Classification of Clinical Outcomes in Hospitalized Asian Elephants Using Machine Learning and Survival Analysis: A Retrospective Study (2019–2024)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Variables
2.2. Descriptive Analysis
2.3. Model Development
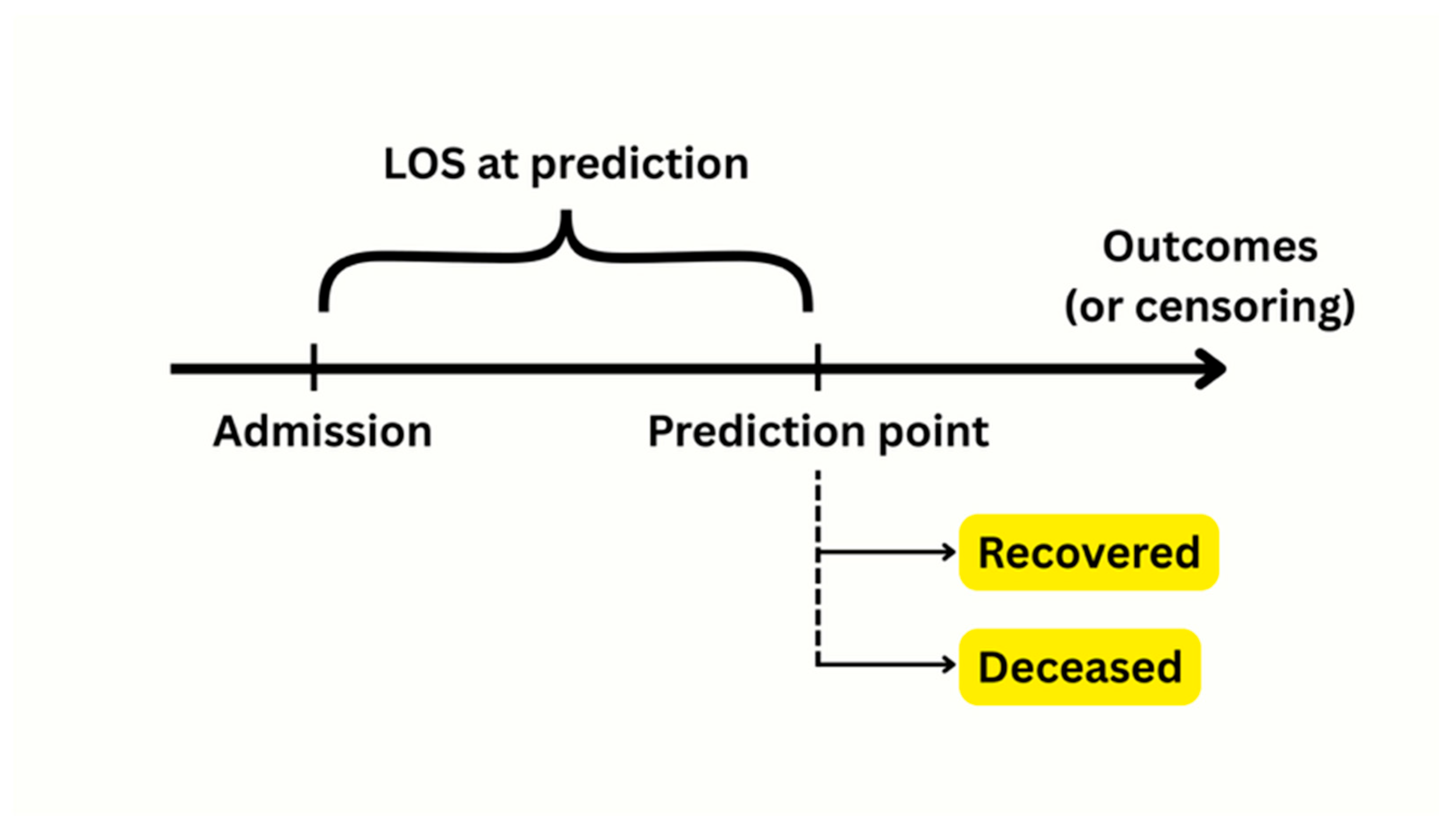
2.4. Survival Analysis
2.5. Software and Reproducibility
3. Results
3.1. Descriptive Findings
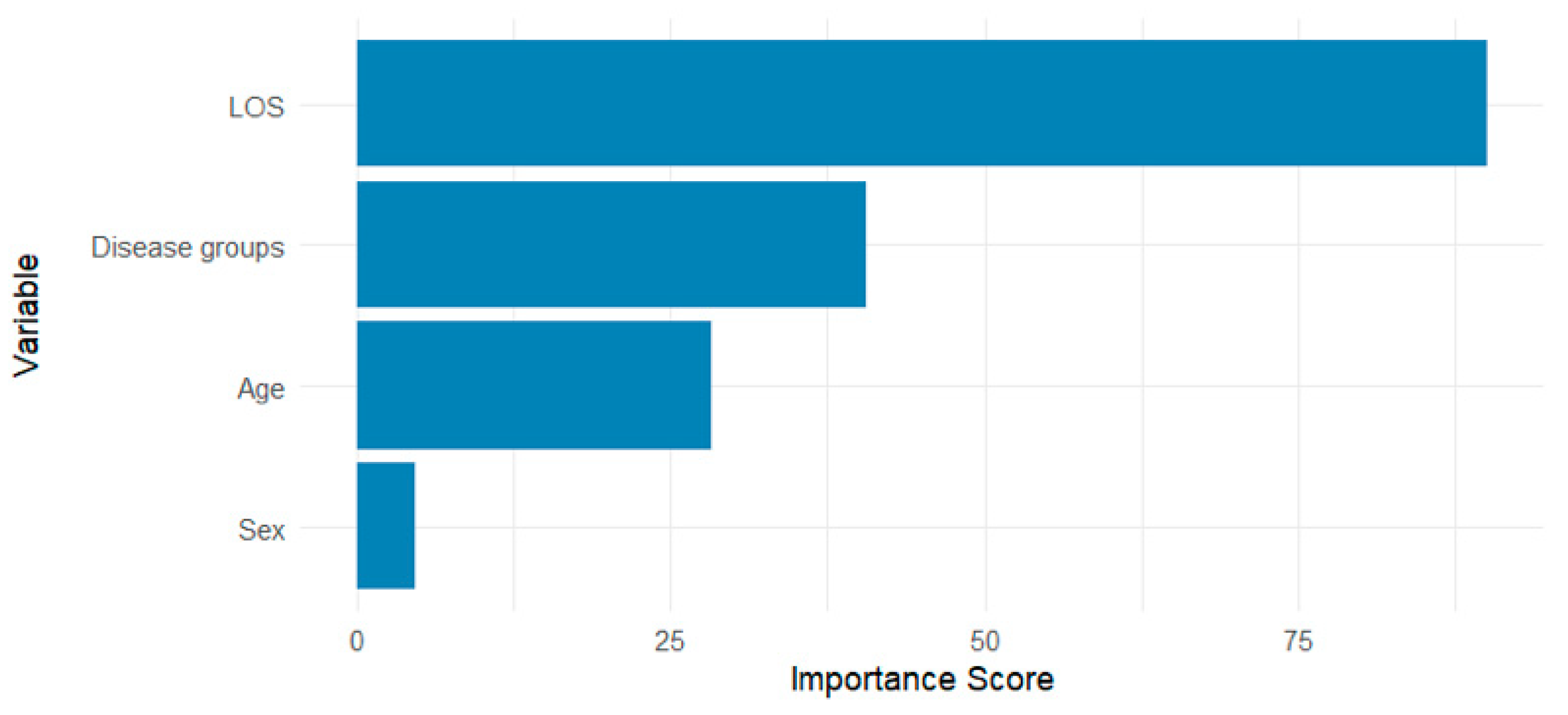
3.2. Classification Model Performance
3.3. Time-to-Event Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AUC | Area Under the Curve |
| CI | Confidence Interval |
| EEHV | Elephant Endotheliotropic Herpesvirus |
| EEHV-HD | Elephant Endotheliotropic Herpesvirus Hemorrhagic Disease |
| ICU | Intensive Care Unit |
| LOS | Length of Stay |
| NA | Not Available |
| NEI | National Elephant Institute |
| ROC | Receiver Operating Characteristic |
| SE | Standard Error |
| XGBoost | eXtreme Gradient Boosting |
References
- Bansiddhi, P.; Brown, J.L.; Thitaram, C. Welfare Assessment and Activities of Captive Elephants in Thailand. Animals 2020, 10, 919. [Google Scholar] [CrossRef]
- Sukmasuang, R.; Phumpakphan, N.; Deungkae, P.; Chaiyarat, R.; Pla-Ard, M.; Khiowsree, N.; Charaspet, K.; Paansri, P.; Noowong, J. Review: Status of Wild Elephant, Conflict and Conservation Actions in Thailand. Biodiversitas J. Biol. Divers. 2024, 25, 1479–1498. [Google Scholar] [CrossRef]
- Angkawanish, T.; Boonprasert, K.; Homkong, P.; Sombutputorn, P.; Mahasawangkul, S.; Keratimanochaya, T.; Clausen, B. Elephant Health Status in Thailand: The Role of Mobile Elephant Clinic and Elephant Hospital. Gajah 2009, 31, 6. [Google Scholar]
- Kosaruk, W.; Brown, J.L.; Towiboon, P.; Punyapornwithaya, V.; Pringproa, K.; Thitaram, C. Measures of Oxidative Status Markers in Relation to Age, Sex, and Season in Sick and Healthy Captive Asian Elephants in Thailand. Animals 2023, 13, 1548. [Google Scholar] [CrossRef] [PubMed]
