64 |
| [137] | Bougouin et al. (2018) | France | Holstein cows | RC | a 4 × 4 Latin square design | 50:50 |
| [138] | Bayat et al. (2018) | Finland | Nordic Red dairy cows | SF6-T | a 5 × 5 Latin square design | 40:60 |
| [139] | Stoldt et al. (2016) | Germany | German Holstein cows | RC | a crossover design | 42:58 |
| [140] | Lopes et al. (2016) | USA | Holstein cows | GFS | a 2 × 2 crossover design | 44:56 |
| [141] | Pirondini et al. (2015) | Italy | Italian Friesian cows | RC | a 4 × 4 Latin square design | 48:52 |
| [142] | Reynolds et al. (2014) | UK | Holstein-Friesian cows | RC | 3 × 3 Latin square design | 49:51 |
References
- O’Connor, S.; Noonan, F.; Savage, D.; Walsh, J. Advancements in real-time monitoring of enteric methane emissions from ruminants. Agriculture 2024, 14, 1096. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Martínez-Marín, G.; Toledo-Alvarado, H.; Amalfitano, N.; Gallo, L.; Bittante, G. Lactation modeling and the effects of rotational crossbreeding on milk production traits and milk-spectra-predicted enteric methane emissions. J. Dairy Sci. 2024, 107, 1485–1499. [Google Scholar] [CrossRef] [PubMed]
- Denninger, T.M.; Schwarm, A.; Dohme-Meier, F.; Münger, A.; Bapst, B.; Wegmann, S.; Grandl, F.; Vanlierde, A.; Sorg, D.; Ortmann, S.; et al. Accuracy of methane emissions predicted from milk mid-infrared spectra and measured by laser methane detectors in Brown Swiss dairy cows. J. Dairy Sci. 2020, 103, 2024–2039. [Google Scholar] [CrossRef] [PubMed]
- Bittante, G.; Cipolat-Gotet, C. Direct and indirect predictions of enteric methane daily production, yield, and intensity per unit of milk and cheese, from fatty acids and milk Fourier-transform infrared spectra. J. Dairy Sci. 2018, 101, 7219–7235. [Google Scholar] [CrossRef] [PubMed]
- Coppa, M.; Vanlierde, A.; Bouchon, M.; Jurquet, J.; Musati, M.; Dehareng, F.; Martin, C. Methodological guidelines: Cow milk mid-infrared spectra to predict reference enteric methane data collected by an automated head-chamber system. J. Dairy Sci. 2022, 105, 9271–9285. [Google Scholar] [CrossRef]
- Blaxter, K.; Clapperton, J. Prediction of the amount of methane produced by ruminants. Brit. J. Nutr. 1965, 19, 511–522. [Google Scholar] [CrossRef]
- Storm, I.M.L.D.; Hellwing, A.L.F.; Nielsen, N.I.; Madsen, J. Methods for measuring and estimating methane emission from ruminants. Animals 2012, 2, 160–183. [Google Scholar] [CrossRef]
- Johnson, K.; Huyler, M.; Westberg, H.; Lamb, B.; Zimmerman, P. Measurement of methane emissions from ruminant livestock using a sulfur hexafluoride tracer technique. Environ. Sci. Technol. 1994, 28, 359–362. [Google Scholar] [CrossRef]
- Dorich, C.D.; Varner, R.K.; Pereira, A.B.D.; Martineau, R.; Soder, K.J.; Brito, A.F. Short communication: Use of a portable, automated, open-circuit gas quantification system and the sulfur hexafluoride tracer technique for measuring enteric methane emissions in Holstein cows fed ad libitum or restricted. J. Dairy Sci. 2015, 98, 2676–2681. [Google Scholar] [CrossRef]
- Laubach, J.; Kelliher, F.M. Methane emissions from dairy cows: Comparing open-path laser measurements to profile-based techniques. Agric. Meteorol. 2005, 135, 340–345. [Google Scholar] [CrossRef]
- Teye, F.; Alkkiomaki, E.; Simojoki, A.; Pastell, M.; Hautala, M.; Ahokas, J. Instrumentation, measurement and performance of three air quality measurement systems for dairy buildings. Appl. Eng. Agric. 2009, 25, 247–256. [Google Scholar] [CrossRef]
- Kelly, J.M.; Kerrigan, B.; Milligan, L.P.; McBride, B.W. Development of a mobile, open-circuit indirect calorimetry system. Can. J. Anim. Sci. 1994, 74, 65–71. [Google Scholar] [CrossRef]
- Madsen, J.; Bjerg, B.S.; Hvelplund, T.; Weisbjerg, M.R.; Lund, P. Methane and carbon dioxide ratio in excreted air for quantification of the methane production from ruminants. Livest. Sci. 2010, 129, 223–227. [Google Scholar] [CrossRef]
- Hristov, A.N.; Lee, C.; Cassidy, T.; Heyler, K.; Tekippe, J.A.; Varga, G.A.; Corl, B.; Brandt, R.C. Effect of Origanum vulgare L. leaves on rumen fermentation, production, and milk fatty acid composition in lactating dairy cows. J. Dairy Sci. 2013, 96, 1189–1202. [Google Scholar] [CrossRef]
- Charmley, E.; Williams, S.; Moate, P.; Hegarty, R.; Herd, R.M.; Oddy, V.; Reyenga, P.; Staunton, K.; Anderson, A.; Hannah, M. A universal equation to predict methane production of forage-fed cattle in Australia. Anim. Prod. Sci. 2015, 56, 169–180. [Google Scholar] [CrossRef]
- Moraes, L.E.; Strathe, A.B.; Fadel, J.G.; Casper, D.P.; Kebreab, E. Prediction of enteric methane emissions from cattle. Glob. Change Biol. 2014, 20, 2140–2148. [Google Scholar] [CrossRef]
- Ramin, M.; Huhtanen, P. Development of equations for predicting methane emissions from ruminants. J. Dairy Sci. 2013, 96, 2476–2493. [Google Scholar] [CrossRef]
- Neves, A.L.A.; Vieira, R.A.M.; Vargas-Bello-Pérez, E.; Chen, Y.; McAllister, T.; Ominski, K.H.; Lin, L.; Guan, L.L. Impact of feed composition on rumen microbial dynamics and phenotypic traits in beef cattle. Microorganisms 2025, 13, 310. [Google Scholar] [CrossRef]
- Molina-Botero, I.C.; Díaz-Céspedes, M.A.; Mayorga-Mogollón, O.L.; Ku-Vera, J.C.; Arceo-Castillo, J.I.; Montoya-Flores, M.D.; Arango, J.; Gómez-Bravo, C. Validation of enteric methane emissions by cattle estimated from mathematical models using data from in vivo experiments. Acta Sci. Anim. Sci. 2024, 47, e69328. [Google Scholar] [CrossRef]
- Mills, J.; Kebreab, E.; Yates, C.; Crompton, L.; Cammell, S.; Dhanoa, M.; Agnew, R.; France, J. Alternative approaches to predicting methane emissions from dairy cows. J. Anim. Sci. 2003, 81, 3141–3150. [Google Scholar] [CrossRef]
- Kriss, M. Quantitative relations of the dry matter of the food consumed, the heat production, the gaseous outgo, and the insensible loss in body weight of cattle. J. Agric. Res. 1930, 40, 283–295. [Google Scholar]
