Effects of Ultrasonic Treatment of Chicken Yolk on the Cryopreservation of Boar Semen
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Management
2.2. Reagents
2.3. Solution Preparation and Processing
2.3.1. Particle Size Analysis and Microstructural Observation
2.3.2. Emulsification Properties
2.4. Semen Collection and Processing
2.5. Semen Cryopreservation and Thawing
2.5.1. Analysis of Sperm Motility Parameters
2.5.2. Assessment of Sperm Acrosome Integrity
2.5.3. Plasma Membrane Integrity Rate
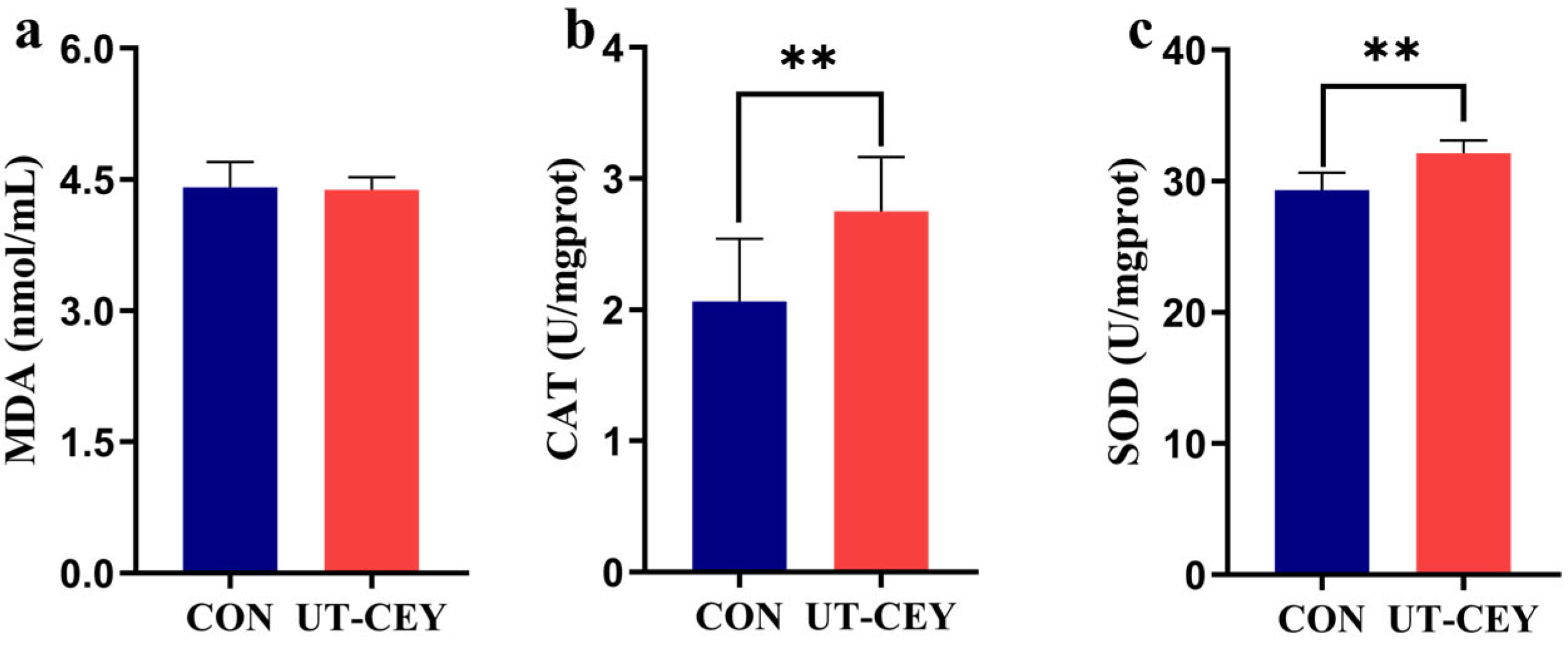
2.5.4. Detection of MDA, SOD and CAT
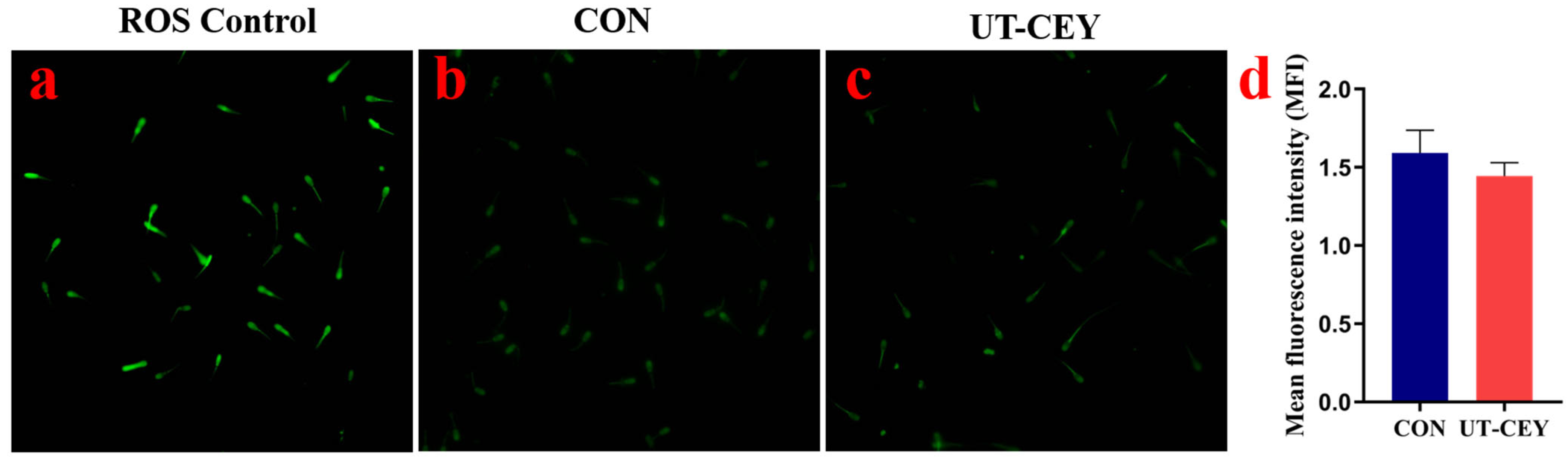
2.5.5. Detection of ROS Levels
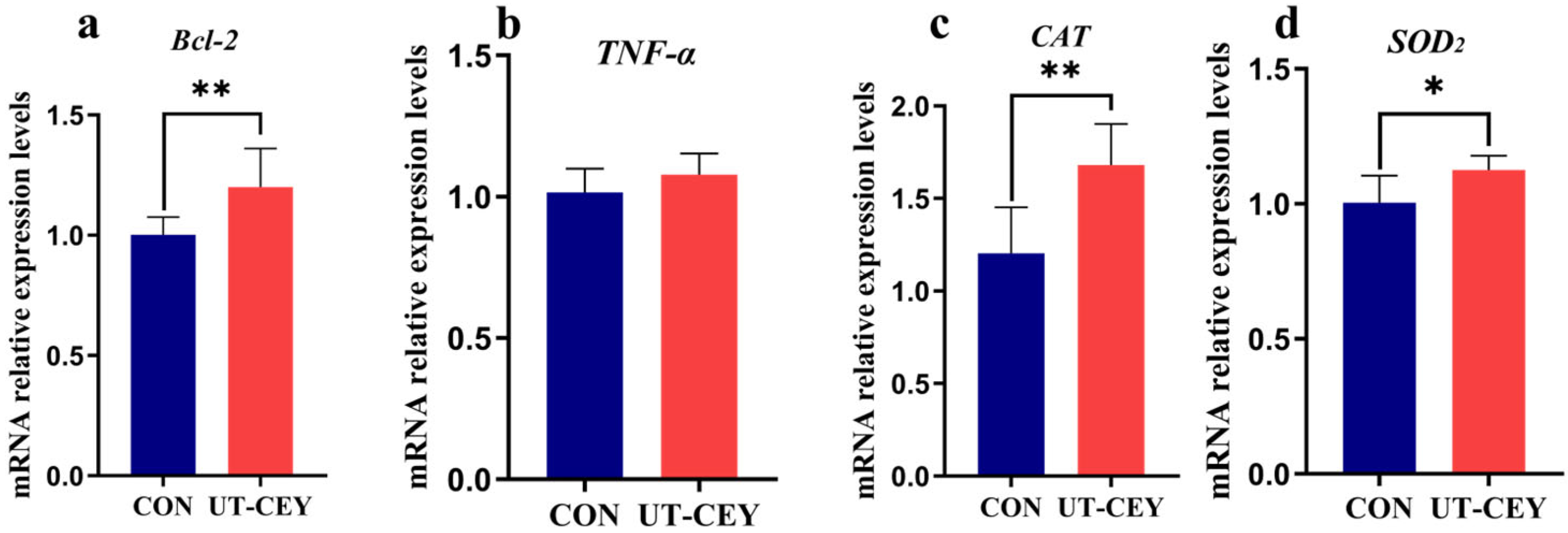
2.5.6. Detection of Apoptosis-Related Genes
2.6. Statistical Analysis
3. Results
3.1. Effect of Ultrasonic Treatment on the Particle Size of Yolk Diluent
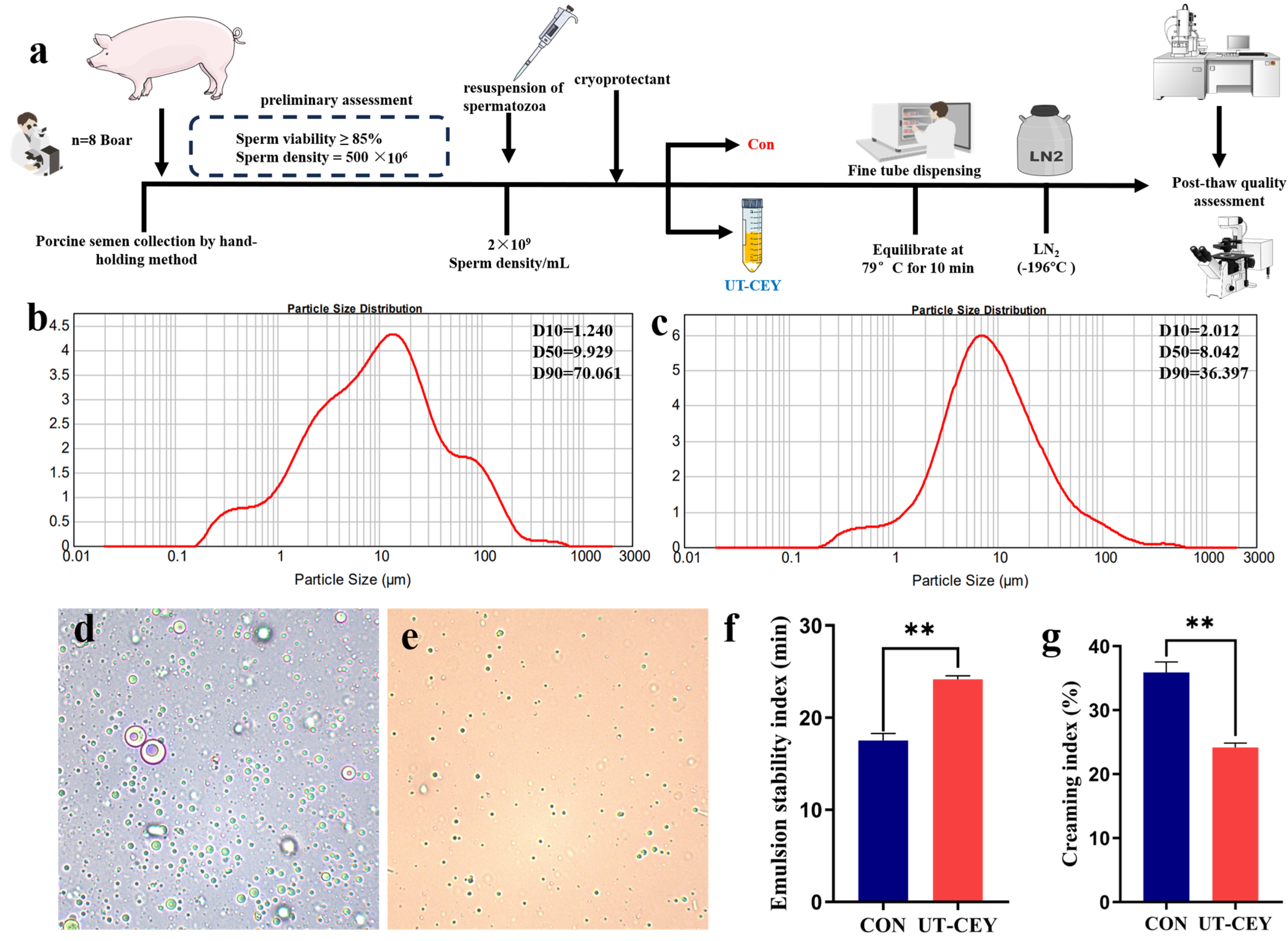
3.2. Effect of Ultrasonicated Chicken Egg Yolk on the Kinematic Parameters of Post-Thaw Boar Sperm
3.3. Effect of Ultrasonicated Chicken Egg Yolk on Acrosome Integrity and Plasma Membrane Integrity of Post-Thaw Boar Sperm
3.4. Effect of Ultrasonicated Egg Yolk on MDA, CAT, and SOD Levels in Post-Thaw Boar Sperm
3.5. Effect of Ultrasonicated Chicken Egg Yolk on ROS Levels in Post-Thaw Boar Sperm
3.6. Effect of Ultrasonic Treatment of Chicken Yolk on mRNA Expression Levels of Apoptosis-Related Genes in Boar Sperm After Freeze-Thawing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Prasad, J.K.; Srivastava, N.; Ghosh, S.K. Strategies to Minimize Various Stress-Related Freeze-Thaw Damages During Conventional Cryopreservation of Mammalian Spermatozoa. Biopreservation Biobanking 2019, 17, 603–612. [Google Scholar] [CrossRef]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm Cryopreservation: A Review on Current Molecular Cryobiology and Advanced Approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef]