- Boonprasert, K.; Punyapornwithaya, V.; Tankaew, P.; Angkawanish, T.; Sriphiboon, S.; Titharam, C.; Brown, J.L.; Somgird, C. Survival Analysis of Confirmed Elephant Endotheliotropic Herpes Virus Cases in Thailand from 2006–2018. PLoS ONE 2019, 14, e0219288. [Google Scholar] [CrossRef]
- Akbarein, H.; Taaghi, M.H.; Mohebbi, M.; Soufizadeh, P. Applications and Considerations of Artificial Intelligence in Veterinary Sciences: A Narrative Review. Vet. Med. Sci. 2025, 11, e70315. [Google Scholar] [CrossRef]
- Schofield, I.; Brodbelt, D.C.; Kennedy, N.; Niessen, S.J.M.; Church, D.B.; Geddes, R.F.; O’Neill, D.G. Machine-Learning Based Prediction of Cushing’s Syndrome in Dogs Attending UK Primary-Care Veterinary Practice. Sci. Rep. 2021, 11, 9035. [Google Scholar] [CrossRef]
- Ohno-Machado, L.; Resnic, F.S.; Matheny, M.E. Prognosis in Critical Care. Annu. Rev. Biomed. Eng. 2006, 8, 567–599. [Google Scholar] [CrossRef]
- Haniffa, R.; Isaam, I.; De Silva, A.P.; Dondorp, A.M.; De Keizer, N.F. Performance of Critical Care Prognostic Scoring Systems in Low and Middle-Income Countries: A Systematic Review. Crit. Care 2018, 22, 18. [Google Scholar] [CrossRef]
- Iwase, S.; Nakada, T.; Shimada, T.; Oami, T.; Shimazui, T.; Takahashi, N.; Yamabe, J.; Yamao, Y.; Kawakami, E. Prediction Algorithm for ICU Mortality and Length of Stay Using Machine Learning. Sci. Rep. 2022, 12, 12912. [Google Scholar] [CrossRef]
- Sanaei, N.; Zamani-Ahmadmahmudi, M.; Nassiri, S.M. Development of Machine Learning Models to Predict Clinical Outcome and Recovery Time in Dogs with Parvovirus Enteritis. Front. Vet. Sci. 2025, 12, 1555714. [Google Scholar] [CrossRef]
- Satoła, A.; Satoła, K. Performance Comparison of Machine Learning Models Used for Predicting Subclinical Mastitis in Dairy Cows: Bagging, Boosting, Stacking, and Super-Learner Ensembles versus Single Machine Learning Models. J. Dairy Sci. 2024, 107, 3959–3972. [Google Scholar] [CrossRef]
