The Combined Application of Intra-Articular Platelet-Rich Plasma Injections and Photobiomodulation Improves Clinical Outcomes in Dogs with Osteoarthritis—Results of a Long-Term, Double-Blinded, Crossover Study
Simple Summary
Abstract
1. Introduction
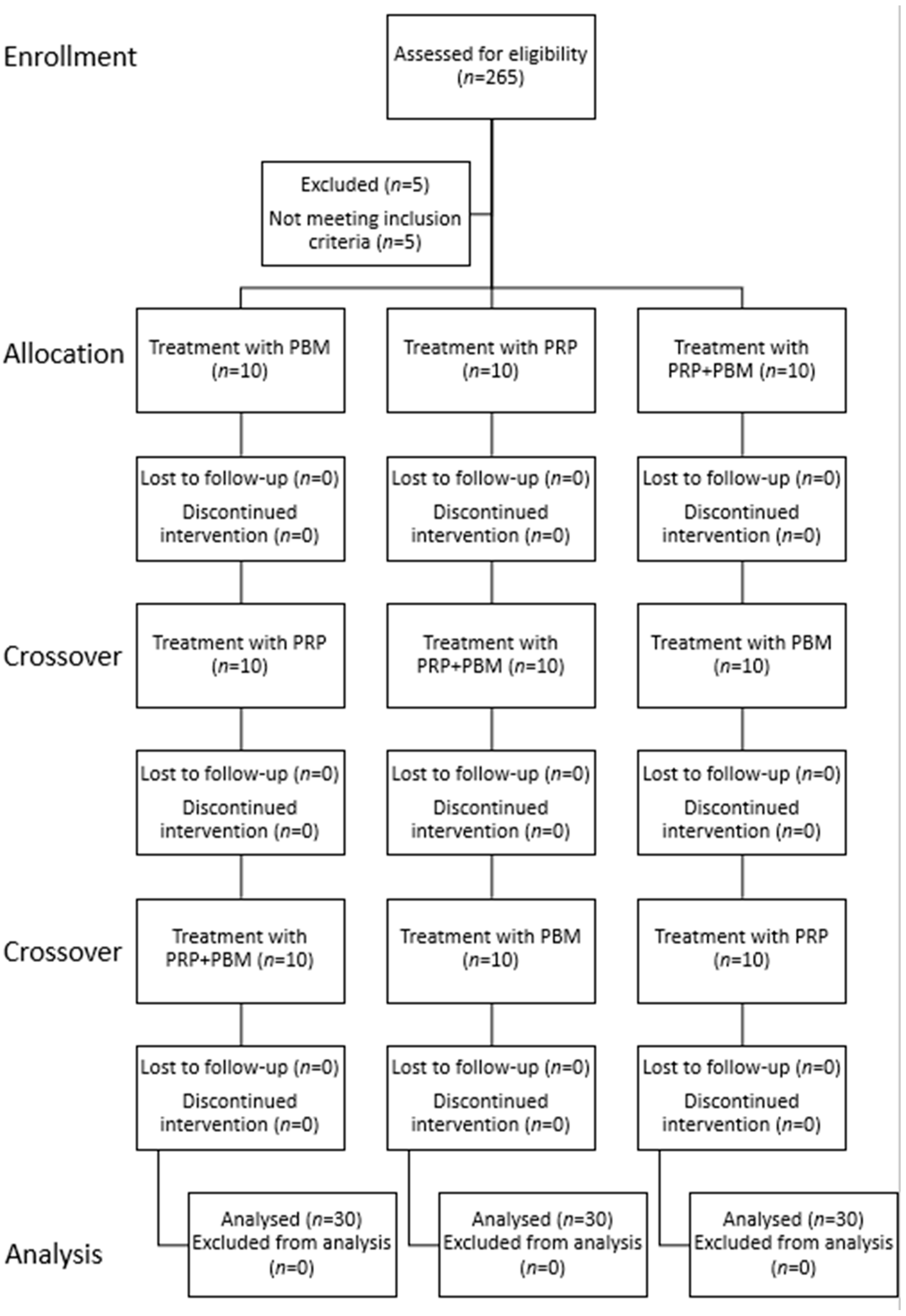
2. Materials and Methods
2.1. Procedures
2.2. Outcome Measures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, K.L.; O’Neill, D.G.; Brodbelt, D.C.; Church, D.B.; Meeson, R.L.; Sargan, D.; Summers, J.F.; Zulch, H.; Collins, L.M. Prevalence, Duration and Risk Factors for Appendicular Osteoarthritis in a UK Dog Population under Primary Veterinary Care. Sci. Rep. 2018, 8, 5641. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.C.; Santos, A.; Jorge, P.; Lavrador, C.; Carreira, L.M. Clinical and Diagnostic Imaging Findings in Police Working Dogs Referred for Hip Osteoarthritis. BMC Vet. Res. 2020, 16, 425. [Google Scholar] [CrossRef]
- Anderson, K.L.; Zulch, H.; O’Neill, D.G.; Meeson, R.L.; Collins, L.M. Risk Factors for Canine Osteoarthritis and Its Predisposing Arthropathies: A Systematic Review. Front. Vet. Sci. 2020, 7, 220. [Google Scholar] [CrossRef]
- Alves, J.C.A.; Jorge, P.I.F.; dos Santos, A.M.M.P. A Survey on the Orthopedic and Functional Assessment in a Portuguese Population of Police Working Dogs. BMC Vet. Res. 2022, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, M.; Falcone, A.; Alibrandi, A.; Zirilli, A.; Passantino, A. Risk Factors Regarding Dog Euthanasia and Causes of Death at a Veterinary Teaching Hospital in Italy: Preliminary Results. Vet. Sci. 2022, 9, 554. [Google Scholar] [CrossRef]
- Pegram, C.; Gray, C.; Packer, R.M.A.; Richards, Y.; Church, D.B.; Brodbelt, D.C.; O’Neill, D.G. Proportion and Risk Factors for Death by Euthanasia in Dogs in the UK. Sci. Rep. 2021, 11, 9145. [Google Scholar] [CrossRef]
- Enomoto, M.; de Castro, N.; Hash, J.; Thomson, A.; Nakanishi-Hester, A.; Perry, E.; Aker, S.; Haupt, E.; Opperman, L.; Roe, S.; et al. Prevalence of Radiographic Appendicular Osteoarthritis and Associated Clinical Signs in Young Dogs. Sci. Rep. 2024, 14, 2827. [Google Scholar] [CrossRef]
- Alves, J.C.; Santos, A.; Jorge, P.; Lafuente, P. A Multiple-Session Mesotherapy Protocol for the Management of Hip Osteoarthritis in Police Working Dogs. Am. J. Vet. Res. 2022, 84, 1–8. [Google Scholar] [CrossRef]
- Alves, J.C.C.; Santos, A.; Jorge, P.; Lavrador, C.; Carreira, L.M.M. Intraarticular Triamcinolone Hexacetonide, Stanozolol, Hylan G-F 20 and Platelet Concentrate in a Naturally Occurring Canine Osteoarthritis Model. Sci. Rep. 2021, 11, 3118. [Google Scholar] [CrossRef]
- Alves, J.C.; Santos, A.; Jorge, P.; Lafuente, P. Multiple Session Mesotherapy for Management of Coxofemoral Osteoarthritis Pain in 10 Working Dogs: A Case Series. Can. Vet. J. 2022, 63, 597–602. [Google Scholar] [PubMed]
- Hunt, J.R.; Dean, R.S.; Davis, G.N.D.; Murrell, J.C. An Analysis of the Relative Frequencies of Reported Adverse Events Associated with NSAID Administration in Dogs and Cats in the United Kingdom. Vet. J. 2015, 206, 183–190. [Google Scholar] [CrossRef]
- Mabry, K.; Hill, T.; Tolbert, M.K. Prevalence of Gastrointestinal Lesions in Dogs Chronically Treated with Nonsteroidal Anti-inflammatory Drugs. J. Vet. Intern. Med. 2021, 35, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Cachon, T.; Frykman, O.; Innes, J.F.; Lascelles, B.D.X.; Okumura, M.; Sousa, P.; Staffieri, F.; Steagall, P.V.; Van Ryssen, B. COAST Development Group’s International Consensus Guidelines for the Treatment of Canine Osteoarthritis. Front. Vet. Sci. 2023, 10, 1137888. [Google Scholar] [CrossRef]
- Mosley, C.; Edwards, T.; Romano, L.; Truchetti, G.; Dunbar, L.; Schiller, T.; Gibson, T.; Bruce, C.; Troncy, E. Proposed Canadian Consensus Guidelines on Osteoarthritis Treatment Based on OA-COAST Stages 1–4. Front. Vet. Sci. 2022, 9, 830098. [Google Scholar] [CrossRef]