- Bratzler, J.W.; Forbes, E. The estimation of methane production by cattle. J. Nutr. 1940, 19, 611–613. [Google Scholar] [CrossRef]
- Holter, J.; Young, A. Methane prediction in dry and lactating Holstein cows. J. Dairy Sci. 1992, 75, 2165–2175. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Juarez, B.; Moraes, L.E.; Appuhamy, J.A.D.R.N.; Pellikaan, W.F.; Casper, D.P.; Tricarico, J.; Kebreab, E. Prediction and evaluation of enteric methane emissions from lactating dairy cows using different levels of covariate information. Anim. Prod. Sci. 2016, 56, 557–564. [Google Scholar] [CrossRef]
- IPCC. Agriculture, forestry and other land use: Emissions from livestock and manure management. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006; Volume 4, pp. 11–87. [Google Scholar]
- IPCC. Emissions from livestock and manure management. In 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; IPCC: Geneva, Switzerland, 2019; Volume 4, pp. 11–207. [Google Scholar]
- Benaouda, M.; González-Ronquillo, M.; Appuhamy, J.A.D.R.N.; Kebreab, E.; Molina, L.T.; Herrera-Camacho, J.; Ku-Vera, J.C.; Ángeles-Hernández, J.C.; Castelán-Ortega, O.A. Development of mathematical models to predict enteric methane emission by cattle in Latin America. Livest. Sci. 2020, 241, 104177. [Google Scholar] [CrossRef]
- Niu, M.; Kebreab, E.; Hristov, A.N.; Oh, J.; Arndt, C.; Bannink, A.; Bayat, A.R.; Brito, A.F.; Boland, T.; Casper, D.; et al. Prediction of enteric methane production, yield, and intensity in dairy cattle using an intercontinental database. Glob. Change Biol. 2018, 24, 3368–3389. [Google Scholar] [CrossRef]
- Bibby, J.; Toutenburg, H. Prediction and Improved Estimation in Linear Models; Wiley: Chichester, UK, 1977; p. 188. [Google Scholar]
- Tedeschi, L.O. Assessment of the adequacy of mathematical models. Agric. Syst. 2006, 89, 225–247. [Google Scholar] [CrossRef]
- Lawrence, I.K.L. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef]
- Moriasi, D.N.; Arnold, J.G.; Liew, M.W.V.; Bingner, R.L.; Harmel, R.D.; Veith, T.L. Model evaluation guidelines for systematic quantification of accuracy in watershed simulations. Trans. ASABE 2007, 50, 885–900. [Google Scholar] [CrossRef]
- St-Pierre, N.R. Invited review: Integrating quantitative findings from multiple studies using mixed model methodology. J. Dairy Sci. 2001, 84, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Donadia, A.B.; Torres, R.N.S.; Silva, H.M.d.; Soares, S.R.; Hoshide, A.K.; Oliveira, A.S.d. Factors affecting enteric emission methane and predictive models for dairy cows. Animals 2023, 13, 1857. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Kebreab, E.; Odongo, N.; McBride, B.; Okine, E.; France, J. Prediction of methane production from dairy and beef cattle. J. Dairy Sci. 2007, 90, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Oh, J.; Firkins, J.L.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.P.S.; Adesogan, A.T.; Yang, W.; Lee, C.; et al. SPECIAL TOPICS—Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options1. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef]
- Yan, T.; Agnew, R.E.; Gordon, F.J.; Porter, M.G. Prediction of methane energy output in dairy and beef cattle offered grass silage-based diets. Livest. Prod. Sci. 2000, 64, 253–263. [Google Scholar] [CrossRef]
- Axelsson, J. The amount of produced methane energy in the European metabolic experiments with adult cattle. K. Lantbrukshögsk. Ann. 1949, 16, 404–419. [Google Scholar]
- Moate, P.J.; Williams, S.; Grainger, C.; Hannah, M.; Ponnampalam, E.; Eckard, R. Influence of cold-pressed canola, brewers grains and hominy meal as dietary supplements suitable for reducing enteric methane emissions from lactating dairy cows. Anim. Feed Sci. Technol. 2011, 166, 254–264. [Google Scholar] [CrossRef]
- Nielsen, N.; Volden, H.; Åkerlind, M.; Brask, M.; Hellwing, A.L.F.; Storlien, T.; Bertilsson, J. A prediction equation for enteric methane emission from dairy cows for use in NorFor. Acta Agric. Scand. A Anim. Sci. 2013, 63, 126–130. [Google Scholar] [CrossRef]
- Storlien, T.M.; Volden, H.; Almøy, T.; Beauchemin, K.A.; McAllister, T.A.; Harstad, O.M. Prediction of enteric methane production from dairy cows. Acta Agric. Scand. A Anim. Sci. 2014, 64, 98–109. [Google Scholar] [CrossRef]
- Patra, A.K. Prediction of enteric methane emission from cattle using linear and non-linear statistical models in tropical production systems. Mitig. Adapt. Strat. Glob. Change 2017, 22, 629–650. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Rodrigues, J.P.P.; Maurício, R.M.; Borges, A.L.C.C.; Silva, R.R.e.; Berchielli, T.T.; Filho, S.C.V.; Machado, F.S.; Campos, M.M.; Ferreira, A.L.; et al. Predicting enteric methane production from cattle in the tropics. Animal 2020, 14, s438–s452. [Google Scholar] [CrossRef]
- Wang, Y.; Song, W.; Wang, Q.; Yang, F.; Yan, Z. Predicting enteric methane emissions from dairy and beef cattle using nutrient composition and intake variables. Animals 2024, 14, 3452. [Google Scholar] [CrossRef] [PubMed]
- Larios, A.D.; Kaur Brar, S.; Avalos Ramírez, A.; Godbout, S.; Sandoval-Salas, F.; Palacios, J.H. Challenges in the measurement of emissions of nitrous oxide and methane from livestock sector. Rev. Environ. Sci. Biotechnol. 2016, 15, 285–297. [Google Scholar] [CrossRef]
- Vargas, J.; Swenson, M.; Schilling-Hazlett, A.K.; Reis, I.A.; Velasquez, C.; Martins, E.C.; Sitorski, L.; Campos, L.M.; Carvalho, P.H.V.; Stackhouse-Lawson, K.R.; et al. Evaluation of models of enteric methane emissions in finishing steers. Animal 2025, 19, 101536. [Google Scholar] [CrossRef]
- Cottle, D.J.; Eckard, R.J. Global beef cattle methane emissions: Yield prediction by cluster and meta-analyses. Anim. Prod. Sci. 2018, 58, 2167–2177. [Google Scholar] [CrossRef]
- Patra, A.K.; Lalhriatpuii, M. Development of statistical models for prediction of enteric methane emission from goats using nutrient composition and intake variables. Agric. Ecosyst. Environ. 2016, 215, 89–99. [Google Scholar] [CrossRef]