- Fraiwan, M.A.; Abutarbush, S.M. Using Artificial Intelligence to Predict Survivability Likelihood and Need for Surgery in Horses Presented with Acute Abdomen (Colic). J. Equine Vet. Sci. 2020, 90, 102973. [Google Scholar] [CrossRef]
- Cope, H.R.; McArthur, C.; Dickman, C.R.; Newsome, T.M.; Gray, R.; Herbert, C.A. A Systematic Review of Factors Affecting Wildlife Survival during Rehabilitation and Release. PLoS ONE 2022, 17, e0265514. [Google Scholar] [CrossRef]
- Whitmer, E.R.; Borremans, B.; Duignan, P.J.; Johnson, S.P.; Lloyd-Smith, J.O.; McClain, A.M.; Field, C.L.; Prager, K.C. Classification and Regression Tree Analysis for Predicting Prognosis in Wildlife Rehabilitation: A Case Study of Leptospirosis in California Sea Lions (Zalophus Californianus). J. Zoo Wildl. Med. 2021, 52, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Brickson, L.; Zhang, L.; Vollrath, F.; Douglas-Hamilton, I.; Titus, A.J. Elephants and Algorithms: A Review of the Current and Future Role of AI in Elephant Monitoring. J. R. Soc. Interface 2023, 20, 20230367. [Google Scholar] [CrossRef] [PubMed]
- Ghavi Hossein-Zadeh, N. Artificial Intelligence in Veterinary and Animal Science: Applications, Challenges, and Future Prospects. Comput. Electron. Agric. 2025, 235, 110395. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. arXiv 2016, arXiv:1603.02754. [Google Scholar] [CrossRef]
- Zhang, H. The Optimality of Naive Bayes. In Proceedings of the Seventeenth International Florida Artificial Intelligence Research Society Conference, Miami Beach, FL, USA, 12–14 May 2004. [Google Scholar]
- Agresti, A. Foundations of Linear and Generalized Linear Models. Hoboken: Wiley. Biometrics 2017, 73, 1060–1061. [Google Scholar] [CrossRef]