- Jung, S.-E.; Ryu, B.-Y. New Strategies for Germ Cell Cryopreservation: Cryoinjury Modulation. Clin. Exp. Reprod. Med. 2023, 50, 213–222. [Google Scholar] [CrossRef]
- Fleming, S.D.; Thomson, L.K. The Oxidative Stress of Human Sperm Cryopreservation. Antioxidants 2025, 14, 402. [Google Scholar] [CrossRef]
- Liang, J.; Larbi, A.; Lv, C.; Ali, S.; Wu, G.; Quan, G. Fertility Results after Exocervical Insemination Using Goat Semen Cryopreserved with Extenders Based on Egg Yolk, Skim Milk, or Soybean Lecithin. Reprod. Domest. Anim. Zuchthyg. 2023, 58, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, C.; Wang, B.; Zeng, S.; Mu, R.; Li, G.; Li, B.; Lv, W. Research Progress and Application of Ultrasonic- and Microwave-Assisted Food Processing Technology. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3707–3731. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Xie, Y.; Wang, Y.; Wang, J. Depolymerization of Chicken Egg Yolk Granules Induced by High-Intensity Ultrasound. Food Chem. 2021, 354, 129580. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiang, D. Stability of Oil-in-Water Emulsions Performed by Ultrasound Power or High-Pressure Homogenization. PLoS ONE 2019, 14, e0213189. [Google Scholar] [CrossRef]
- He, W.-H.; Zhai, X.-H.; Duan, X.-J.; Di, H.-S. Effect of Resveratrol Treatment on Apoptosis and Apoptotic Pathways during Boar Semen Freezing. J. Zhejiang Univ. Sci. B 2020, 21, 485–494. [Google Scholar] [CrossRef]
- Zheng, D.; Yu, D.; Lin, S.; Ji, L.; Sun, Y.; Liu, C.; Zhang, X.; Yu, Z. Enhancing Salt-Induced Gelation of Egg Yolk Granules through pH-Ultrasound Combined Treatment: A Physicochemical and Microstructural Analysis. Ultrason. Sonochem. 2024, 111, 107101. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Liu, L.; Cheng, L.; Huang, Q.; Wu, D.; Peng, L.; Shi, X.; Li, S.; Geng, F. Quantitative N-Glycoproteomic Analyses Provide Insights into the Effects of Thermal Processes on Egg White Functional Properties. Food Chem. 2021, 342, 128252. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Wang, Y.; Wu, D.; Liang, D.; Ye, H.; Cai, Z.; Ma, M.; Geng, F. Effects of High-Intensity Ultrasonic (HIU) Treatment on the Functional Properties and Assemblage Structure of Egg Yolk. Ultrason. Sonochem. 2020, 60, 104767. [Google Scholar] [CrossRef]
- Xu, C.; Yang, X.; Sui, H.; Tong, X.; Zhang, D.; Zheng, X.; Jiao, J.; Wang, C.; Cao, Z.; Zhang, Y. Effects of Different Ages on Frozen Semen Quality and in Vitro Fertilization Efficiency in Wannan Black Pigs. Front. Vet. Sci. 2024, 11, 1395718. [Google Scholar] [CrossRef]
- Fraser, L.; Brym, P.; Pareek, C.S.; Mogielnicka-Brzozowska, M.; Paukszto, Ł.; Jastrzębski, J.P.; Wasilewska-Sakowska, K.; Mańkowska, A.; Sobiech, P.; Żukowski, K. Transcriptome Analysis of Boar Spermatozoa with Different Freezability Using RNA-Seq. Theriogenology 2020, 142, 400–413. [Google Scholar] [CrossRef]
- Salmani, H.; Towhidi, A.; Zhandi, M.; Bahreini, M.; Sharafi, M. In Vitro Assessment of Soybean Lecithin and Egg Yolk Based Diluents for Cryopreservation of Goat Semen. Cryobiology 2014, 68, 276–280. [Google Scholar] [CrossRef]
- Brum, A.M.; Thomas, A.D.; Sabeur, K.; Ball, B.A. Evaluation of Coomassie Blue Staining of the Acrosome of Equine and Canine Spermatozoa. Am. J. Vet. Res. 2006, 67, 358–362. [Google Scholar] [CrossRef]
- Cakir, C.; Kuspinar, G.; Ganiyev, A.; Aslan, K.; Kasapoglu, I.; Kilicarslan, H.; Ata, B.; Uncu, G.; Avcı, B. Reliability of Hypo-Osmotic Swelling Test on Fresh and Frozen-Thawed Ejaculated or Testicular Immotile Sperm: A Sibling Oocyte Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 293, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Toni, L.S.; Garcia, A.M.; Jeffrey, D.A.; Jiang, X.; Stauffer, B.L.; Miyamoto, S.D.; Sucharov, C.C. Optimization