- Patra, A.K.; Lalhriatpuii, M.; Debnath, B.C. Predicting enteric methane emission in sheep using linear and non-linear statistical models from dietary variables. Anim. Prod. Sci. 2016, 56, 574–584. [Google Scholar] [CrossRef]
- Brask, M.; Lund, P.; Weisbjerg, M.R.; Hellwing, A.L.F.; Poulsen, M.; Larsen, M.K.; Hvelplund, T. Methane production and digestion of different physical forms of rapeseed as fat supplements in dairy cows. J. Dairy Sci. 2013, 96, 2356–2365. [Google Scholar] [CrossRef]
- Benaouda, M.; Martin, C.; Li, X.; Kebreab, E.; Hristov, A.N.; Yu, Z.; Yáñez-Ruiz, D.R.; Reynolds, C.K.; Crompton, L.A.; Dijkstra, J.; et al. Evaluation of the performance of existing mathematical models predicting enteric methane emissions from ruminants: Animal categories and dietary mitigation strategies. Anim. Feed Sci. Technol. 2019, 255, 114207. [Google Scholar] [CrossRef]
- Oikawa, K.; Terada, F.; Kurihara, M.; Suzuki, T.; Nonaka, I.; Hosoda, K.; Kamiya, Y.; Roh, S.; Haga, S. Methane emission prediction models for lactating cows based on feed intake, body weight, and milk yield and composition: Variable methane conversion factor-based approach. J. Dairy Sci. 2025, 108, 7248–7261. [Google Scholar] [CrossRef]
- Dong, R.; Dong, H.; Beauchemin, K.A.; Xin, H. Performance evaluation of manure nitrogen output models suitable for lactating dairy cows in China. Trans. ASABE 2018, 61, 1713–1727. [Google Scholar] [CrossRef]
- Min, B.-R.; Lee, S.; Jung, H.; Miller, D.N.; Chen, R. Enteric methane emissions and animal performance in dairy and beef cattle production: Strategies, opportunities, and Impact of reducing emissions. Animals 2022, 12, 948. [Google Scholar] [CrossRef] [PubMed]
- Marumo, J.L.; LaPierre, P.A.; Van Amburgh, M.E. Enteric methane emissions prediction in dairy cattle and effects of monensin on methane emissions: A meta-analysis. Animals 2023, 13, 1392. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.F.; Jia, P.; Li, B.C.; Wang, B.; Yang, C.L.; Liu, Z.H.; Diao, Q.Y. Quantification and prediction of enteric methane emissions from Chinese lactating Holstein dairy cows fed diets with different dietary neutral detergent fiber/non-fibrous carbohydrate (NDF/NFC) ratios. J. Integr. Agric. 2022, 21, 797–811. [Google Scholar] [CrossRef]
- Moe, P.W.; Tyrrell, H.F. Methane production in dairy cows. J. Dairy Sci. 1979, 62, 1583–1586. [Google Scholar] [CrossRef]
- Hippenstiel, F.; Pries, M.; Büscher, W.; Südekum, K.H. Comparative evaluation of equations predicting methane production of dairy cattle from feed characteristics. Arch. Anim. Nutr. 2013, 67, 279–288. [Google Scholar] [CrossRef]
- Pickering, N.K.; Chagunda, M.G.G.; Banos, G.; Mrode, R.; McEwan, J.C.; Wall, E. Genetic parameters for predicted methane production and laser methane detector measurements. J. Anim. Sci. 2015, 93, 11–20. [Google Scholar] [CrossRef]
- Islam, M.; Kim, S.-H.; Son, A.R.; Lee, S.-S.; Lee, S.-S. Breed and season-specific methane conversion factors influence methane emission factor for enteric methane of dairy steers. Sustainability 2022, 14, 7030. [Google Scholar] [CrossRef]
- Ormston, S.; Yan, T.; Chen, X.; Gordon, A.W.; Theodoridou, K.; Huws, S.; Stergiadis, S. Impact of dietary forage proportion and crossbreeding on feed efficiency and methane emissions in lactating dairy cows. Anim. Nutr. 2025, 20, 419–429. [Google Scholar] [CrossRef]
- Olijhoek, D.W.; Hellwing, A.L.F.; Noel, S.J.; Lund, P.; Larsen, M.; Weisbjerg, M.R.; Børsting, C.F. Feeding up to 91% concentrate to Holstein and Jersey dairy cows: Effects on enteric methane emission, rumen fermentation and bacterial community, digestibility, production, and feeding behavior. J. Dairy Sci. 2022, 105, 9523–9541. [Google Scholar] [CrossRef]
- Grainger, C.; Clarke, T.; McGinn, S.M.; Auldist, M.J.; Beauchemin, K.A.; Hannah, M.C.; Waghorn, G.C.; Clark, H.; Eckard, R.J. Methane emissions from dairy cows measured using the sulfur hexafluoride (SF6) tracer and chamber techniques. J. Dairy Sci. 2007, 90, 2755–2766. [Google Scholar] [CrossRef]
- Ma, X.; Räisänen, S.E.; Wang, K.; Amelchanka, S.; Giller, K.; Islam, M.Z.; Li, Y.; Peng, R.; Reichenbach, M.; Serviento, A.M.; et al. Evaluating GreenFeed and respiration chambers for daily and intraday measurements of enteric gaseous exchange in dairy cows housed in tiestalls. J. Dairy Sci. 2024, 107, 10913–10931. [Google Scholar] [CrossRef]
- Giagnoni, G.; Lund, P.; Johansen, M.; Hellwing, A.L.F.; Noel, S.J.; Thomsen, J.P.S.; Poulsen, N.A.; Weisbjerg, M.R. Effect of carbohydrate type in silages and concentrates on feed intake, enteric methane, and milk yield from dairy cows. J. Dairy Sci. 2024, 107, 7851–7866. [Google Scholar] [CrossRef] [PubMed]
- van Gastelen, S.; Yáñez-Ruiz, D.; Khelil-Arfa, H.; Blanchard, A.; Bannink, A. Effect of a blend of cinnamaldehyde, eugenol, and Capsicum oleoresin on methane emission and lactation performance of Holstein-Friesian dairy cows. J. Dairy Sci. 2024, 107, 857–869. [Google Scholar] [CrossRef] [PubMed]
- van Gastelen, S.; Burgers, E.E.A.; Dijkstra, J.; de Mol, R.; Muizelaar, W.; Walker, N.; Bannink, A. Long-term effects of 3-nitrooxypropanol on methane emission and milk production characteristics in Holstein-Friesian dairy cows. J. Dairy Sci. 2024, 107, 5556–5573. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, S.E.; Sigurðardóttir, Þ.H.; Halmemies-Beauchet-Filleau, A.; Pitkänen, O.; Vanhatalo, A.; Sairanen, A.; Kokkonen, T. Ruminal methane emission and lactational performance of cows fed rapeseed cake and oats on a grass silage–based diet. J. Dairy Sci. 2024, 107, 6732–6741. [Google Scholar] [CrossRef]
- Martins, L.F.; Cueva, S.F.; Wasson, D.E.; Silvestre, T.; Stepanchenko, N.; Hile, M.L.; Hristov, A.N. Separate offering of forages and concentrates to lactating dairy cows: Effects on lactational performance, enteric methane emission, and efficiency of nutrient utilization. J. Dairy Sci. 2024, 107, 4587–4604. [Google Scholar] [CrossRef]