- CiHan, P. Horse Surgery and Survival Prediction with Artificial Intelligence Models: Performance Comparison of Original, Imputed, Balanced, and Feature-Selected Datasets. Kafkas Univ. Vet. Fak. Derg. 2024, 30, 233–241. [Google Scholar] [CrossRef]
- Cetintav, B.; Yalcin, A. From Prediction to Precision: Explainable AI-Driven Insights for Targeted Treatment in Equine Colic. Animals 2025, 15, 126. [Google Scholar] [CrossRef]
- Yun, Y.; Sripiboon, S.; Pringproa, K.; Chuammitri, P.; Punyapornwithaya, V.; Boonprasert, K.; Tankaew, P.; Angkawanish, T.; Namwongprom, K.; Arjkumpa, O.; et al. Clinical Characteristics of Elephant Endotheliotropic Herpesvirus (EEHV) Cases in Asian Elephants (Elephas maximus) in Thailand during 2006–2019. Vet. Q. 2021, 41, 268–279. [Google Scholar] [CrossRef]
- Fontes, G.S.; McCarthy, R.J.; Kutzler, M.A.; Zitek-Morrison, E. The Effects of Sex and Neuter Status on Trauma Survival in Dogs: A Veterinary Committee on Trauma Registry Study. J. Vet. Emerg. Crit. Care 2022, 32, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Castelain, D.L.; Dufourni, A.; Pas, M.L.; Bokma, J.; De Bruijn, E.; Paulussen, E.; Lefère, L.; Van Loon, G.; Pardon, B. Retrospective Cohort Study on Diseases and Risk Factors Associated with Death in Hospitalized Neonatal Foals. J. Vet. Intern. Med. 2025, 39, e17269. [Google Scholar] [CrossRef] [PubMed]
- Thitaram, C.; Pongsopawijit, P.; Thongtip, N.; Angkavanich, T.; Chansittivej, S.; Wongkalasin, W.; Somgird, C.; Suwankong, N.; Prachsilpchai, W.; Suchit, K.; et al. Dystocia Following Prolonged Retention of a Dead Fetus in an Asian Elephant (Elephas maximus). Theriogenology 2006, 66, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Battineni, G.; Sagaro, G.G.; Chinatalapudi, N.; Amenta, F. Applications of Machine Learning Predictive Models in the Chronic Disease Diagnosis. J. Pers. Med. 2020, 10, 21. [Google Scholar] [CrossRef]