- Innes, J.F.; Clayton, J.; Lascelles, B.D.X. Review of the Safety and Efficacy of Long-Term NSAID Use in the Treatment of Canine Osteoarthritis. Vet. Rec. 2010, 166, 226–230. [Google Scholar] [CrossRef]
- Walton, M.B.; Cowderoy, E.C.; Wustefeld-Janssens, B.; Lascelles, B.D.X.; Innes, J.F. Mavacoxib and Meloxicam for Canine Osteoarthritis: A Randomised Clinical Comparator Trial. Vet. Rec. 2014, 175, 280. [Google Scholar] [CrossRef]
- Moreau, M.; Dupuis, J.; Bonneau, N.H. Clinical Evaluation of a Nutraceutical, Carprofen and Meloxicam for the Treatment of Dogs with Osteoarthritis. Vet. Rec. 2003, 152, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.; Waibel, F.W.A.; Carrera, I.; Spattini, G.; Clark, L.; Adams, R.J.; Von Pfeil, D.J.F.; De Sousa, R.J.R.; Villagrà, D.B.; Amengual-Vila, M.; et al. Musculoskeletal Adverse Events in Dogs Receiving Bedinvetmab (Librela). Front. Vet. Sci. 2025, 12, 1581490. [Google Scholar] [CrossRef] [PubMed]
- Juhakoski, R.; Heliovaara, M.; Impivaara, O.; Kroger, H.; Knekt, P.; Lauren, H.; Arokoski, J.P.A. Risk Factors for the Development of Hip Osteoarthritis: A Population-Based Prospective Study. Rheumatology 2008, 48, 83–87. [Google Scholar] [CrossRef]
- Frizziero, A.; Giannotti, E.; Ferraro, C.; Masiero, S. Platelet Rich Plasma Intra-Articular Injections: A New Therapeutic Strategy for the Treatment of Knee Osteoarthritis in Sport Rehabilitation. A Systematic Review. Sport. Sci. Health 2012, 8, 15–22. [Google Scholar] [CrossRef]
- Alves, J.C.A.; dos Santos, A.M.M.P.; Jorge, P.I.F.; Lavrador, C.F.T.V.B.; Carreira, L.M.A. Management of Osteoarthritis Using 1 Intra-Articular Platelet Concentrate Administration in a Canine Osteoarthritis Model. Am. J. Sports Med. 2021, 49, 599–608. [Google Scholar] [CrossRef]
- Alves, J.C.; Santos, A.; Jorge, P.; Lavrador, C.; Carreira, L.M. A Report on the Use of a Single Intra-Articular Administration of Autologous Platelet Therapy in a Naturally Occurring Canine Osteoarthritis Model—A Preliminary Study. BMC Musculoskelet. Disord. 2020, 21, 127. [Google Scholar] [CrossRef]
- Alves, J.C.; Santos, A.; Carreira, L.M. A Preliminary Report on the Combined Effect of Intra-Articular Platelet-Rich Plasma Injections and Photobiomodulation in Canine Osteoarthritis. Animals 2023, 13, 3247. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.R.; Geeslin, A.G.; Goudie, E.B.; Petrigliano, F.A.; LaPrade, R.F. Minimum Information for Studies Evaluating Biologics in Orthopaedics (MIBO). J. Bone Jt. Surg. 2017, 99, 809–819. [Google Scholar] [CrossRef]
- Arican, M.; Şimşek, A.; Parlak, K.; Atli, K.; Sönmez, G. Matrix Metalloproteinases 2 and 9 Activity after Intra-Articular Injection of Autologous Platelet-Rich Plasma for the Treatment of Osteoarthritis in Dogs. Acta Vet. Brno 2018, 87, 127–135. [Google Scholar] [CrossRef]
- Fahie, M.A.; Ortolano, G.A.; Guercio, V.; Schaffer, J.A.; Johnston, G.; Au, J.; Hettlich, B.A.; Phillips, T.; Allen, M.J.; Bertone, A.L. A Randomized Controlled Trial of the Efficacy of Autologous Platelet Therapy for the Treatment of Osteoarthritis in Dogs. J. Am. Vet. Med. Assoc. 2013, 243, 1291–1297. [Google Scholar] [CrossRef]
- Silva, R.F.; Carmona, J.U.; Rezende, C.M.F. Intra-Articular Injections of Autologous Platelet Concentrates in Dogs with Surgical Reparation of Cranial Cruciate Ligament Rupture. Vet. Comp. Orthop. Traumatol. 2013, 26, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Smith, P.A.; Bozynski, C.C.; Kuroki, K.; Cook, C.R.; Stoker, A.M.; Pfeiffer, F.M. Multiple Injections of Leukoreduced Platelet Rich Plasma Reduce Pain and Functional Impairment in a Canine Model of ACL and Meniscal Deficiency. J. Orthop. Res. 2016, 34, 607–615. [Google Scholar] [CrossRef]