of Phenol-Chloroform RNA Extraction. MethodsX 2018, 5, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Sui, J.; Wang, J.; Wang, Y.; Wu, D.; Wang, B.; Geng, F. Research Note: Aggregation-Depolymerization of Chicken Egg Yolk Granule under Different Food Processing Conditions. Poult. Sci. 2023, 102, 102696. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, J.; Wang, N.; Wu, D.; Li, H.; Geng, F. Ultrasound-Assisted pH-Shifting Remodels Egg-Yolk Low-Density Lipoprotein to Enable Construction of a Stable Aqueous Solution of Vitamin D3. Curr. Res. Food Sci. 2022, 5, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luo, T.; Wang, L.; Song, H.; Wang, F.; Weng, Z.; Zhou, J.; Xiang, X.; Xiong, L.; Shen, X. Emulsifying Properties of Wheat Germ Protein: Effect of Different Ultrasonic Treatment. Ultrason. Sonochem. 2023, 98, 106479. [Google Scholar] [CrossRef]
- Xu, Q.; Li, X.; Lv, Y.; Liu, Y.; Yin, C. Effects of Ultrasonic Treatment on Ovomucin: Structure, Functional Properties and Bioactivity. Ultrason. Sonochem. 2022, 89, 106153. [Google Scholar] [CrossRef]
- Divar, M.R.; Mogheiseh, A.; Mohammadi, F.; Mavalizadeh, L. Effects of Extender Filtration and Egg Yolk Concentration on Canine Semen Cryopreservation. Reprod. Domest. Anim. Zuchthyg. 2023, 58, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-T.; Yan, B.; Guo, L.-N.; Liu, M.; Li, Y.-H.; Shao, Z.-Y.; Diao, H.; Liu, S.-Y.; Yu, H.-G. Scriptaid Is a Prospective Agent for Improving Human Asthenozoospermic Sample Quality and Fertilization Rate in Vitro. Asian J. Androl. 2024, 26, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Ramu, S.; Jeyendran, R.S. The Hypo-Osmotic Swelling Test for Evaluation of Sperm Membrane Integrity. Methods Mol. Biol. Clifton NJ 2013, 927, 21–25. [Google Scholar] [CrossRef]