- Thorsteinsson, M.; Chassé, É.; Curtasu, M.V.; Battelli, M.; Bruhn, A.; Hellwing, A.L.F.; Weisbjerg, M.R.; Nielsen, M.O. Potential of 2 northern European brown seaweeds (Fucus serratus and Fucus vesiculosus) as enteric methane inhibitors in dairy cows. J. Dairy Sci. 2024, 107, 10628–10640. [Google Scholar] [CrossRef]
- Redoy, M.R.A.; Ahmed, S.; Bulnes, M.; Kleinschmit, D.H.; Uddin, M.E. Isoacid supplementation influences feed sorting, chewing behaviors, and enteric methane emissions differentially in mid-lactation dairy cows depending on dietary forage level. J. Dairy Sci. 2025, 108, 1419–1430. [Google Scholar] [CrossRef]
- Akter, A.; Li, X.; Grey, E.; Wang, S.C.; Kebreab, E. Grape pomace supplementation reduced methane emissions and improved milk quality in lactating dairy cows. J. Dairy Sci. 2025, 108, 2468–2480. [Google Scholar] [CrossRef]
- Colin, R.L.; Sperber, J.L.; Buse, K.K.; Kononoff, P.J.; Watson, A.K.; Erickson, G.E. Effect of an algae feed additive on reducing enteric methane emissions from cattle. Transl. Anim. Sci. 2024, 8, txae109. [Google Scholar] [CrossRef]
- Ahvenjärvi, S.; Bayat, A.-R.; Toivanen, M.; Mäntysaari, P.; Tapio, I. The effects of residual energy intake on nutrient use, methane emissions and microbial composition in dairy cows. Sci. Rep. 2024, 14, 613. [Google Scholar] [CrossRef]
- Molina-Botero, I.C.; Gaviria-Uribe, X.; Rios-Betancur, J.P.; Medina-Campuzano, M.; Toro-Trujillo, M.; González-Quintero, R.; Ospina, B.; Arango, J. Methane emission, carbon footprint and productivity of specialized dairy cows supplemented with bitter cassava (Manihot esculenta Crantz). Animals 2024, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fu, S.; Li, G.; Yao, Z.; Du, X.; Zhang, Y.; Gao, T. Enteric methane emissions, rumen fermentation, and milk composition of dairy cows fed 3-nitrooxypropanol and L-malate supplements. Front. Vet. Sci. 2024, 11, 1479535. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, M.T.; Baki, C.; Terranova, M.; Amelchanka, S.L.; Dubois, S.; Wiget, A.; Leiber, F.; Krause, H.-M.; Baumann, S. The effect of biochar supplementation on feed utilization, milk production and methane emission in lactating dairy cows. Anim. Feed Sci. Technol. 2024, 318, 116127. [Google Scholar] [CrossRef]
- Kabsuk, J.; Nusri-un, J.; Binsulong, B.; Gunha, T.; Sommart, K. Effects of substituting cassava pulp with broken rice and cassava chips in crossbred Holstein diets: Rumen fermentation, enteric methane emission, and energy utilization. Animals 2024, 14, 2257. [Google Scholar] [CrossRef]
- Ruchita, K.; Tassilo, B.; Ilma, T.; AliReza, B. Effect of a garlic and citrus extract supplement on performance, rumen fermentation, methane production, and rumen microbiome of dairy cows. J. Dairy Sci. 2023, 106, 4608–4621. [Google Scholar] [CrossRef]
- Starsmore, K.; Lopez-Villalobos, N.; Shalloo, L.; Egan, M.; Burke, J.; Lahart, B. Animal factors that affect enteric methane production measured using the GreenFeed monitoring system in grazing dairy cows. J. Dairy Sci. 2023, 107, 2930–2940. [Google Scholar] [CrossRef]
- Peng, J.; LiFeng, D.; Yan, T.; QiYu, D. Bacillus subtilis and Macleaya cordata extract regulate the rumen microbiota associated with enteric methane emission in dairy cows. Microbiome 2023, 11, 229. [Google Scholar] [CrossRef]
- Reyes, D.C.; Meredith, J.; Puro, L.; Berry, K.; Kersbergen, R.; Soder, K.J.; Quigley, C.; Donihue, M.; Cox, D.; Price, N.N.; et al. Maine organic dairy producers’ receptiveness to seaweed supplementation and effect of Chondrus crispus on enteric methane emissions in lactating cows. Front. Vet. Sci. 2023, 10, 1153097. [Google Scholar] [CrossRef]
- Fresco, S.; Boichard, D.; Fritz, S.; Lefebvre, R.; Barbey, S.; Gaborit, M.; Martin, P. Comparison of methane production, intensity, and yield throughout lactation in Holstein cows. J. Dairy Sci. 2023, 106, 4147–4157. [Google Scholar] [CrossRef]
- Lifeng, D.; Lei, Z.; Bowei, L.; Yanhua, G.; Tianhai, Y.; Peter, L.; Zhuofan, L.; Qiyu, D. Dietary supplementation with xylooligosaccharides and exogenous enzyme improves milk production, energy utilization efficiency and reduces enteric methane emissions of Jersey cows. J. Anim. Sci. Biotechnol. 2023, 14, 71. [Google Scholar] [CrossRef]
- Noe, A.J.; Cecilia, M.C.; Hebert, H.J.; Efrén, R.J.; Epigmenio, C.; Alejandro, P.; Alejandro, C.F.; Luis, C. Effect of canola oil supplementation level on total tract digestion, ruminal fermentation, and methane emissions of cows grazing Urochloa sp. supplemented with a fixed amount of concentrate. Trop. Anim. Health Prod. 2023, 55, 77. [Google Scholar] [CrossRef]
- Benchaar, C.; Hassanat, F.; Beauchemin, K.A.; Ouellet, D.R.; Lapierre, H.; Côrtes, C. Effect of metabolizable protein supply on milk performance, ruminal fermentation, apparent total-tract digestibility, energy and nitrogen utilization, and enteric methane production of Ayrshire and Holstein Cows. Animals 2023, 13, 832. [Google Scholar] [CrossRef] [PubMed]
- Muizelaar, W.; van Duinkerken, G.; Khan, Z.; Dijkstra, J. Evaluation of 3 northwest European seaweed species on enteric methane production and lactational performance of Holstein-Friesian dairy cows. J. Dairy Sci. 2023, 106, 4622–4633. [Google Scholar] [CrossRef]
- Lazzari, G.; Münger, A.; Eggerschwiler, L.; Borda-Molina, D.; Seifert, J.; Camarinha-Silva, A.; Schrade, S.; Zähner, M.; Zeyer, K.; Kreuzer, M.; et al. Effects of Acacia mearnsii added to silages differing in nutrient composition and condensed tannins on ruminal and manure-derived methane emissions of dairy cows. J. Dairy Sci. 2023, 106, 6816–6833. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, L.R.; Eastridge, M.L.; Firkins, J.L.; Lee, C. Effects of corn silage and grain expressing α-amylase on ruminal nutrient digestibility, microbial protein synthesis, and enteric methane emissions in lactating cows. J. Dairy Sci. 2023, 106, 3932–3946. [Google Scholar] [CrossRef]