- Parlak, K.; Arican, M. Effect of Intra-Articular Administration of Autologous PRP and Activated PRP on Inflammatory Mediators in Dogs with Osteoarthritis. Vet. Med. 2020, 65, 62–70. [Google Scholar] [CrossRef]
- Alves, J.C.; Santos, A.; Jorge, P. Platelet-Rich Plasma Therapy in Dogs with Bilateral Hip Osteoarthritis. BMC Vet. Res. 2021, 17, 207. [Google Scholar] [CrossRef]
- Anders, J.; Kobiela Kertz, A.; Wu, X. Basic Principles of Photobiomodulation and Its Effects at the Cellular, Tissue, and System Levels. In Laser Therapy in Veterinary Medicine: Photobiomodulation; Riegel, R.J., Goldbold, J., Eds.; Wiley Blackwell: Ames, IA, USA, 2017; pp. 36–52. [Google Scholar]
- Wardlaw, J.L.; Gazzola, K.M.; Wagoner, A.; Brinkman, E.; Burt, J.; Butler, R.; Gunter, J.M.; Senter, L.H. Laser Therapy for Incision Healing in 9 Dogs. Front. Vet. Sci. 2019, 5, 349. [Google Scholar] [CrossRef] [PubMed]
- Looney, A.L.; Huntingford, J.L.; Blaeser, L.L.; Mann, S. A Randomized Blind Placebo-Controlled Trial Investigating the Effects of Photobiomodulation Therapy (PBMT) on Canine Elbow Osteoarthritis. Can. Vet. J. 2018, 59, 959–966. [Google Scholar]
- Alves, J.C.; Santos, A.; Jorge, P.; Carreira, L.M. A Randomized Double-Blinded Controlled Trial on the Effects of Photobiomodulation Therapy in Dogs with Osteoarthritis. Am. J. Vet. Res. 2022, 83, ajvr.22.03.0036. [Google Scholar] [CrossRef]
- Alves, J.C.; Jorge, P.; Santos, A. The Effect of Photobiomodulation Therapy on the Management of Chronic Idiopathic Large-Bowel Diarrhea in Dogs. Lasers Med. Sci. 2021, 37, 2045–2051. [Google Scholar] [CrossRef]
- Alves, J.C.; Jorge, P.; Santos, A. The Effect of Photobiomodulation Therapy on Inflammation Following Dental Prophylaxis. J. Vet. Dent. 2023, 41, 26–30. [Google Scholar] [CrossRef]
- Flückiger, M. Scoring Radiographs for Canine Hip Dysplasia—The Big Three Organisations in the World. Eur. J. Compagnion Anim. Pract. 2008, 2, 135–140. [Google Scholar]
- Alves, J.C.; Santos, A.; Jorge, P.; Lavrador, C.; Carreira, L.M. Characterization of Weight-Bearing Compensation in Dogs With Bilateral Hip Osteoarthritis. Top. Companion Anim. Med. 2022, 49, 100655. [Google Scholar] [CrossRef]
- Clough, W.; Canapp, S.; Taboada, L.; Dycus, D.; Leasure, C. Sensitivity and Specificity of a Weight Distribution Platform for the Detection of Objective Lameness and Orthopaedic Disease. Vet. Comp. Orthop. Traumatol. 2018, 31, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.B.; Cowderoy, E.; Lascelles, D.; Innes, J.F. Evaluation of Construct and Criterion Validity for the ‘Liverpool Osteoarthritis in Dogs’ (LOAD) Clinical Metrology Instrument and Comparison to Two Other Instruments. PLoS ONE 2013, 8, e58125. [Google Scholar] [CrossRef]
- Volstad, N.; Sandberg, G.; Robb, S.; Budsberg, S. The Evaluation of Limb Symmetry Indices Using Ground Reaction Forces Collected with One or Two Force Plates in Healthy Dogs. Vet. Comp. Orthop. Traumatol. 2017, 30, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.C.; Santos, A.; Jorge, P. Initial Psychometric Evaluation of the Portuguese Version of the Canine Brief Pain Inventory. Am. J. Vet. Res. 2022, 84, ajvr.22.09.0166. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.C.; Jorge, P.; Santos, A. Initial Psychometric Evaluation of the Portuguese Version of the Liverpool Osteoarthritis in Dogs. BMC Vet. Res. 2022, 18, 367. [Google Scholar] [CrossRef]
- Alves, J.C. Initial Psychometric Evaluation of the Portuguese Version of the Canine Orthopedic Index. Vet. Comp. Orthop. Traumatol. 2023, 36, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.C.; Santos, A.; Jorge, P.; Lavrador, C.; Carreira, L.M. Evaluation of Four Clinical Metrology Instruments for the Assessment of Osteoarthritis in Dogs. Animals 2022, 12, 2808. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.C.; Santos, A.; Lavrador, C.; Carreira, L.M. Minimal Clinically Important Differences for a Weight Distribution Platform in Dogs with Osteoarthritis. Animals 2023, 14, 128. [Google Scholar] [CrossRef]