- Grampurohit, S.; Sagarnal, C. Disease Prediction Using Machine Learning Algorithms. In Proceedings of the 2020 International Conference for Emerging Technology (INCET), Belgaum, India, 5–7 June 2020; IEEE: New York, NY, USA, 2020. [Google Scholar]
- Hadar, B.N.; Poljak, Z.; Bonnett, B.; Coe, J.; Stone, E.A.; Bernardo, T.M. Machine Learning Predicts Selected Cat Diseases Using Insurance Data amid Challenges in Interpretability. Am. J. Vet. Res. 2025, 86, S52–S62. [Google Scholar] [CrossRef]
- Prosser, H.M.; Bortoluzzi, E.M.; Valeris-Chacin, R.J.; Baker, E.C.; Scott, M.A. Application of Artificial Intelligence and Machine Learning in Bovine Respiratory Disease Prevention, Diagnosis, and Classification. Am. J. Vet. Res. 2025, 86, S22–S26. [Google Scholar] [CrossRef]
- Kwananocha, I.; Niyom, S.; Budsayaplakorn, P.; Kasemsuwan, S.; Theerapan, W.; Warrit, K. Prognostic Factors for Mortality Following Diaphragmatic Herniorrhaphy in Dogs and Cats: Multivariable Logistic Regression and Machine Learning Approaches. Vet. Sci. 2025, 12, 819. [Google Scholar] [CrossRef]
- Richman, L.K.; Zong, J.-C.; Latimer, E.M.; Lock, J.; Fleischer, R.C.; Heaggans, S.Y.; Hayward, G.S. Elephant Endotheliotropic Herpesviruses EEHV1A, EEHV1B, and EEHV2 from Cases of Hemorrhagic Disease Are Highly Diverged from Other Mammalian Herpesviruses and May Form a New Subfamily. J. Virol. 2014, 88, 13523–13546. [Google Scholar] [CrossRef] [PubMed]
- Sripiboon, S.; Jackson, B.; Ditcham, W.; Holyoake, C.; Robertson, I.; Thitaram, C.; Tankaew, P.; Letwatcharasarakul, P.; Warren, K. Molecular Characterisation and Genetic Variation of Elephant Endotheliotropic Herpesvirus Infection in Captive Young Asian Elephants in Thailand. Infect. Genet. Evol. 2016, 44, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.J.; Remnant, J.G.; Bollard, N.J.; Burrows, A.; Whay, H.R.; Bell, N.J.; Mason, C.; Huxley, J.N. Recovery of Chronically Lame Dairy Cows Following Treatment for Claw Horn Lesions: A Randomised Controlled Trial. Vet. Rec. 2016, 178, 116. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.A.; Martins, A.D.S.; Alves, L.R.; Pereira, L.W.B.; Saraiva, J.R.; Duarte, J.M.B.; Zanetti, E.D.S.; Schweitzer, C.M.; Dutra, I.S.; Borsanelli, A.C. Prevalence and Risk Factors of Bone and Dental Lesions in Neotropical Deer. Animals 2024, 14, 1892. [Google Scholar] [CrossRef]
- Back, J.; Jin, Y.; Jin, T.; Lee, S. Development and Validation of an Automated Sepsis Risk Assessment System. Res. Nurs. Health 2016, 39, 317–327. [Google Scholar] [CrossRef]
- Jana, S.; Dasgupta, T.; Dey, L. Predicting Medical Events and ICU Requirements Using a Multimodal Multiobjective Transformer Network. Exp. Biol. Med. 2022, 247, 1988–2002. [Google Scholar] [CrossRef]
- Scantlebury, C.E.; Perkins, E.; Pinchbeck, G.L.; Archer, D.C.; Christley, R.M. Could It Be Colic? Horse-Owner Decision Making and Practices in Response to Equine Colic. BMC Vet. Res. 2014, 10, S1. [Google Scholar] [CrossRef]
- Burrell, K.; Sutton-Walker, G.; England, G.C.W.; Burford, J.H.; Freeman, S.L. Prospective Case Study of Critical Decision Making for Horses Referred for Treatment of Colic. Vet. Rec. 2024, 194, e3615. [Google Scholar] [CrossRef]
- De Angeli, K.; Gao, S.; Danciu, I.; Durbin, E.B.; Wu, X.-C.; Stroup, A.; Doherty, J.; Schwartz, S.; Wiggins, C.; Damesyn, M.; et al. Class Imbalance in Out-of-Distribution Datasets: Improving the Robustness of the TextCNN for the Classification of Rare Cancer Types. J. Biomed. Inform. 2022, 125, 103957. [Google Scholar] [CrossRef]
- Abdulsadig, R.S.; Rodriguez-Villegas, E. A Comparative Study in Class Imbalance Mitigation When Working with Physiological Signals. Front. Digit. Health 2024, 6, 1377165. [Google Scholar] [CrossRef]
- Japkowicz, N.; Stephen, S. The Class Imbalance Problem: A Systematic Study. Intell. Data Anal. 2002, 6, 429–449. [Google Scholar] [CrossRef]