- Niu, P.; Kreuzer, M.; Liesegang, A.; Kunz, C.; Schwarm, A.; Giller, K. Effects of graded levels of dietary pomegranate peel on methane and nitrogen losses, and metabolic and health indicators in dairy cows. J. Dairy Sci. 2023, 106, 8627–8641. [Google Scholar] [CrossRef]
- Thorsteinsson, M.; Weisbjerg, M.R.; Lund, P.; Bruhn, A.; Hellwing, A.L.F.; Nielsen, M.O. Effects of dietary inclusion of 3 Nordic brown macroalgae on enteric methane emission and productivity of dairy cows. J. Dairy Sci. 2023, 106, 6921–6937. [Google Scholar] [CrossRef]
- Mirka, T.; Peter, L.; Riis, W.M.; Joan, N.S.; Amanda, S.A.; Frydendahl, H.A.L.; Helene, H.H.; Olaf, N.M. Enteric methane emission of dairy cows supplemented with iodoform in a dose-response study. Sci. Rep. 2023, 13, 12797. [Google Scholar] [CrossRef]
- Silvestre, T.; Martins, L.F.; Cueva, S.F.; Wasson, D.E.; Stepanchenko, N.; Räisänen, S.E.; Sommai, S.; Hile, M.L.; Hristov, A.N. Lactational performance, rumen fermentation, nutrient use efficiency, enteric methane emissions, and manure greenhouse gas-emitting potential in dairy cows fed a blend of essential oils. J. Dairy Sci. 2023, 106, 7661–7674. [Google Scholar] [CrossRef] [PubMed]
- Almeida, K.V.; Resende, T.L.; Silva, L.H.P.; Dorich, C.D.; Pereira, A.B.D.; Soder, K.J.; Brito, A.F. Feeding incremental amounts of ground flaxseed: Effects on diversity and relative abundance of ruminal microbiota and enteric methane emissions in lactating dairy cows. Transl. Anim. Sci. 2023, 7, txad050. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Elcoso, G.; Escartín, M.; Spengler, K.; Jouve, A. Modulation of milking performance, methane emissions, and rumen microbiome on dairy cows by dietary supplementation of a blend of essential oils. Animal 2023, 17, 100825. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.R.O.; Jacobs, J.L.; Chandra, S.; Soust, M.; Russo, V.M.; Douglas, M.L.; Hess, P.S.A. The effect of direct-fed lactobacillus species on milk production and methane emissions of dairy cows. Animals 2023, 13, 1018. [Google Scholar] [CrossRef]
- Khan, G.Q.; Prestløkken, E.; Lund, P.; Hellwing, A.L.F.; Larsen, M. Effects of the density of extruded pellets on starch digestion kinetics, rumen fermentation, fiber digestibility and enteric methane production in dairy cows. J. Anim. Physiol. Anim. Nutr. 2022, 107, 981–994. [Google Scholar] [CrossRef]
- Della Rosa, M.M.; Sandoval, E.; Luo, D.; Pacheco, D.; Jonker, A. Effect of feeding fresh forage plantain (Plantago lanceolata) or ryegrass-based pasture on methane emissions, total-tract digestibility, and rumen fermentation of nonlactating dairy cows. J. Dairy Sci. 2022, 105, 6628–6638. [Google Scholar] [CrossRef]
- Silvestre, T.; Räisänen, S.E.; Cueva, S.F.; Wasson, D.E.; Lage, C.F.A.; Martins, L.F.; Wall, E.; Hristov, A.N. Effects of a combination of Capsicum oleoresin and clove essential oil on metabolic status, lactational performance, and enteric methane emissions in dairy cows. J. Dairy Sci. 2022, 105, 9610–9622. [Google Scholar] [CrossRef]
- Garcia, F.; Muñoz, C.; Martínez-Ferrer, J.; Urrutia, N.L.; Martínez, E.D.; Saldivia, M.; Immig, I.; Kindermann, M.; Walker, N.; Ungerfeld, E.M. 3-Nitrooxypropanol substantially decreased enteric methane emissions of dairy cows fed true protein- or urea-containing diets. Heliyon 2022, 8, e09738. [Google Scholar] [CrossRef]
- Peng, J.; Yan, T.; Zhihao, L.; Fadi, L.; Tianhai, Y.; Shulin, M.; Lifeng, D.; Qiyu, D. Diets supplementation with Bacillus subtilis and Macleaya cordata extract improve production performance and the metabolism of energy and nitrogen, while reduce enteric methane emissions in dairy cows. Anim. Feed Sci. Technol. 2022, 294, 115481. [Google Scholar] [CrossRef]
- Daniel, K.; Richard, E.; John, G.; Claudia, A.; Lutz, M.; Svenja, M. Effects of replacing Brachiaria hay with either Desmodium intortum or dairy concentrate on animal performance and enteric methane emissions of low-yielding dairy cows. Front. Anim. Sci. 2022, 3, 963323. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Y.; Niu, J.; Guo, Y.; Pauline, M.; Zhao, X.; Li, Q.; Cao, Y.; Bi, C.; Zhang, X.; et al. Effect of active dry yeast on lactation performance, methane production, and ruminal fermentation patterns in early-lactating Holstein cows. J. Dairy Sci. 2021, 104, 381–390. [Google Scholar] [CrossRef]
- Bayat, A.R.; Vilkki, J.; Razzaghi, A.; Leskinen, H.; Kettunen, H.; Khurana, R.; Brand, T.; Ahvenjärvi, S. Evaluating the effects of high-oil rapeseed cake or natural additives on methane emissions and performance of dairy cows. J. Dairy Sci. 2021, 105, 1211–1224. [Google Scholar] [CrossRef]
- Civiero, M.; Delagarde, R.; Berndt, A.; Rosseto, J.; de Souza, M.N.; Schaitz, L.H.; Ribeiro-Filho, H.M.N. Progressive inclusion of pearl millet herbage as a supplement for dairy cows fed mixed rations: Effects on methane emissions, dry matter intake, and milk production. J. Dairy Sci. 2021, 104, 2956–2965. [Google Scholar] [CrossRef]
- Stefenoni, H.A.; Räisänen, S.E.; Cueva, S.F.; Wasson, D.E.; Lage, C.F.A.; Melgar, A.; Fetter, M.E.; Smith, P.; Hennessy, M.; Vecchiarelli, B.; et al. Effects of the macroalga Asparagopsis taxiformis and oregano leaves on methane emission, rumen fermentation, and lactational performance of dairy cows. J. Dairy Sci. 2021, 104, 4157–4173. [Google Scholar] [CrossRef]
- Hassanat, F.; Benchaar, C. Corn silage-based diet supplemented with increasing amounts of linseed oil: Effects on methane production, rumen fermentation, nutrient digestibility, nitrogen utilization, and milk production of dairy cows. J. Dairy Sci. 2021, 104, 5375–5390. [Google Scholar] [CrossRef] [PubMed]
- Ramin, M.; Fant, P.; Huhtanen, P. The effects of gradual replacement of barley with oats on enteric methane emissions, rumen fermentation, milk production, and energy utilization in dairy cows. J. Dairy Sci. 2021, 104, 5617–5630. [Google Scholar] [CrossRef]