- Brown, D.C.; Bell, M.; Rhodes, L. Power of Treatment Success Definitions When the Canine Brief Pain Inventory Is Used to Evaluate Carprofen Treatment for the Control of Pain and Inflammation in Dogs with Osteoarthritis. Am. J. Vet. Res. 2013, 74, 1467–1473. [Google Scholar] [CrossRef]
- Innes, J.F.; Morton, M.; Lascelles, B.D.X. Minimal Clinically-Important Difference for “Liverpool Osteoarthritis in Dogs” (LOAD) and Canine Orthopedic Index (COI). In Proceedings of the 21st ESVOT Congress, ESVOT, Nice, France, 21–24 September 2022; pp. 164–166. [Google Scholar]
- Alves, J.C.; Innes, J.F. Minimal Clinically-Important Differences for the “Liverpool Osteoarthritis in Dogs” (LOAD) and the “Canine Orthopedic Index” (COI) in Dogs with Osteoarthritis. PLoS ONE 2023, 18, e0291881. [Google Scholar] [CrossRef]
- Laflamme, D. Development and Validation of a Body Condition Score System for Dogs. Canine Pr. 1997, 22, 10–15. [Google Scholar]
- Cai, X.; Zaki, S. The Effect of Intra-articular Platelet-rich Plasma Injection on Pain and Lameness in Dogs with Osteoarthritis. Aust. Vet. J. 2025, 103, 663–671. [Google Scholar] [CrossRef]
- Walton, B.; Cox, T.; Innes, J. ‘How Do I Know My Animal Got Better?’—Measuring Outcomes in Small Animal Orthopaedics. In Pr. 2018, 40, 42–50. [Google Scholar] [CrossRef]
- Vassão, P.G.; Parisi, J.; Penha, T.F.C.; Balão, A.B.; Renno, A.C.M.; Avila, M.A. Association of Photobiomodulation Therapy (PBMT) and Exercises Programs in Pain and Functional Capacity of Patients with Knee Osteoarthritis (KOA): A Systematic Review of Randomized Trials. Lasers Med. Sci. 2021, 36, 1341–1353. [Google Scholar] [CrossRef]
- Sanches, M.; Assis, L.; Criniti, C.; Fernandes, D.; Tim, C.; Renno, A.C.M. Chondroitin Sulfate and Glucosamine Sulfate Associated to Photobiomodulation Prevents Degenerative Morphological Changes in an Experimental Model of Osteoarthritis in Rats. Lasers Med. Sci. 2018, 33, 549–557. [Google Scholar] [CrossRef]
- Stancker, T.G.; Vieira, S.S.; Serra, A.J.; do Nascimento Lima, R.; dos Santos Feliciano, R.; Silva, J.A.; dos Santos, S.A.; dos Santos Vieira, M.A.; Simões, M.C.B.; Leal-Junior, E.C.; et al. Can Photobiomodulation Associated with Implantation of Mesenchymal Adipose-Derived Stem Cells Attenuate the Expression of MMPs and Decrease Degradation of Type II Collagen in an Experimental Model of Osteoarthritis? Lasers Med. Sci. 2018, 33, 1073–1084. [Google Scholar] [CrossRef]
- Armitage, A.J.; Miller, J.M.; Sparks, T.H.; Georgiou, A.E.; Reid, J. Efficacy of Autologous Mesenchymal Stromal Cell Treatment for Chronic Degenerative Musculoskeletal Conditions in Dogs: A Retrospective Study. Front. Vet. Sci. 2023, 9, 1014687. [Google Scholar] [CrossRef] [PubMed]
- Irmak, G.; Demirtaş, T.T.; Gümüşderelioğlu, M. Sustained Release of Growth Factors from Photoactivated Platelet Rich Plasma (PRP). Eur. J. Pharm. Biopharm. 2020, 148, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.J.; Miller, A.V.; Colbath, A.C.; Peralta, S.; Frye, C.W. Literature Review Details and Supports the Application of Platelet-Rich Plasma Products in Canine Medicine, Particularly as an Orthobiologic Agent for Osteoarthritis. J. Am. Vet. Med. Assoc. 2024, 262, S8–S15. [Google Scholar] [CrossRef] [PubMed]
- Lees, P. Pharmacology of Drugs Used to Treat Osteoarthritis in Veterinary Practice. Inflammopharmacology 2003, 11, 385–399. [Google Scholar] [CrossRef]
- Venable, R.O.; Stoker, A.M.; Cook, C.R.; Cockrell, M.K.; Cook, J.L. Examination of Synovial Fluid Hyaluronan Quantity and Quality in Stifle Joints of Dogs with Osteoarthritis. Am. J. Vet. Res. 2008, 69, 1569–1573. [Google Scholar] [CrossRef]
- Johnston, S.A. Osteoarthritis. Joint Anatomy, Physiology, and Pathobiology. Vet. Clin. N. Am. Small Anim. Pr. 1997, 27, 699–723. [Google Scholar] [CrossRef]