- Breitbart, H.; Grinshtein, E. Mechanisms That Protect Mammalian Sperm from the Spontaneous Acrosome Reaction. Int. J. Mol. Sci. 2023, 24, 17005. [Google Scholar] [CrossRef]
- Hermansson, U.; Johannisson, A.; Axnér, E. Cryopreservation of Dog Semen in a Tris Extender with Two Different 1% Soybean Preparations Compared with a Tris Egg Yolk Extender. Vet. Med. Sci. 2021, 7, 812–819. [Google Scholar] [CrossRef]
- Strzezek, R.; Koziorowska-Gilun, M.; Stawiszyńska, M. Cryopreservation of Canine Semen: The Effect of Two Extender Variants on the Quality and Antioxidant Properties of Spermatozoa. Pol. J. Vet. Sci. 2012, 15, 721–726. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Shi, Y.; Wang, Y.; Cheng, L.; Liu, L.; Wang, N.; Li, H.; Wu, D.; Geng, F. Molecular Aggregation and Property Changes of Egg Yolk Low-Density Lipoprotein Induced by Ethanol and High-Density Ultrasound. Ultrason. Sonochem. 2020, 63, 104933. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.; Wang, X.; Wu, S.; Li, D.; Liu, C.; Feng, Z. Ultrasonic Thermal-Assisted Extraction of Phosvitin from Egg Yolk and Evaluation of Its Properties. Polymers 2019, 11, 1353. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Feng, C.; Liu, R.; Zheng, Y.; Hoque, S.A.M.; Wu, D.; Lu, H.; Zhang, T.; Zeng, W. Exogenous Oleic Acid and Palmitic Acid Improve Boar Sperm Motility via Enhancing Mitochondrial Β-Oxidation for ATP Generation. Animal 2020, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of Oxidative Stress and Antioxidants on Semen Functions. Vet. Med. Int. 2010, 2010, 686137. [Google Scholar] [CrossRef]
- Trzcińska, M.; Bryła, M. Apoptotic-like Changes of Boar Spermatozoa in Freezing Media Supplemented with Different Antioxidants. Pol. J. Vet. Sci. 2015, 18, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Onoe, S.; Sugita, Y.; Paquignon, M.; Dacheux, F.; Dacheux, J.L. Water Insoluble Fraction of Egg Yolk Maintains Porcine Sperm Motility by Activating Adenylate Cyclase. Mol. Reprod. Dev. 1991, 28, 136–142. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Min, L.; Cao, H.; Adetunji, A.O.; Zhou, K.; Zhu, Z. Pyrroloquinoline Quinone Improved Boar Sperm Quality via Maintaining Mitochondrial Function During Cryopreservation. Antioxidants 2025, 14, 102. [Google Scholar] [CrossRef]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M. Storage of Boar Semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef]
- Deng, Y.; Li, K.; Yan, W.; Li, K.; Wang, C. Gene Expression Regulation and the Signal Transduction of Programmed Cell Death. Curr. Issues Mol. Biol. 2024, 46, 10264–10298. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Kawai, T.; Umehara, T.; Hoque, S.A.M.; Zeng, W.; Shimada, M. Negative Effects of ROS Generated during Linear Sperm Motility on Gene Expression and ATP Generation in Boar Sperm Mitochondria. Free Radic. Biol. Med. 2019, 141, 159–171. [Google Scholar] [CrossRef]
- Naseri, F.; Siasy, E.; Angaji, S.A.; Peyvandi, M. RNA Sequencing Data Analysis of Oligoasthenoteratozoospermia Patients’ Sperms and Effects of Curcumin on the Expression of Some Genes during Sperm Cryopreservation and Freeze-Thawing Process. Iran. J. Public Health 2025, 54, 214–224. [Google Scholar] [CrossRef]
- Moradi Gardeshi, T.; Shahandeh, E.; Tavakolpoor Saleh, N.; Karami, S.; Mirzaei Azandaryani, Z.; Mazaheri, F.; Mohammadi, H. Evaluation of the Effect of Mitoquinone on Functional Parameters, DNA Structure, and Genes Expression Related to the Apoptotic and Antioxidants of Human Sperm after Freezing-Thawing. Mol. Biol. Rep. 2024, 51, 183. [Google Scholar] [CrossRef]

| Genes | Forward Sequence (5′-3′) | Reverse Sequence (5′-3’) | Product Length | NCBI ID |
|---|---|---|---|---|
| GAPDH | TTCCACGGCACAGTCAAGGC | CATGGTCGTGAAGACACCAG | 150 bp | NM_001206359.1 |
| TNF-α | ATTCAGGGATGTGTGGCCTG | CCAGATGTCCCAGGTTGCAT | 120 bp | NM_214022.1 |