- Benchaar, C.; Hassanat, F.; Beauchemin, K.A.; Gislon, G.; Ouellet, D.R. Diet supplementation with canola meal improves milk production, reduces enteric methane emissions, and shifts nitrogen excretion from urine to feces in dairy cows. J. Dairy Sci. 2021, 104, 9645–9663. [Google Scholar] [CrossRef]
- Cueva, S.F.; Stefenoni, H.; Melgar, A.; Räisänen, S.E.; Lage, C.F.A.; Wasson, D.E.; Fetter, M.E.; Pelaez, A.M.; Roth, G.W.; Hristov, A.N. Lactational performance, rumen fermentation, and enteric methane emission of dairy cows fed an amylase-enabled corn silage. J. Dairy Sci. 2021, 104, 9827–9841. [Google Scholar] [CrossRef]
- Fant, P.; Ramin, M.; Huhtanen, P. Replacement of barley with oats and dehulled oats: Effects on milk production, enteric methane emissions, and energy utilization in dairy cows fed a grass silage-based diet. J. Dairy Sci. 2021, 104, 12540–12552. [Google Scholar] [CrossRef]
- Darabighane, B.; Tapio, I.; Ventto, L.; Kairenius, P.; Stefański, T.; Leskinen, H.; Shingfield, K.J.; Vilkki, J.; Bayat, A.-R. Effects of starch level and a mixture of sunflower and fish oils on nutrient intake and digestibility, rumen fermentation, and ruminal methane emissions in dairy cows. Animals 2021, 11, 1310. [Google Scholar] [CrossRef]
- Schilde, M.; von Soosten, D.; Hüther, L.; Meyer, U.; Zeyner, A.; Dänicke, S. Effects of 3-nitrooxypropanol and varying concentrate feed proportions in the ration on methane emission, rumen fermentation and performance of periparturient dairy cows. Arch. Anim. Nutr. 2021, 75, 79–104. [Google Scholar] [CrossRef]
- Melgar, A.; Lage, C.F.A.; Nedelkov, K.; Räisänen, S.E.; Stefenoni, H.; Fetter, M.E.; Chen, X.; Oh, J.; Duval, S.; Kindermann, M.; et al. Enteric methane emission, milk production, and composition of dairy cows fed 3-nitrooxypropanol. J. Dairy Sci. 2021, 104, 357–366. [Google Scholar] [CrossRef]
- Børsting, C.F.; Brask, M.; Hellwing, A.L.F.; Weisbjerg, M.R.; Lund, P. Enteric methane emission and digestion in dairy cows fed wheat or molasses. J. Dairy Sci. 2020, 103, 1448–1462. [Google Scholar] [CrossRef] [PubMed]
- Moate, P.J.; Deighton, M.H.; Jacobs, J.; Ribaux, B.E.; Morris, G.L.; Hannah, M.C.; Mapleson, D.; Islam, M.S.; Wales, W.J.; Williams, S.R.O. Influence of proportion of wheat in a pasture-based diet on milk yield, methane emissions, methane yield, and ruminal protozoa of dairy cows. J. Dairy Sci. 2020, 103, 2373–2386. [Google Scholar] [CrossRef] [PubMed]
- Melgar, A.; Welter, K.C.; Nedelkov, K.; Martins, C.M.M.R.; Harper, M.T.; Oh, J.; Räisänen, S.E.; Chen, X.; Cueva, S.F.; Duval, S.; et al. Dose-response effect of 3-nitrooxypropanol on enteric methane emissions in dairy cows. J. Dairy Sci. 2020, 103, 6145–6156. [Google Scholar] [CrossRef]
- van Gastelen, S.; Dijkstra, J.; Binnendijk, G.; Duval, S.M.; Heck, J.M.L.; Kindermann, M.; Zandstra, T.; Bannink, A. 3-Nitrooxypropanol decreases methane emissions and increases hydrogen emissions of early lactation dairy cows, with associated changes in nutrient digestibility and energy metabolism. J. Dairy Sci. 2020, 103, 8074–8093. [Google Scholar] [CrossRef]
- Williams, S.R.O.; Hannah, M.C.; Eckard, R.J.; Wales, W.J.; Moate, P.J. Supplementing the diet of dairy cows with fat or tannin reduces methane yield, and additively when fed in combination. Animal 2020, 14, s464–s472. [Google Scholar] [CrossRef]
- Moate, P.J.; Jacobs, J.L.; Hixson, J.L.; Deighton, M.H.; Hannah, M.C.; Morris, G.L.; Ribaux, B.E.; Wales, W.J.; Williams, S.R.O. Effects of feeding either red or white grape marc on milk production and methane emissions from early-lactation dairy cows. Animals 2020, 10, 976. [Google Scholar] [CrossRef]
- Mekuriaw, S.; Tsunekawa, A.; Ichinohe, T.; Tegegne, F.; Haregeweyn, N.; Kobayashi, N.; Tassew, A.; Mekuriaw, Y.; Walie, M.; Tsubo, M.; et al. Effect of feeding improved grass hays and eragrostis tef straw silage on milk yield, nitrogen utilization, and methane emission of lactating fogera dairy cows in Ethiopia. Animals 2020, 10, 1021. [Google Scholar] [CrossRef]
- Boland, T.M.; Pierce, K.M.; Kelly, A.K.; Kenny, D.A.; Lynch, M.B.; Waters, S.M.; Whelan, S.J.; McKay, Z.C. Feed intake, methane emissions, milk production and rumen methanogen populations of grazing dairy cows supplemented with various C 18 fatty acid sources. Animals 2020, 10, 2380. [Google Scholar] [CrossRef]
- Enriquez-Hidalgo, D.; Teixeira, D.L.; Pinheiro Machado Filho, L.C.; Hennessy, D.; Toro-Mujica, P.; Williams, S.R.O.; Pereira, F.C. Incorporating a fresh mixed annual ryegrass and berseem clover forage into the winter diet of dairy cows resulted in reduced milk yield, but reduced nitrogen excretion and reduced methane yield. Front. Vet. Sci. 2020, 7, 576944. [Google Scholar] [CrossRef]
- Benchaar, C. Feeding oregano oil and its main component carvacrol does not affect ruminal fermentation, nutrient utilization, methane emissions, milk production, or milk fatty acid composition of dairy cows. J. Dairy Sci. 2020, 103, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Bougouin, A.; Martin, C.; Doreau, M.; Ferlay, A. Effects of starch-rich or lipid-supplemented diets that induce milk fat depression on rumen biohydrogenation of fatty acids and methanogenesis in lactating dairy cows. Animal 2019, 13, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Van Wesemael, D.; Vandaele, L.; Ampe, B.; Cattrysse, H.; Duval, S.; Kindermann, M.; Fievez, V.; De Campeneere, S.; Peiren, N. Reducing enteric methane emissions from dairy cattle: Two ways to supplement 3-nitrooxypropanol. J. Dairy Sci. 2019, 102, 1780–1787. [Google Scholar] [CrossRef]
- Focant, M.; Froidmont, E.; Archambeau, Q.; Dang Van, Q.C.; Larondelle, Y. The effect of oak tannin (Quercus robur) and hops (Humulus lupulus) on dietary nitrogen efficiency, methane emission, and milk fatty acid composition of dairy cows fed a low-protein diet including linseed. J. Dairy Sci. 2019, 102, 1144–1159. [Google Scholar] [CrossRef]