- Vina, E.R.; Kwoh, C.K. Epidemiology of Osteoarthritis. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef]
- Ornetti, P.; Nourissat, G.; Berenbaum, F.; Sellam, J.; Richette, P.; Chevalier, X. Does Platelet-Rich Plasma Have a Role in the Treatment of Osteoarthritis? Jt. Bone Spine 2016, 83, 31–36. [Google Scholar] [CrossRef] [PubMed]
| Light Parameters (Dose) | |
|---|---|
| Wavelength (nm) | 980 nm |
| Radiant Power (W) | 13 |
| Irradiance (W/cm2) at the skin surface | 2.6 (using a large contact treatment head) |
| Fluence (J/cm2) | 15 |
| Total Joules | 5250 |
| Treatment Protocol | Continuously moving grid pattern in contact over the treatment area at a speed of 2.5–7.5 cm/s, according to manufacturer recommendations. |
| Treatment Area (cm2) | 350 (entire hip area) |
| Treatment Time | 6 min, 44 s |
| Parameter | Whole Blood | Platelet Concentrate | ||
|---|---|---|---|---|
| Mean Value | SD | Mean Value | SD | |
| Platelets (×103/mm3) | 327.93 | 86.13 | 1708.53 | 440.99 |
| RBC (×106/mm3) | 6.82 | 1.21 | 0.48 | 0.06 |
| WDC (×103/mm3) | 11.18 | 4.36 | 4.47 | 3.40 |
| Lymphocytes (×103/mm3) | 2.26 | 0.86 | 2.71 | 1.79 |
| Monocytes (×103/mm3) | 0.76 | 0.41 | 0.57 | 0.35 |
| Neutrophils (×103/mm3) | 7.73 | 3.49 | 0.85 | 0.35 |
| Eosinophils (×103/mm3) | 0.42 | 0.47 | 0.35 | 0.41 |
| Basophils (×103/mm3) | 0.01 | 0.02 | 0.00 | 0.00 |
| Measure | Group | T0 | p | +8 d | p | ES | +15 d | p | ES | +30 d | p | ES | +60 d | p | ES | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Med | IQR | Med | IQR | Δ | Med | IQR | Δ | Med | IQR | Δ | Med | IQR | Δ | ||||||||||||||||||||||||||||||||
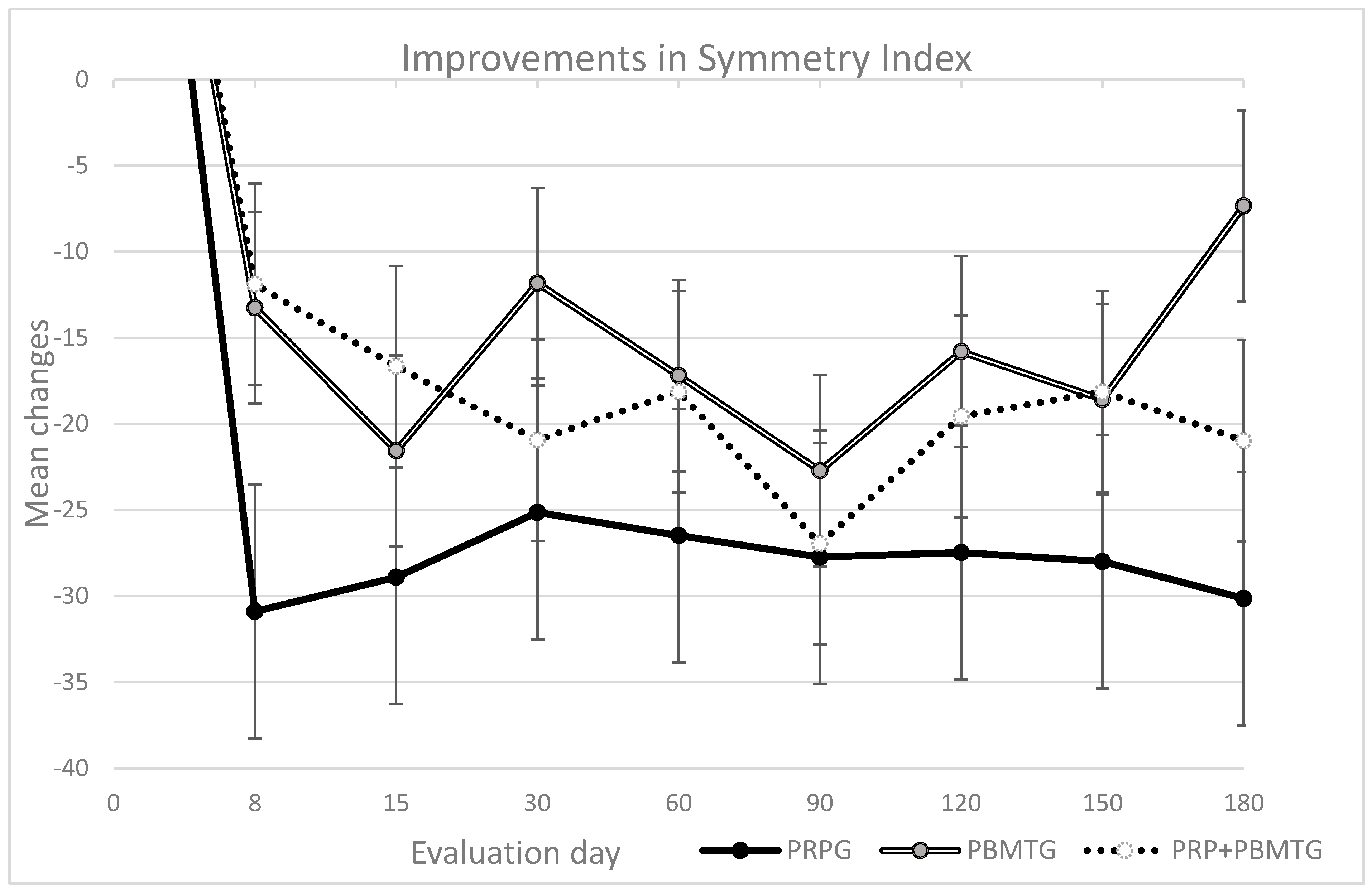
| Weight bearing | Symmetry Index (CSI ≥ 10) | PRPG | 38.0 | 58.7 | 0.90 | 7.1 | 10.9 | −30.9 | 0.03 * | 0.16 | 9.1 | 10.9 | −28.9 | 0.02 * | 0.38 | 12.9 | 22.4 | −25.1 | 0.03 * | 0.30 | 11.5 | 28.7 | −26.5 | 0.77 | 0.17 | ||||||||||||||||||||
| PBMTG | 31.8 | 39.6 | 18.6 | 23.5 | −13.3 | 10.3 | 13.3 | −21.6 | 20.0 | 46.3 | −11.8 | 14.6 | 20.5 | −17.2 | |||||||||||||||||||||||||||||||
| PRP + PBMTG | 32.1 | 39.6 | 20.2 | 27.8 | −11.9 | 15.4 | 27.8 | −16.7 | 11.1 | 17.5 | −20.9 | 14.0 | 17.1 | −18.1 | |||||||||||||||||||||||||||||||
| Deviation (CSI ≥ 2) | PRPG | 4.7 | 5.3 | 0.42 | 1.5 | 2.0 | −3.2 | 0.08 | 0.28 | 2.0 | 2.0 | −2.7 | 0.16 | 0.38 | 3.0 | 4.8 | −1.7 | 0.85 | 0.20 | 2.5 | 4.8 | −2.2 | 0.21 | 0.13 | |||||||||||||||||||||
| PBMTG | 7.3 | 10.0 | 2.0 | 3.8 | −5.3 | 3.5 | 3.5 | −3.8 | 3.5 | 5.8 | −3.8 | 4.0 | 5.8 | −3.3 | |||||||||||||||||||||||||||||||
| PRP + PBMTG | 6.0 | 6.7 | 2.5 | 2.8 | −3.5 | 2.0 | 3.0 | −4.0 | 3.0 | 4.0 | −3.0 | 3.0 | 3.0 | −3.0 | |||||||||||||||||||||||||||||||
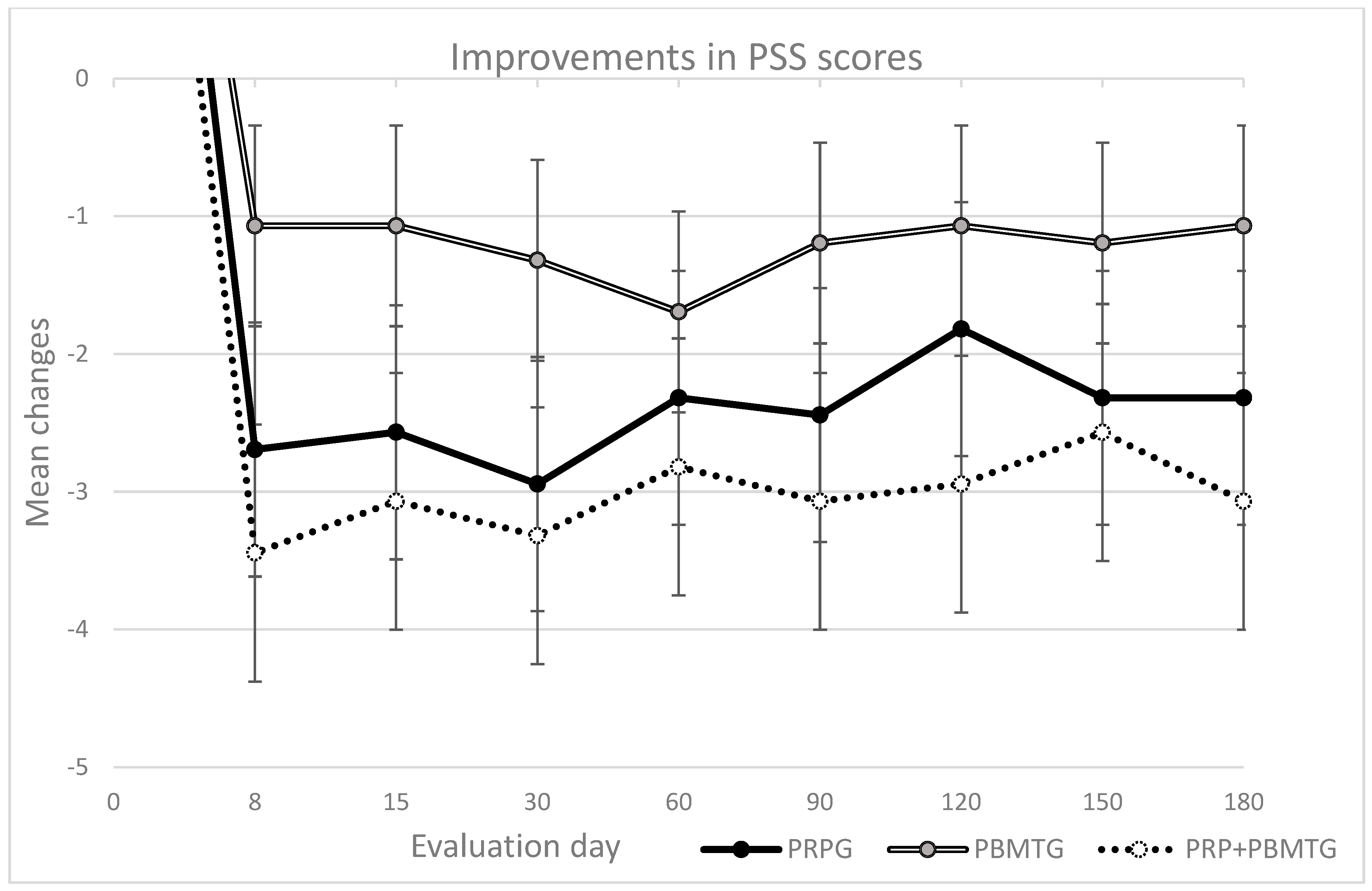
| CBPI | PSS (range 0–10; CSI ≥ 1) | PRPG | 5.8 | 2.2 | 0.11 | 3.1 | 3.8 | −2.7 | 0.17 | 0.10 | 3.3 | 3.9 | −2.6 | 0.30 | 0.38 | 2.9 | 3.5 | −2.9 | 0.01 * | 0.41 | 3.5 | 3.6 | −2.3 | 0.51 | 0.07 | ||||||||||||||||||||
| PBMTG | 5.3 | 1.8 | 4.3 | 3.8 | −1.1 | 4.3 | 2.0 | −1.1 | 4.0 | 2.2 | −1.3 | 3.6 | 2.2 | −1.7 | |||||||||||||||||||||||||||||||
| PRP + PBMTG | 5.3 | 3.0 | 1.9 | 2.5 | −3.4 | 2.3 | 3.8 | −3.1 | 2.0 | 3.4 | −3.3 | 2.5 | 3.9 | −2.8 | |||||||||||||||||||||||||||||||
| PIS (range 0–10; CSI ≥ 2) | PRPG | 5.5 | 3.8 | 0.24 | 2.7 | 5.0 | −2.8 | 0.18 | 0.15 | 3.4 | 5.0 | −2.1 | 0.77 | 0.38 | 2.6 | 4.3 | −2.9 | 0.16 | 0.21 | 3.6 | 3.8 | −1.9 | 0.69 | 0.12 | |||||||||||||||||||||
| PBMTG | 5.8 | 4.1 | 4.0 | 2.9 | −1.8 | 4.0 | 2.6 | −1.8 | 3.9 | 3.9 | −1.9 | 4.0 | 3.2 | −1.8 | |||||||||||||||||||||||||||||||
| PRP + PBMTG | 4.4 | 4.7 | 1.9 | 3.7 | −2.5 | 1.9 | 5.2 | −2.5 | 2.0 | 4.0 | −2.4 | 2.0 | 4.7 | −2.4 | |||||||||||||||||||||||||||||||
| LOAD (range 0–52; CSI ≥ 4) | PRPG | 22.6 | 14.0 | 0.46 | 14.0 | 9.5 | −8.6 | 0.01 * | 0.43 | 14.0 | 11.0 | −8.6 | 0.03 * | 0.38 | 14.0 | 10.8 | −8.6 | 0.04 * | 0.45 | 18.5 | 13.8 | −4.1 | 0.04 * | 0.38 | |||||||||||||||||||||