- Buda, M.; Maki, A.; Mazurowski, M.A. A Systematic Study of the Class Imbalance Problem in Convolutional Neural Networks. Neural Netw. 2018, 106, 249–259. [Google Scholar] [CrossRef]
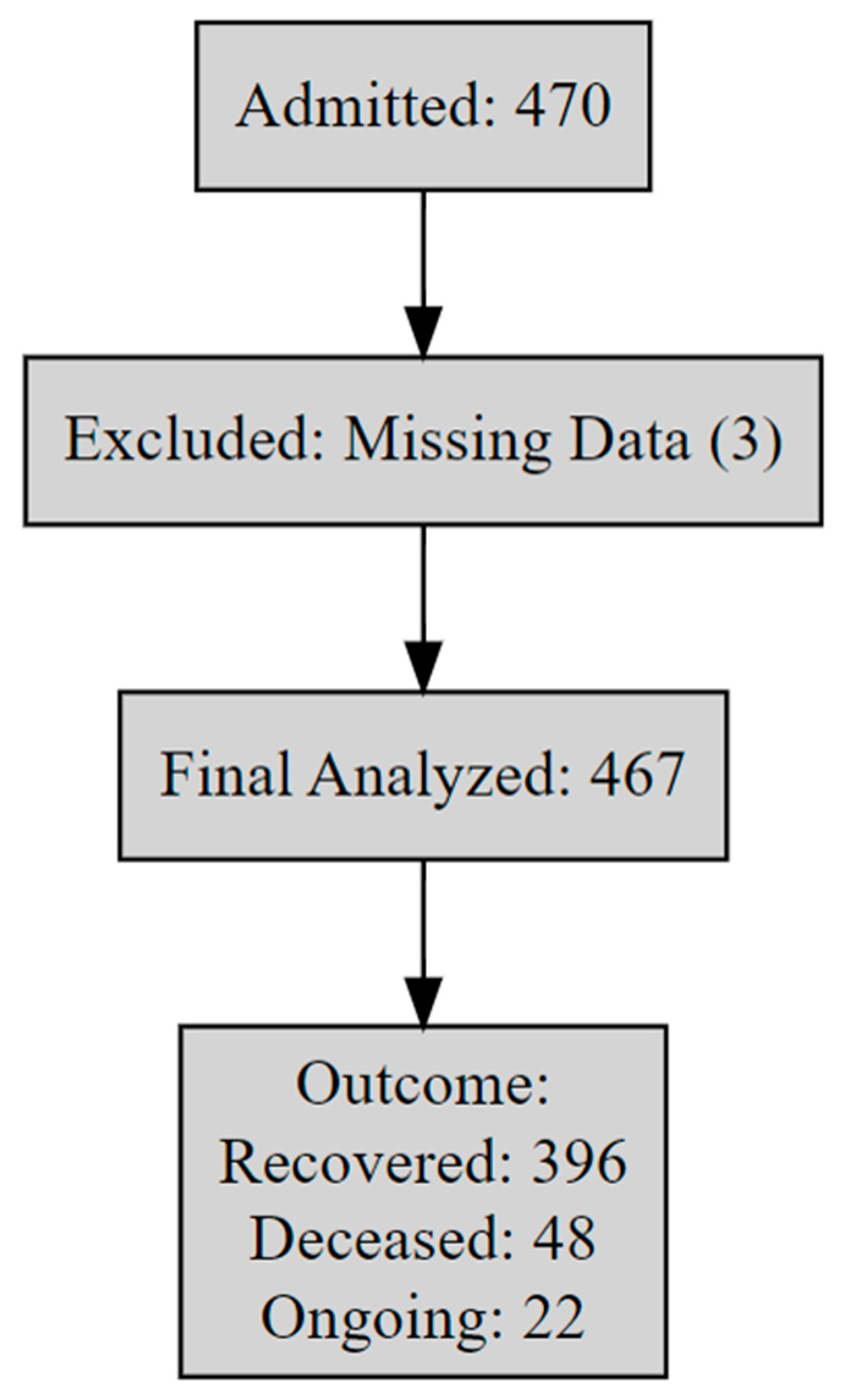
| Variable | Overall (%) | Deceased (%) | Ongoing (%) | Recovered (%) | Length of Stay (Day) Mean ± SE (Range) |
|---|---|---|---|---|---|
| Sex | |||||
| 258 (55.25%) | 27 (10.47%) | 13 (5.04%) | 218 (84.50%) | 102.88 ± 19.38 (0 to 2380) |
| 209 (44.75%) | 21 (10.05%) | 10 (4.78%) | 178 (85.17%) | 80.95 ± 17.57 (0 to 1919) |
| Disease group | |||||
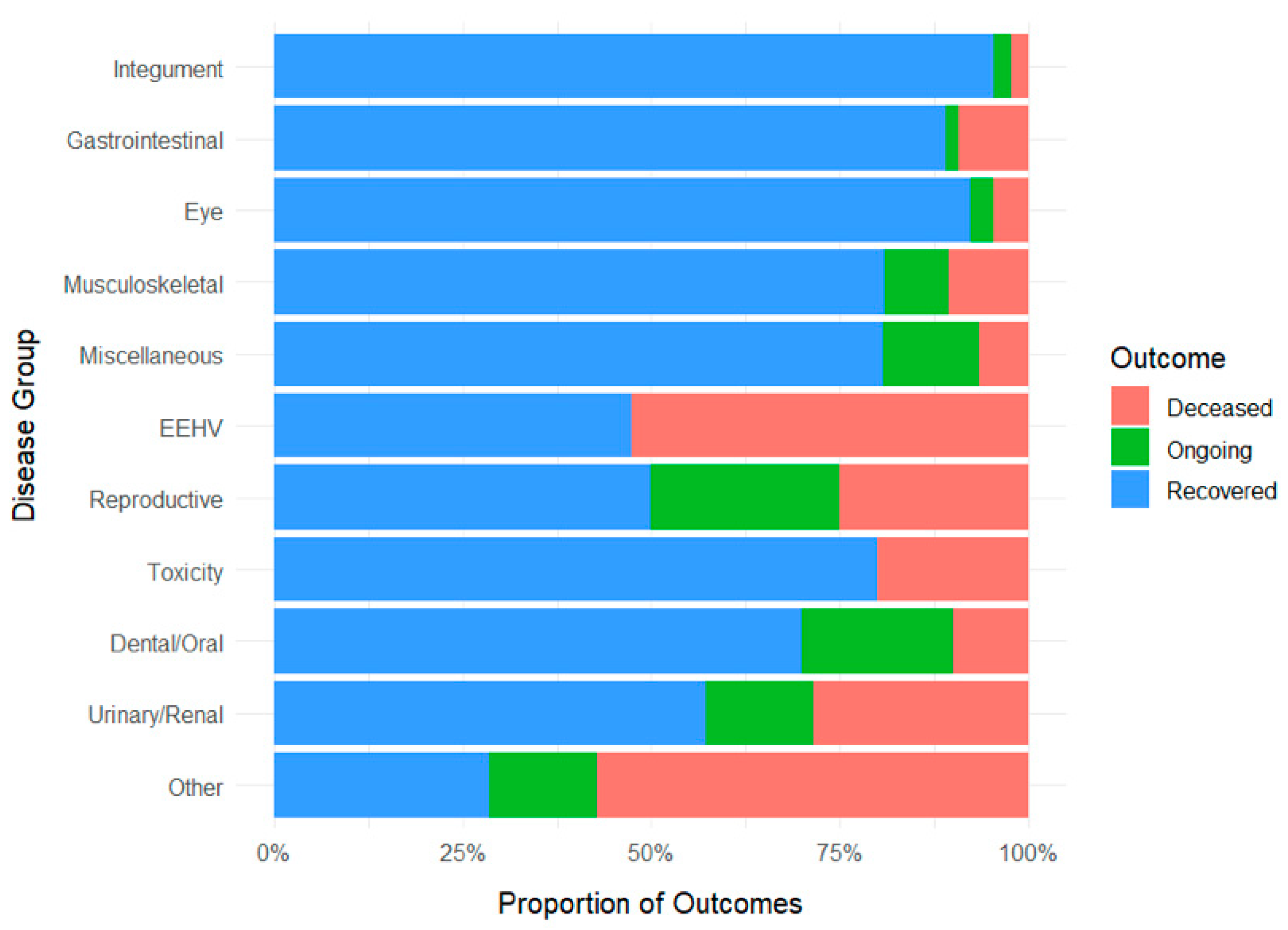
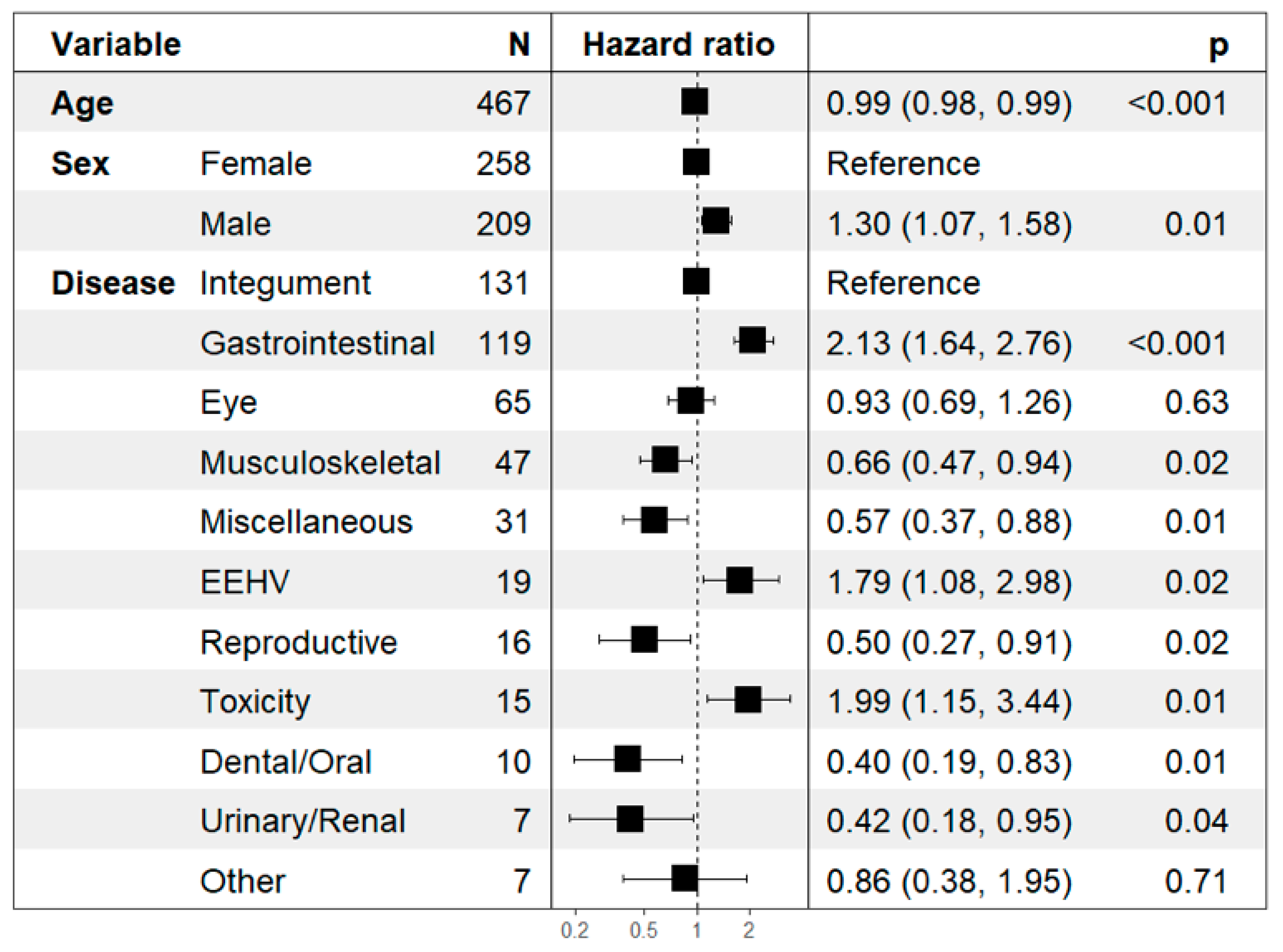
| 131 (28.05%) | 3 (2.29%) | 3 (2.29%) | 125 (95.42%) | 88.63 ± 23.05 (0 to 1523) |
| 119 (25.48%) | 11 (9.24%) | 2 (1.68%) | 106 (89.08%) | 41.24 ± 21.91 (0 to 2380) |
| 65 (13.92%) | 3 (4.62%) | 2 (3.08%) | 60 (92.31%) | 47.72 ± 13.11 (2 to 821) |
| 47 (10.06%) | 5 (10.64%) | 4 (8.51%) | 38 (80.85%) | 240.66 ± 76.24 (1 to 1934) |
| 31 (6.64%) | 2 (6.45%) | 4 (12.90%) | 25 (80.65%) | 201.32 ± 69.10 (2 to 1257) |
| 19 (4.07%) | 10 (52.63%) | - | 9 (47.37%) | 7.95 ± 2.21 (0 to 35) |
| 16 (3.43%) | 4 (25.0%) | 4 (25.0%) | 8 (50.0%) | 174.19 ± 73.33 (5 to 1058) |
| 15 (3.21%) | 3 (20.0%) | - | 12 (80.0%) | 8.60 ± 2.19 (0 to 35) |
| 10 (2.14%) | 1 (10.0%) | 2 (20.0%) | 7 (70.0%) | 220.30 ± 110.25 (4 to 1035) |
| 7 (1.50%) | 2 (28.57%) | 1 (14.29%) | 4 (57.14%) | 113.14 ± 49.42 (27 to 396) |
| 7 (1.50%) | 4 (57.14%) | 1 (14.29%) | 2 (28.57%) | 32.14 ± 12.83 (2 to 96) |
| Total | 467 (100%) | 48 (10.30%) | 22 (4.72%) | 396 (84.98%) | 93.06 ± 13.28 (0 to 2380) |
| Model | Accuracy (Mean ± SE) | Log-Loss (Mean ± SE) |
|---|---|---|
| Random Forest | 0.863 ± 0.017 | 0.374 ± 0.046 |
| XGBoost | 0.860 ± 0.021 | 0.559 ± 0.103 |
| Naïve Bayes | 0.849 ± 0.014 | 2.13 ± 0.217 |