- Sun, F.; Aguerre, M.J.; Wattiaux, M.A. Starch and dextrose at 2 levels of rumen-degradable protein in iso-nitrogenous diets: Effects on lactation performance, ruminal measurements, methane emission, digestibility, and nitrogen balance of dairy cows. J. Dairy Sci. 2019, 102, 1281–1293. [Google Scholar] [CrossRef]
- Kliem, K.E.; Humphries, D.J.; Kirton, P.; Givens, D.I.; Reynolds, C.K. Differential effects of oilseed supplements on methane production and milk fatty acid concentrations in dairy cows. Animal 2019, 13, 309–317. [Google Scholar] [CrossRef]
- Judy, J.V.; Bachman, G.C.; Brown-Brandl, T.M.; Fernando, S.C.; Hales, K.E.; Miller, P.S.; Stowell, R.R.; Kononoff, P.J. Energy balance and diurnal variation in methane production as affected by feeding frequency in Jersey cows in late lactation. J. Dairy Sci. 2018, 101, 10899–10910. [Google Scholar] [CrossRef]
- Cherif, C.; Hassanat, F.; Claveau, S.; Girard, J.; Gervais, R.; Benchaar, C. Faba bean (Vicia faba) inclusion in dairy cow diets: Effect on nutrient digestion, rumen fermentation, nitrogen utilization, methane production, and milk performance. J. Dairy Sci. 2018, 101, 8916–8928. [Google Scholar] [CrossRef]
- van Wyngaard, J.D.V.; Meeske, R.; Erasmus, L.J. Effect of concentrate level on enteric methane emissions, production performance, and rumen fermentation of Jersey cows grazing kikuyu-dominant pasture during summer. J. Dairy Sci. 2018, 101, 9954–9966. [Google Scholar] [CrossRef]
- Kidane, A.; Øverland, M.; Mydland, L.T.; Prestløkken, E. Interaction between feed use efficiency and level of dietary crude protein on enteric methane emission and apparent nitrogen use efficiency with Norwegian Red dairy cows. J. Anim. Sci. 2018, 96, 3967–3982. [Google Scholar] [CrossRef] [PubMed]
- Kolling, G.J.; Stivanin, S.C.B.; Gabbi, A.M.; Machado, F.S.; Ferreira, A.L.; Campos, M.M.; Tomich, T.R.; Cunha, C.S.; Dill, S.W.; Pereira, L.G.R.; et al. Performance and methane emissions in dairy cows fed oregano and green tea extracts as feed additives. J. Dairy Sci. 2018, 101, 4221–4234. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.R.O.; Chaves, A.V.; Deighton, M.H.; Jacobs, J.L.; Hannah, M.C.; Ribaux, B.E.; Morris, G.L.; Wales, W.J.; Moate, P.J. Influence of feeding supplements of almond hulls and ensiled citrus pulp on the milk production, milk composition, and methane emissions of dairy cows. J. Dairy Sci. 2018, 101, 2072–2083. [Google Scholar] [CrossRef] [PubMed]
- Bougouin, A.; Ferlay, A.; Doreau, M.; Martin, C. Effects of carbohydrate type or bicarbonate addition to grass silage-based diets on enteric methane emissions and milk fatty acid composition in dairy cows. J. Dairy Sci. 2018, 101, 6085–6097. [Google Scholar] [CrossRef]
- Bayat, A.R.; Tapio, I.; Vilkki, J.; Shingfield, K.J.; Leskinen, H. Plant oil supplements reduce methane emissions and improve milk fatty acid composition in dairy cows fed grass silage-based diets without affecting milk yield. J. Dairy Sci. 2018, 101, 1136–1151. [Google Scholar] [CrossRef]
- Stoldt, A.-K.; Derno, M.; Das, G.; Weitzel, J.M.; Wolffram, S.; Metges, C.C. Effects of rutin and buckwheat seeds on energy metabolism and methane production in dairy cows. J. Dairy Sci. 2016, 99, 2161–2168. [Google Scholar] [CrossRef]
- Lopes, J.C.; de Matos, L.F.; Harper, M.T.; Giallongo, F.; Oh, J.; Gruen, D.; Ono, S.; Kindermann, M.; Duval, S.; Hristov, A.N. Effect of 3-nitrooxypropanol on methane and hydrogen emissions, methane isotopic signature, and ruminal fermentation in dairy cows. J. Dairy Sci. 2016, 99, 5335–5344. [Google Scholar] [CrossRef]
- Pirondini, M.; Colombini, S.; Mele, M.; Malagutti, L.; Rapetti, L.; Galassi, G.; Crovetto, G.M. Effect of dietary starch concentration and fish oil supplementation on milk yield and composition, diet digestibility, and methane emissions in lactating dairy cows. J. Dairy Sci. 2015, 98, 357–372. [Google Scholar] [CrossRef]
- Reynolds, C.K.; Humphries, D.J.; Kirton, P.; Kindermann, M.; Duval, S.; Steinberg, W. Effects of 3-nitrooxypropanol on methane emission, digestion, and energy and nitrogen balance of lactating dairy cows. J. Dairy Sci. 2014, 97, 3777–3789. [Google Scholar] [CrossRef]

| Items | Mean | SD | Min | Max | CV | Median | n |
|---|---|---|---|---|---|---|---|
| BW (kg) | 610.90 | 66.52 | 456.00 | 739.00 | 10.89 | 622.50 | 102 |
| NDF (g/kg DM) | 346.18 | 64.37 | 225.00 | 519.00 | 18.60 | 342.00 | 117 |
| EE (g/kg DM) | 33.24 | 8.96 | 7.60 | 58.00 | 26.94 | 34.00 | 99 |
| DMI (kg/d) | 21.17 | 3.81 | 9.96 | 28.90 | 18.01 | 21.90 | 125 |
| OMI (g/d) | 20.37 | 2.69 | 15.60 | 25.90 | 13.22 | 20.70 | 55 |
| GEI (MJ/d) | 361.34 | 88.50 | 149.00 | 500.00 | 24.49 | 367.26 | 70 |
| MEI (MJ/d) | 246.38 | 32.50 | 189.12 | 299.00 | 13.19 | 241.42 | 28 |
| NDFI (kg/d) | 7.10 | 1.71 | 2.58 | 10.90 | 24.12 | 7.21 | 118 |
| ADFI (kg/d) | 4.09 | 1.33 | 0.79 | 7.26 | 32.38 | 4.55 | 97 |
| OMD (%) | 71.39 | 1.99 | 66.30 | 76.10 | 2.79 | 71.50 | 63 |
| NDFD (%) | 51.47 | 10.20 | 27.80 | 68.20 | 19.81 | 49.40 | 71 |
| Forage (%) | 58.71 | 15.99 | 9.00 | 100.00 | 27.23 | 60.00 | 128 |
| CH4 (g/d) | 381.34 | 85.15 | 129.00 | 510.00 | 22.33 | 392.00 | 117 |
| CH4 (MJ/d) | 22.52 | 5.23 | 8.81 | 34.17 | 23.23 | 22.68 | 134 |
| Model | Author | Prediction Model | Animal | Study |
|---|---|---|---|---|
| 1 | Kriss | CH4 (MJ/d) = 75.42 + 94.28 × DMI (kg/d) × 0.05524 (MJ/g of CH4) | Dairy | [22] |
| 2 | Axelsson | CH4 (MJ/d) = −2.07 + 2.636 × DMI − 0.105 × DMI2 | Dairy | [39] |
| 3 | Yan et al. | CH4 (g/d) = (3.23 + 0.055 × GEI)/0.05565 | Dairy | [38] |
| 4 | Mills et al. | CH4 (MJ/d) = 5.93 + 0.92 × DMI | Dairy | [21] |
| 5 | Mills et al. | CH4 (MJ/d) = 8.25 + 0.07 × MEI (MJ/d) | Dairy | [21] |
| 6 | Mills et al. | CH4 (g/d) = 56.27 × (1 − exp (−0.028 × DMI)/0.05565) | Dairy | [21] |