| PBMTG | 27.9 | 17.0 | 21.5 | 14.8 | −6.4 | 22.5 | 14.5 | −5.4 | 22.5 | 17.5 | −5.4 | 21.0 | 18.5 | −6.9 | |||||||||||||||||||||||||||||||
| PRP + PBMTG | 26.6 | 15.0 | 13.0 | 13.5 | −13.6 | 13.5 | 16.3 | −13.1 | 15.0 | 15.3 | −11.6 | 16.0 | 13.8 | −10.6 | |||||||||||||||||||||||||||||||
| COI | Stiffness (range 0–16; CSI ≥ 4) | PRPG | 10.6 | 4.0 | 0.44 | 4.0 | 4.0 | −6.6 | 0.19 | 0.01 | 4.5 | 4.0 | −6.1 | 0.52 | 0.38 | 4.0 | 2.8 | −6.6 | 0.04 * | 0.35 | 4.5 | 4.0 | −6.1 | 0.67 | 0.19 | ||||||||||||||||||||
| PBMTG | 10.6 | 4.0 | 6.0 | 4.0 | −4.6 | 7.0 | 4.0 | −3.6 | 7.0 | 4.0 | −3.6 | 5.5 | 4.0 | −5.1 | |||||||||||||||||||||||||||||||
| PRP + PBMTG | 9.3 | 5.3 | 4.0 | 5.8 | −5.3 | 4.0 | 5.5 | −5.3 | 4.0 | 4.0 | −5.3 | 4.5 | 4.0 | −4.8 | |||||||||||||||||||||||||||||||
| Function (range 0–16; CSI ≥ 4) | PRPG | 9.3 | 5.3 | 0.27 | 4.0 | 5.0 | −5.3 | 0.02 * | 0.47 | 4.0 | 3.8 | −5.3 | 0.45 | 0.38 | 4.0 | 3.8 | −5.3 | 0.02 * | 0.43 | 4.0 | 5.0 | −5.3 | 0.04 * | 0.31 | |||||||||||||||||||||
| PBMTG | 9.3 | 4.7 | 6.5 | 5.8 | −2.8 | 6.5 | 6.5 | −2.8 | 6.5 | 5.8 | −2.8 | 6.0 | 5.8 | −3.3 | |||||||||||||||||||||||||||||||
| PRP + PBMTG | 8.6 | 4.0 | 4.0 | 6.8 | −4.6 | 4.0 | 7.3 | −4.6 | 4.0 | 5.5 | −4.6 | 4.0 | 4.0 | −4.6 | |||||||||||||||||||||||||||||||
| Gait (range 0–20; CSI ≥ 4) | PRPG | 10.6 | 4.0 | 0.29 | 6.0 | 5.8 | −4.6 | 0.02 * | 0.61 | 7.0 | 5.8 | −3.6 | 0.33 | 0.38 | 5.0 | 5.8 | −5.6 | 0.15 | 0.26 | 8.0 | 5.8 | −2.6 | 0.56 | 0.08 | |||||||||||||||||||||
| PBMTG | 12.6 | 6.3 | 9.0 | 6.8 | −3.6 | 9.0 | 6.8 | −3.6 | 9.0 | 9.5 | −3.6 | 9.0 | 6.0 | −3.6 | |||||||||||||||||||||||||||||||
| PRP + PBMTG | 10.6 | 8.6 | 5.0 | 7.5 | −5.6 | 6.0 | 9.8 | −4.6 | 5.5 | 7.8 | −5.1 | 8.0 | 8.8 | −2.6 | |||||||||||||||||||||||||||||||
| QOL (range 0–12; CSI ≥ 3) | PRPG | 6.7 | 4.0 | 0.76 | 5.0 | 4.0 | −1.7 | 0.22 | 0.16 | 6.0 | 3.8 | −0.7 | 0.04 * | 0.38 | 4.0 | 3.0 | −2.7 | 0.04 * | 0.50 | 5.5 | 4.0 | −1.2 | 0.02 * | 0.34 | |||||||||||||||||||||
| PBMTG | 8.0 | 4.0 | 4.0 | 3.8 | −4.0 | 4.0 | 4.5 | −4.0 | 4.0 | 4.8 | −4.0 | 4.0 | 4.0 | −4.0 | |||||||||||||||||||||||||||||||
| PRP + PBMTG | 6.0 | 6.3 | 3.0 | 5.0 | −3.0 | 3.5 | 5.0 | −2.5 | 4.5 | 4.8 | −1.5 | 4.0 | 4.0 | −2.0 | |||||||||||||||||||||||||||||||
| Overall (range 0–64; CSI ≥ 14) | PRPG | 37.2 | 17.3 | 0.39 | 19.0 | 18.8 | −18.2 | 0.70 | 0.24 | 21.5 | 17.3 | −15.7 | 0.53 | 0.38 | 17.0 | 15.3 | −20.2 | 0.32 | 0.13 | 22.0 | 18.8 | −15.2 | 0.71 | 0.11 | |||||||||||||||||||||
| PBMTG | 40.6 | 19.0 | 25.5 | 20.3 | −15.1 | 26.5 | 21.8 | −14.1 | 26.5 | 24.0 | −14.1 | 24.5 | 19.8 | −16.1 | |||||||||||||||||||||||||||||||
| PRP + PBMTG | 34.6 | 24.3 | 16.0 | 25.0 | −18.6 | 17.5 | 27.5 | −17.1 | 18.0 | 22.0 | −16.6 | 20.5 | 20.8 | −14.1 | |||||||||||||||||||||||||||||||
| Measure | Group | +90 d | p | ES | +120 d | p | ES | +150 d | p | ES | +180 d | p | ES | ||||||||||||||||||||||||||||||||
| Med | IQR | Δ | Med | IQR | Δ | Med | IQR | Δ | Med | IQR | Δ | ||||||||||||||||||||||||||||||||||
| Weight bearing | Symmetry Index (CSI ≥ 10) | PRPG | 10.3 | 28.6 | −27.7 | 0.29 | 0.22 | 10.5 | 15.4 | −27.5 | 0.02 * | 0.05 | 10.0 | 21.8 | −28.0 | 0.47 | 0.23 | 7.9 | 25.6 | −30.1 | 0.01 * | 0.82 | |||||||||||||||||||||||
| PBMTG | 9.1 | 14.9 | −22.7 | 16.0 | 26.9 | −15.8 | 13.2 | 20.1 | −18.6 | 24.5 | 33.5 | −7.3 | |||||||||||||||||||||||||||||||||
| PRP + PBMTG | 5.1 | 14.0 | −27.0 | 12.5 | 16.9 | −19.6 | 14.0 | 19.7 | −18.1 | 11.1 | 13.1 | −21.0 | |||||||||||||||||||||||||||||||||
| Deviation (CSI ≥ 2) | PRPG | 3.5 | 6.0 | −1.2 | <0.01 * | 0.56 | 2.0 | 3.0 | −2.7 | 0.01 * | 0.22 | 2.0 | 3.8 | −2.7 | 0.07 | 0.16 | 2.5 | 4.0 | −2.2 | 0.05 * | 0.42 | ||||||||||||||||||||||||
| PBMTG | 4.5 | 4.0 | −2.8 | 3.0 | 4.0 | −4.3 | 3.5 | 4.8 | −3.8 | 5.0 | 6.0 | −2.3 | |||||||||||||||||||||||||||||||||