| Bcl-2 | GGCAACCCATCCTGGCACCT | AACTCATCGCCCGCCTCCCT | 134 bp | NM_001322240.2 |
| CAT | GCTGAGTCCGAAGTCGTCTA | GCCTTACAGAACGTTGCAGG | 78 bp | NM_214301.2 |
| SOD2 | TTTGTAGGAGCGCCGAATAC | TAACCTCCTGGCTCTTTCCA | 217 bp | NM_214127.2 |
| Item | CON | UT-CEY | p-Value |
|---|---|---|---|
| TM (%) | 31.43 ± 0.76 B | 35.00 ± 0.59 A | 0.000 |
| PM (%) | 25.60 ± 1.06 B | 30.25 ± 0.83 A | 0.000 |
| VSL (μm/s) | 20.59 ± 0.50 B | 21.32 ± 0.60 A | 0.004 |
| VCL (μm/s) | 35.22 ± 1.07 B | 36.93 ± 0.72 A | 0.000 |
| VAP (μm/s) | 28.68 ± 0.97 B | 31.20 ± 1.04 A | 0.000 |
| LIN (%) | 58.53 ± 2.31 | 57.74 ± 1.26 | 0.311 |
| STR (%) | 71.86 ± 2.43 A | 68.42 ± 3.06 B | 0.006 |
| WOB (%) | 81.48 ± 2.90 b | 84.49 ± 2.78 a | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chang, F.; Zhang, B.; Liu, H.; Zhou, M.; Zhang, X.; Sang, S.; Li, X.; Li, J.; Hu, Q.; et al. Effects of Ultrasonic Treatment of Chicken Yolk on the Cryopreservation of Boar Semen. Vet. Sci. 2025, 12, 1024. https://doi.org/10.3390/vetsci12111024
Liu Y, Chang F, Zhang B, Liu H, Zhou M, Zhang X, Sang S, Li X, Li J, Hu Q, et al. Effects of Ultrasonic Treatment of Chicken Yolk on the Cryopreservation of Boar Semen. Veterinary Sciences. 2025; 12(11):1024. https://doi.org/10.3390/vetsci12111024
Chicago/Turabian StyleLiu, Yanyan, Fuqiang Chang, Biyu Zhang, Haidong Liu, Meng Zhou, Xin Zhang, Shouqian Sang, Xiu Li, Jing Li, Qianqian Hu, and et al. 2025. "Effects of Ultrasonic Treatment of Chicken Yolk on the Cryopreservation of Boar Semen" Veterinary Sciences 12, no. 11: 1024. https://doi.org/10.3390/vetsci12111024
APA StyleLiu, Y., Chang, F., Zhang, B., Liu, H., Zhou, M., Zhang, X., Sang, S., Li, X., Li, J., Hu, Q., Gu, Y., & Ruan, C. (2025). Effects of Ultrasonic Treatment of Chicken Yolk on the Cryopreservation of Boar Semen. Veterinary Sciences, 12(11), 1024. https://doi.org/10.3390/vetsci12111024