| 7 | IPCC | CH4 (g/d) = 0.065 × GEI/0.05565 | All | [26] |
| 8 | Ellis et al. | CH4 (g/d) = (3.14 + 2.11 × NDFI (kg/d))/0.05565 | Dairy | [36] |
| 9 | Ellis et al. | CH4 (g/d) = (2.16 + 0.493 × DMI − 1.36 × ADFI (kg/d) + 1.97 × NDFI (kg/d))/0.05565 | Dairy | [36] |
| 10 | Ellis et al. | CH4 (g/d) = (3.23 + 0.809 × DMI)/0.05565 | Dairy | [36] |
| 11 | Ellis et al. | CH4 (g/d) = (4.08 + 0.068 × MEI)/0.05565 | Dairy | [36] |
| 12 | Ellis et al. | CH4 (g/d) = (1.21 + 0.059 × MEI + 0.093 × Forage (%))/0.05565 | Dairy | [36] |
| 13 | Ellis et al. | CH4 (g/d) = (8.56 + 0.139 × Forage)/0.05565 | Dairy | [36] |
| 14 | Ellis et al. | CH4 (g/d) = (5.87 + 2.43 × ADFI)/0.05565 | Dairy | [36] |
| 15 | Ellis et al. | CH4 (MJ/d) = 3.41 + 0.520× DMI − 0.996 × ADFI + 1.15 × NDFI | All | [36] |
| 16 | Ellis et al. | CH4 (MJ/d) = 3.272 + 0.736 × DMI | All | [36] |
| 17 | Moate et al. | CH4 (g/d) = (24.51 − 0.0788 × EE (g/kg DM)) × DMI | Dairy | [40] |
| 18 | Hristov et al. | CH4 (g/d) = 2.54 + 19.14 × DMI | Dairy | [37] |
| 19 | Nielsen et al. | CH4 (g/d) = (1.26 × DMI)/0.05565 | Dairy | [41] |
| 20 | Ramin and Huhtanen | CH4 (g/d) = (62 + 25 × DMI) × 16.0/22.4 | Dairy | [18] |
| 21 | Ramin and Huhtanen | CH4 (g/d) = (20 + 35.8 × DMI − 0.5 × DMI2) × 16.0/22.4 | All | [18] |
| 22 | Ramin and Huhtanen | CH4 (MJ/d) = 0.797 + 1.427 × DMI − 0.020 × DMI2 | All | [18] |
| 23 | Storlien et al. | CH4 (g/d) = (−1.47 + 1.28 × DMI)/0.05565 | Dairy | [42] |
| 24 | Storlien et al. | CH4 (g/d) = (−2.76 + 3.74 × NDFI)/0.05565 | Dairy | [42] |
| 25 | Moraes et al. | CH4 (g/d) = (0.225 + 0.042 × GEI + 0.0125 × NDF (g/kg DM) − 0.0329 × EE)/0.05565 | Dairy | [17] |
| 26 | Moraes et al. | CH4 (g/d) = (3.247 + 0.043 × GEI)/0.05565 | Dairy | [17] |
| 27 | Charmley et al. | CH4 (g/d) = 38 + 19.22 × DMI | Dairy | [16] |
| 28 | Charmley et al. | CH4 (g/d) = (2.14 + 0.058 × GEI)/0.05565 | Dairy | [16] |
| 29 | Charmley et al. | CH4 (g/d) = 20.7 × DMI | All | [16] |
| 30 | Santiago-Juarez et al. | CH4 (g/d) = (4.544 + 0.773 × DMI)/0.05565 | Dairy | [25] |
| 31 | Patra | CH4 (MJ/d) = 35.21 − (35.21 + 0.25) × exp (−0.0354 × DMI) | All | [43] |
| 32 | Niu et al. | CH4 (g/d) = 107 + 14.5 × DMI | Dairy | [29] |
| 33 | Niu et al. | CH4 (g/d) = 160 + 14.2 × DMI − 13.5 × EE/10 | Dairy | [29] |
| 34 | Niu et al. | CH4 (g/d) = 26.0 + 15.3 × DMI + 3.42 × NDF/10 | Dairy | [29] |
| 35 | Ribeiro et al. | CH4 (g/d) = (4.15 + 0.822 × DMI)/0.05565 | Dairy | [44] |
| 36 | Ribeiro et al. | CH4 (g/d) = (3.35 + 0.047 × GEI)/0.05566 | Dairy | [44] |
| 37 | Donadia et al. | CH4 (g/d) = 550.21 − 0.669 × EE − 0.094 × OMD | Dairy | [35] |
| 38 | Donadia et al. | CH4 (g/d) = 133.49 − 0.025× EE × DMI + 0.021 × OMD × DMI | Dairy | [35] |
| 39 | Wang et al. | CH4 (MJ/d) = −0.3496 + 0.5941× DMI + 1.388 × NDFI + (−0.027) × ADFI | All | [45] |
| 40 | Wang et al. | CH4 (MJ/d) = 0.3989 + 0.8685 × DMI + 0.6675 × NDFI | Dairy | [45] |
| Rank | Model | Observed | Predicted | R2 | r | CCC | μ | MSPE (g/d, or MJ/d) | RMSPE (%) | MSPE | RSR | n | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ECT (%) | ER (%) | ED (%) | ||||||||||
| 1 | 38 | 392.87 ± 91.04 | 430.31 ± 65.10 | 0.66 | 0.81 | 0.69 | −0.49 | 64.86 | 16.51 | 33.32 | 1.97 | 66.16 | 0.71 | 47 |
| 2 | 12 | 415.84 ± 58.86 | 381.94 ± 32.92 | 0.72 | 0.85 | 0.58 | 0.77 | 48.51 | 11.67 | 48.83 | 12.38 | 41.01 | 0.82 | 24 |
| 3 | 21 | 396.71 ± 68.58 | 386.14 ± 44.15 | 0.33 | 0.57 | 0.51 | 0.19 | 57.18 | 14.41 | 3.42 | 0.74 | 96.75 | 0.83 | 107 |
| 4 | 33 | 398.59 ± 69.92 | 410.82 ± 51.63 | 0.34 | 0.58 | 0.55 | −0.20 | 58.69 | 14.72 | 4.34 | 3.36 | 93.46 | 0.84 | 83 |
| 5 | 5 | 24.08 ± 3.84 | 25.50 ± 2.32 | 0.40 | 0.63 | 0.51 | −0.48 | 3.26 | 13.54 | 19.03 | 0.09 | 83.88 | 0.85 | 28 |
| 6 | 39 | 23.22 ± 4.48 | 23.78 ± 4.10 | 0.35 | 0.59 | 0.58 | −0.13 | 3.92 | 16.86 | 2.02 | 13.79 | 85.08 | 0.87 | 111 |
| 7 | 22 | 23.24 ± 4.56 | 21.75 ± 2.39 | 0.32 | 0.57 | 0.43 | 0.45 | 4.04 | 17.37 | 13.57 | 0.21 | 86.92 | 0.88 | 125 |
| 8 | 32 | 396.71 ± 68.58 | 408.27 ± 55.99 | 0.30 | 0.55 | 0.53 | −0.19 | 60.95 | 15.36 | 3.59 | 8.98 | 88.33 | 0.89 | 107 |
| 9 | 35 | 396.71 ± 68.58 | 381.47 ± 57.03 | 0.30 | 0.55 | 0.53 | 0.24 | 62.07 | 15.65 | 6.03 | 9.68 | 85.17 | 0.90 | 107 |
| 10 | 3 | 371.13 ± 82.79 | 397.45 ± 87.17 | 0.38 | 0.62 | 0.59 | −0.31 | 78.11 | 21.05 | 11.35 | 21.08 | 69.18 | 0.94 | 56 |
| 11 | 30 | 396.71 ± 68.58 | 370.26 ± 53.63 | 0.30 | 0.55 | 0.49 | 0.44 | 64.82 | 16.34 | 16.66 | 6.03 | 78.10 | 0.95 | 107 |
| 12 | 25 | 376.39 ± 76.41 | 327.89 ± 53.50 | 0.47 | 0.69 | 0.51 | 0.76 | 73.30 | 19.47 | 43.78 | 0.02 | 57.54 | 0.96 | 43 |
| 13 | 4 | 23.24 ± 4.56 | 25.40 ± 3.52 | 0.30 | 0.55 | 0.47 | −0.54 | 4.49 | 19.31 | 23.31 | 5.13 | 72.19 | 0.98 | 125 |
| 14 | 18 | 396.71 ± 68.58 | 400.21 ± 73.90 | 0.30 | 0.55 | 0.55 | −0.05 | 67.52 | 17.02 | 0.27 | 28.71 | 71.96 | 0.98 | 107 |
| 15 | 20 | 396.71 ± 68.58 | 415.31 ± 68.95 | 0.30 | 0.55 | 0.53 | −0.27 | 67.54 | 17.03 | 7.58 | 21.38 | 71.92 | 0.98 | 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Ren, Y.; Li, Z.; Dong, R. Performance Comparison of the Prediction Models for Enteric Methane Emissions from Dairy Cattle. Vet. Sci. 2025, 12, 1036. https://doi.org/10.3390/vetsci12111036
Song M, Ren Y, Li Z, Dong R. Performance Comparison of the Prediction Models for Enteric Methane Emissions from Dairy Cattle. Veterinary Sciences. 2025; 12(11):1036. https://doi.org/10.3390/vetsci12111036
Chicago/Turabian StyleSong, Mimi, Yongliang Ren, Zenghui Li, and Ruilan Dong. 2025. "Performance Comparison of the Prediction Models for Enteric Methane Emissions from Dairy Cattle" Veterinary Sciences 12, no. 11: 1036. https://doi.org/10.3390/vetsci12111036
APA StyleSong, M., Ren, Y., Li, Z., & Dong, R. (2025). Performance Comparison of the Prediction Models for Enteric Methane Emissions from Dairy Cattle. Veterinary Sciences, 12(11), 1036. https://doi.org/10.3390/vetsci12111036