| PRP + PBMTG | 1.0 | 4.0 | −5.0 | 3.0 | 3.0 | −3.0 | 3.0 | 3.0 | −3.0 | 2.5 | 3.0 | −3.5 | |||||||||||||||||||||||||||||||||
| CBPI | PSS (range 0–10; CSI ≥ 1) | PRPG | 3.4 | 4.0 | −2.4 | 0.04 * | 0.33 | 4.0 | 3.4 | −1.8 | 0.03 * | 0.39 | 3.5 | 3.4 | −2.3 | 0.04 * | 0.34 | 3.5 | 3.2 | −2.3 | 0.04 * | 0.40 | |||||||||||||||||||||||
| PBMTG | 4.1 | 1.9 | −1.2 | 4.3 | 2.4 | −1.1 | 4.1 | 1.9 | −1.2 | 4.3 | 2.6 | −1.1 | |||||||||||||||||||||||||||||||||
| PRP + PBMTG | 2.3 | 4.0 | −3.1 | 2.4 | 3.9 | −2.9 | 2.8 | 3.9 | −2.6 | 2.3 | 3.9 | −3.1 | |||||||||||||||||||||||||||||||||
| PIS (range 0–10; CSI ≥ 2) | PRPG | 3.0 | 4.3 | −2.5 | 0.53 | 0.29 | 4.1 | 3.7 | −1.4 | 0.32 | 0.18 | 3.1 | 4.2 | −2.4 | 0.56 | 0.07 | 3.6 | 4.1 | −1.9 | 0.62 | 0.11 | ||||||||||||||||||||||||
| PBMTG | 3.9 | 2.1 | −1.9 | 4.0 | 2.4 | −1.8 | 4.0 | 2.7 | −1.8 | 3.9 | 2.5 | −1.9 | |||||||||||||||||||||||||||||||||
| PRP + PBMTG | 2.3 | 4.2 | −2.1 | 2.1 | 4.2 | −2.3 | 2.6 | 4.1 | −1.8 | 2.2 | 4.2 | −2.2 | |||||||||||||||||||||||||||||||||
| LOAD (range 0–52; CSI ≥ 4) | PRPG | 18.5 | 10.8 | −4.1 | 0.04 * | 0.92 | 19.0 | 11.8 | −3.6 | 0.04 * | 0.95 | 18.5 | 12.5 | −4.1 | 0.04 * | 0.99 | 20.5 | 12.0 | −2.1 | 0.04 * | 0.55 | ||||||||||||||||||||||||
| PBMTG | 23.0 | 14.5 | −4.9 | 23.0 | 19.3 | −4.9 | 24.0 | 17.5 | −3.9 | 24.0 | 16.8 | −3.9 | |||||||||||||||||||||||||||||||||
| PRP + PBMTG | 17.0 | 13.8 | −9.6 | 16.5 | 15.5 | −10.1 | 18.0 | 15.5 | −8.6 | 18.0 | 15.5 | −8.6 | |||||||||||||||||||||||||||||||||
| COI | Stiffness (range 0–16; CSI ≥ 4) | PRPG | 5.0 | 4.0 | −5.6 | 0.53 | 0.08 | 5.5 | 4.0 | −5.1 | 0.03 * | 0.39 | 6.5 | 4.8 | −4.1 | 0.02 * | 0.31 | 4.5 | 3.8 | −6.1 | 0.03 * | 0.43 | |||||||||||||||||||||||
| PBMTG | 7.0 | 4.0 | −3.6 | 8.0 | 4.0 | −2.6 | 8.0 | 6.8 | −2.6 | 7.5 | 4.0 | −3.1 | |||||||||||||||||||||||||||||||||
| PRP + PBMTG | 4.0 | 5.8 | −5.3 | 4.5 | 6.0 | −4.8 | 4.5 | 6.0 | −4.8 | 4.5 | 6.0 | −4.8 | |||||||||||||||||||||||||||||||||
| Function (range 0–16; CSI ≥ 4) | PRPG | 6.0 | 4.0 | −3.3 | 0.03 * | 0.10 | 6.5 | 4.0 | −2.8 | 0.02 * | 0.42 | 6.0 | 4.0 | −3.3 | 0.02 * | 0.34 | 6.0 | 4.0 | −3.3 | 0.02 * | 0.33 | ||||||||||||||||||||||||
| PBMTG | 7.0 | 4.5 | −2.3 | 7.0 | 5.8 | −2.3 | 7.0 | 5.8 | −2.3 | 7.0 | 5.8 | −2.3 | |||||||||||||||||||||||||||||||||
| PRP + PBMTG | 4.0 | 5.8 | −4.6 | 4.0 | 4.8 | −4.6 | 4.0 | 4.8 | −4.6 | 4.0 | 5.5 | −4.6 | |||||||||||||||||||||||||||||||||
| Gait (range 0–20; CSI ≥ 4) | PRPG | 7.5 | 5.8 | −3.1 | 0.47 | 0.24 | 8.5 | 5.5 | −2.1 | 0.21 | 0.19 | 8.0 | 5.3 | −2.6 | 0.16 | 0.03 | 8.0 | 5.0 | −2.6 | 0.21 | 0.07 | ||||||||||||||||||||||||
| PBMTG | 9.0 | 5.8 | −3.6 | 10.0 | 7.8 | −2.6 | 10.0 | 7.5 | −2.6 | 9.5 | 7.5 | −3.1 | |||||||||||||||||||||||||||||||||
| PRP + PBMTG | 7.5 | 8.3 | −3.1 | 8.0 | 7.0 | −2.6 | 8.0 | 6.0 | −2.6 | 8.0 | 7.5 | −2.6 | |||||||||||||||||||||||||||||||||
| QOL (range 0–12; CSI ≥ 3) | PRPG | 5.0 | 18.5 | −1.7 | 0.04 * | 0.05 | 6.0 | 4.0 | −0.7 | 0.04 * | 0.48 | 8.0 | 6.0 | 1.4 | 0.04 * | 0.14 | 6.0 | 3.8 | −0.7 | 0.04 * | 0.32 | ||||||||||||||||||||||||
| PBMTG | 4.5 | 3.8 | −3.5 | 5.0 | 3.8 | −3.0 | 5.0 | 3.8 | −3.0 | 5.0 | 3.8 | −3.0 | |||||||||||||||||||||||||||||||||
| PRP + PBMTG | 4.5 | 4.5 | −1.5 | 4.0 | 3.8 | −2.0 | 4.0 | 3.8 | −2.0 | 4.5 | 3.8 | −1.5 | |||||||||||||||||||||||||||||||||
| Overall (range 0–64; CSI ≥ 14) | PRPG | 23.5 | 32.3 | −13.7 | 0.59 | 0.11 | 26.5 | 17.5 | −10.7 | 0.29 | 0.20 | 28.5 | 20.0 | −8.7 | 0.02 * | 0.35 | 24.5 | 16.5 | −12.7 | 0.29 | 0.07 | ||||||||||||||||||||||||
| PBMTG | 27.5 | 18.0 | −13.1 | 30.0 | 21.3 | −10.6 | 30.0 | 23.8 | −10.6 | 29.0 | 21.0 | −11.6 | |||||||||||||||||||||||||||||||||
| PRP + PBMTG | 20.0 | 24.3 | −14.6 | 20.5 | 21.5 | −14.1 | 20.5 | 20.5 | −14.1 | 21.0 | 22.8 | −13.6 | |||||||||||||||||||||||||||||||||
| Outcome Measure | Log Rank Test | PRPG | PBMTG | PRP + PBMTG | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Mean | SD | 95% CI | Mean | SD | 95% CI | ||||||