| Logistic | 0.858 ± 0.011 | 0.750 ± 0.125 |
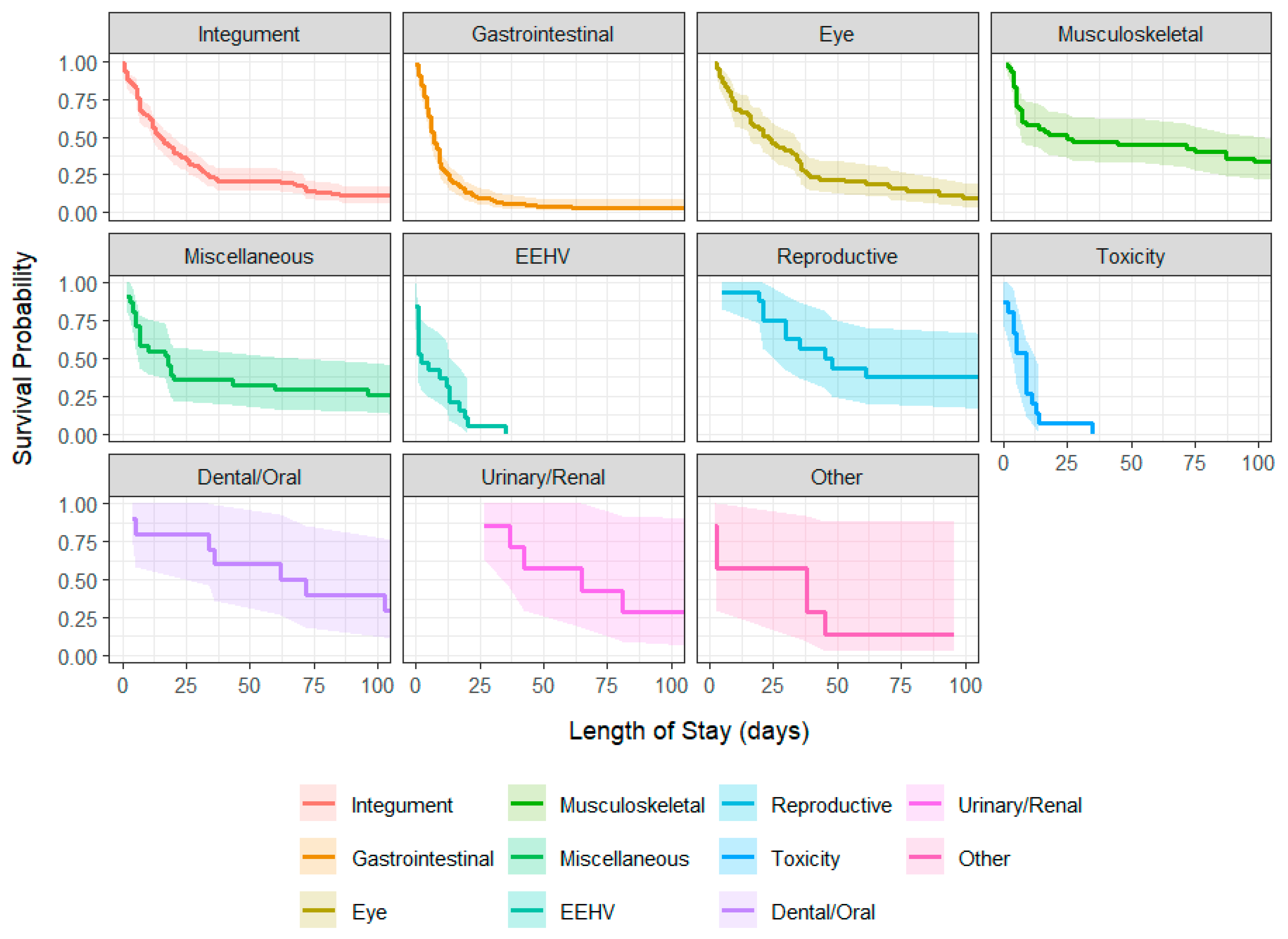
| Disease | Median (Days) | 95% CI Lower | 95% CI Upper |
|---|---|---|---|
| Integument (N = 131) | 15.0 | 12 | 20 |
| Gastrointestinal (N = 119) | 7.0 | 6 | 9 |
| Eye (N = 65) | 23.0 | 16 | 34 |
| Musculoskeletal (N = 47) | 25 | 7 | 111 |
| Miscellaneous (N = 31) | 18.0 | 7 | 96 |
| EEHV (N = 19) | 2.0 | 1 | 17 |
| Reproductive (N = 16) | 46.5 | 30 | - |
| Toxicity (N = 15) | 9.0 | 4 | 13 |
| Dental/Oral (N = 10) | 67.0 | 34 | - |
| Urinary/Renal (N = 7) | 65.0 | 37 | - |
| Other (N = 7) | 38.0 | 3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosaruk, W.; Punyapornwithaya, V.; Ueangpaiboon, P.; Angkawanish, T. Classification of Clinical Outcomes in Hospitalized Asian Elephants Using Machine Learning and Survival Analysis: A Retrospective Study (2019–2024). Vet. Sci. 2025, 12, 998. https://doi.org/10.3390/vetsci12100998
Kosaruk W, Punyapornwithaya V, Ueangpaiboon P, Angkawanish T. Classification of Clinical Outcomes in Hospitalized Asian Elephants Using Machine Learning and Survival Analysis: A Retrospective Study (2019–2024). Veterinary Sciences. 2025; 12(10):998. https://doi.org/10.3390/vetsci12100998
Chicago/Turabian StyleKosaruk, Worapong, Veerasak Punyapornwithaya, Pichamon Ueangpaiboon, and Taweepoke Angkawanish. 2025. "Classification of Clinical Outcomes in Hospitalized Asian Elephants Using Machine Learning and Survival Analysis: A Retrospective Study (2019–2024)" Veterinary Sciences 12, no. 10: 998. https://doi.org/10.3390/vetsci12100998
APA StyleKosaruk, W., Punyapornwithaya, V., Ueangpaiboon, P., & Angkawanish, T. (2025). Classification of Clinical Outcomes in Hospitalized Asian Elephants Using Machine Learning and Survival Analysis: A Retrospective Study (2019–2024). Veterinary Sciences, 12(10), 998. https://doi.org/10.3390/vetsci12100998