| Weight bearing | SI | 0.03 * | 94.7 | 14.8 | 65.6 | 123.7 | 68.3 | 14.2 | 40.4 | 96.1 | 121.6 | 15.2 | 89.2 | 133.0 |
| Deviation | 0.02 * | 95.6 | 16.8 | 62.7 | 128.6 | 79.9 | 15.7 | 49.3 | 110.6 | 162.1 | 14.9 | 123.0 | 154.4 | |
| CBPI | PSS | 0.02 * | 101.4 | 14.9 | 72.2 | 130.6 | 69.8 | 14.1 | 42.0 | 97.5 | 101.8 | 14.2 | 42.5 | 142.0 |
| PIS | 0.04 * | 126.1 | 13.6 | 99.5 | 152.7 | 113.9 | 15.5 | 83.5 | 144.3 | 133.7 | 14.8 | 63.1 | 175.9 | |
| LOAD | <0.01 * | 126.3 | 12.2 | 102.3 | 150.3 | 112.1 | 13.9 | 84.8 | 139.4 | 156.7 | 14.5 | 79.6 | 197.9 | |
| COI | Stiffness | 0.03 * | 66.3 | 14.3 | 38.3 | 94.3 | 49.8 | 12.5 | 25.3 | 74.4 | 70.0 | 12.4 | 23.9 | 105.3 |
| Function | 0.02 * | 61.3 | 13.6 | 34.6 | 88.1 | 59.0 | 12.8 | 33.9 | 84.1 | 80.2 | 13.8 | 28.3 | 119.3 | |
| Gait | 0.01 * | 93.1 | 13.6 | 66.6 | 119.7 | 72.9 | 13.9 | 45.6 | 100.4 | 105.9 | 14.4 | 44.7 | 146.9 | |
| QOL | 0.02 * | 50.8 | 12.7 | 25.9 | 75.7 | 39.6 | 10.3 | 19.4 | 59.7 | 66.8 | 12.4 | 21.8 | 101.9 | |
| Overall | 0.04 * | 73.5 | 14.3 | 45.5 | 101.5 | 66.2 | 13.5 | 39.7 | 92.7 | 96.7 | 7.9 | 51.0 | 119.3 | |
| Outcome Measure | Log Rank Test | PRPG | PBMTG | PRP + PBMTG | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Mean | SD | 95% CI | Mean | SD | 95% CI | ||||||
| Weight bearing | SI | 0.03 * | 118.5 | 13.9 | 91.1 | 145.9 | 96.5 | 13.3 | 70.4 | 122.6 | 129.0 | 7.5 | 100.0 | 129.3 |
| Deviation | 0.02 * | 101.0 | 12.5 | 76.4 | 125.6 | 103.0 | 12.5 | 78.6 | 127.4 | 178.3 | 11.8 | 102.9 | 169.9 | |
| CBPI | PSS | 0.04 * | 124 | 13.7 | 97.2 | 150.8 | 124.0 | 13.2 | 98.0 | 149.9 | 112.0 | 14.1 | 72.8 | 156.2 |
| PIS | 0.04 * | 119.0 | 13.5 | 92.6 | 145.7 | 103.0 | 14.5 | 74.6 | 131.4 | 147.0 | 14.4 | 58.7 | 180.0 | |
| LOAD | 0.03 * | 141.5 | 9.9 | 122.1 | 160.9 | 132.0 | 12.3 | 107.9 | 156.1 | 163.3 | 10.5 | 159.5 | 167.2 | |
| COI | Stiffness | 0.03 * | 68.5 | 13.0 | 42.9 | 94.0 | 64.0 | 12.8 | 38.9 | 89.0 | 81.0 | 14.2 | 53.3 | 108.7 |
| Function | 0.03 * | 73.0 | 12.8 | 47.9 | 98.1 | 79.5 | 11.8 | 56.4 | 102.6 | 112.0 | 9.9 | 92.4 | 131.6 | |
| Gait | 0.03 * | 110.5 | 13.1 | 84.6 | 13.14 | 91.0 | 13.4 | 64.8 | 117.2 | 111.5 | 15.6 | 88.8 | 134.2 | |
| QOL | 0.02 * | 87 | 14.3 | 59.0 | 114.9 | 92.5 | 14.6 | 83.4 | 98.1 | 107.5 | 11.8 | 84.4 | 130.6 | |
| Overall | 0.04 * | 87.0 | 14.3 | 59.0 | 114.9 | 82.5 | 12.7 | 57.7 | 107.3 | 107.5 | 7.5 | 77.6 | 107.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, J.C.; Santos, A.; Carreira, L.M. The Combined Application of Intra-Articular Platelet-Rich Plasma Injections and Photobiomodulation Improves Clinical Outcomes in Dogs with Osteoarthritis—Results of a Long-Term, Double-Blinded, Crossover Study. Vet. Sci. 2025, 12, 1025. https://doi.org/10.3390/vetsci12111025
Alves JC, Santos A, Carreira LM. The Combined Application of Intra-Articular Platelet-Rich Plasma Injections and Photobiomodulation Improves Clinical Outcomes in Dogs with Osteoarthritis—Results of a Long-Term, Double-Blinded, Crossover Study. Veterinary Sciences. 2025; 12(11):1025. https://doi.org/10.3390/vetsci12111025
Chicago/Turabian StyleAlves, J. C., Ana Santos, and L. Miguel Carreira. 2025. "The Combined Application of Intra-Articular Platelet-Rich Plasma Injections and Photobiomodulation Improves Clinical Outcomes in Dogs with Osteoarthritis—Results of a Long-Term, Double-Blinded, Crossover Study" Veterinary Sciences 12, no. 11: 1025. https://doi.org/10.3390/vetsci12111025
APA StyleAlves, J. C., Santos, A., & Carreira, L. M. (2025). The Combined Application of Intra-Articular Platelet-Rich Plasma Injections and Photobiomodulation Improves Clinical Outcomes in Dogs with Osteoarthritis—Results of a Long-Term, Double-Blinded, Crossover Study. Veterinary Sciences, 12(11), 1025. https://doi.org/10.3390/vetsci12111025





