Proliferation-Based WHO Grading and Heterogeneous Gastrin Expression in Canine Gallbladder Neuroendocrine Tumors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Identification
2.2. Histology and Immunohistology
2.3. Statistical Analysis
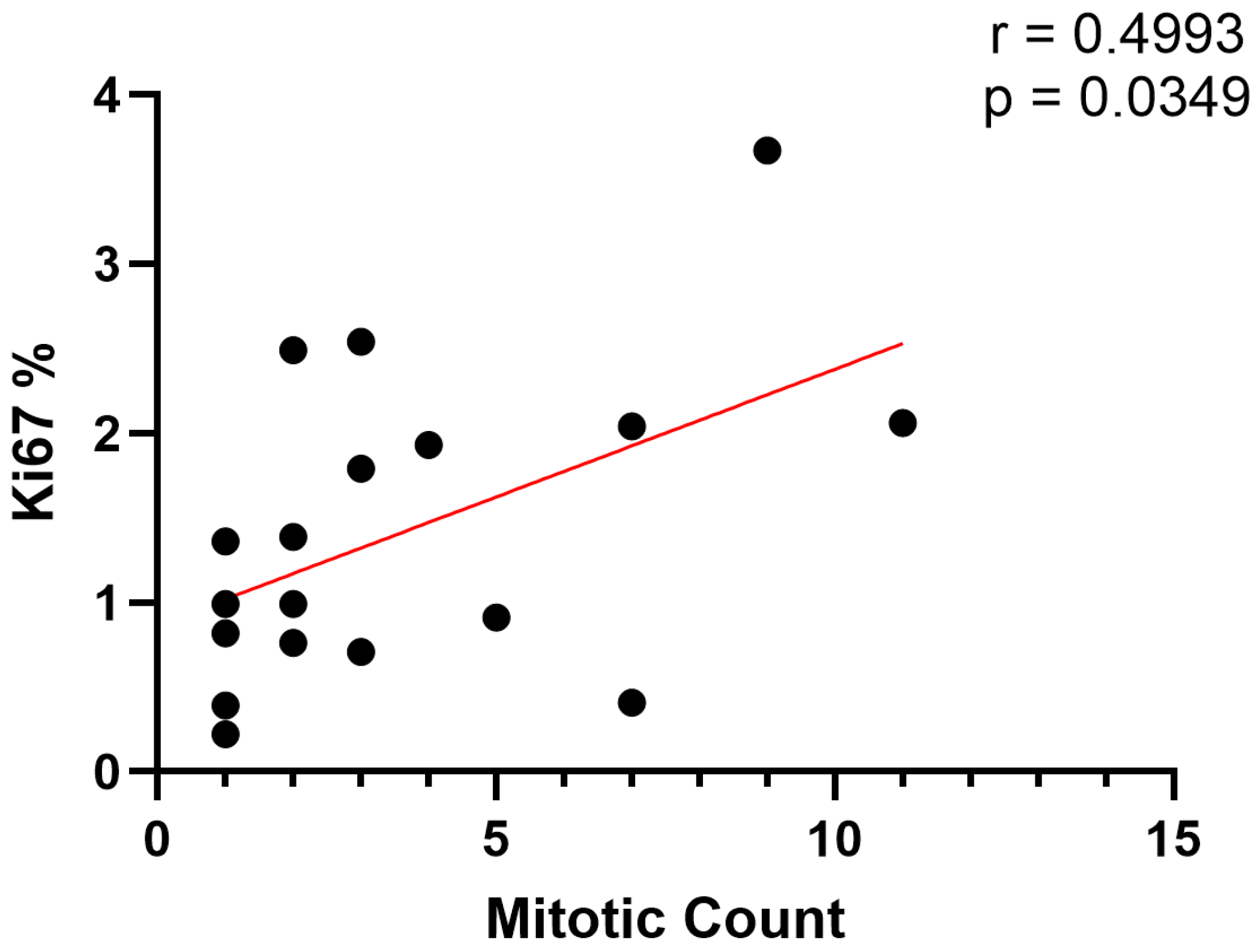
3. Results
3.1. Patient Demographics
3.2. Histologic Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GB | Gallbladder |
| NEC | Neuroendocrine carcinoma |
| NEN | Neuroendocrine neoplasm |
| NET | Neuroendocrine tumor |
References
- Kelly, N.; Wu, Y.-T.; Johnston, A.N. Gallbladder Neuroendocrine Neoplasms in Dogs and Humans. Vet. Sci. 2024, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M.; Bankoff, B.J.; Rosenstein, P.K.; Clendaniel, D.C.; Sánchez, M.D.; Durham, A.C. Clinical, Histopathologic, and Immunohistochemical Features of 13 Cases of Canine Gallbladder Neuroendocrine Carcinoma. J. Vet. Diagn. Investig. 2021, 33, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-G.; Jiang, S.-T.; Zhou, Y.; Zhang, J.-W.; Sang, X.-T.; Zhang, L.; Lu, X.; Xu, Y.-Y. Primary Gastrinoma of the Gallbladder: A Case Report and Review of the Literature. Front. Oncol. 2023, 13, 1279766. [Google Scholar] [CrossRef]
- Duan, S.; Rico, K.; Merchant, J.L. Gastrin: From Physiology to Gastrointestinal Malignancies. Function 2021, 3, zqab062. [Google Scholar] [CrossRef]
- Boyce, M.; Moore, A.R.; Sagatun, L.; Parsons, B.N.; Varro, A.; Campbell, F.; Fossmark, R.; Waldum, H.L.; Pritchard, D.M. Netazepide, a Gastrin/Cholecystokinin-2 Receptor Antagonist, Can Eradicate Gastric Neuroendocrine Tumours in Patients with Autoimmune Chronic Atrophic Gastritis. Br. J. Clin. Pharmacol. 2017, 83, 466–475. [Google Scholar] [CrossRef]
- Lloyd, K.C.; Amirmoazzami, S.; Friedik, F.; Chew, P.; Walsh, J.H. Somatostatin Inhibits Gastrin Release and Acid Secretion by Activating Sst2 in Dogs. Am. J. Physiol.-Gastrointest. Liver Physiol. 1997, 272, G1481–G1488. [Google Scholar] [CrossRef]
- Kim, S.; Hosoya, K.; Takagi, S.; Okumura, M. Treatment of Gastrin-Secreting Tumor with Sustained-Release Octreotide Acetate in a Dog. J. Am. Anim. Hosp. Assoc. 2015, 51, 407–412. [Google Scholar] [CrossRef]
- Hayden, D.W.; Henson, M.S. Gastrin-Secreting Pancreatic Endocrine Tumor in a Dog (Putative Zollinger-Ellison Syndrome). J. Vet. Diagn. Investig. 1997, 9, 100–103. [Google Scholar] [CrossRef]
- Altschul, M.; Simpson, K.W.; Dykes, N.L.; Mauldin, E.A.; Reubi, J.C.; Cummings, J.F. Evaluation of Somatostatin Analogues for the Detection and Treatment of Gastrinoma in a Dog. J. Small Anim. Pract. 1997, 38, 286–291. [Google Scholar] [CrossRef]
- Abrams, J.A.; Del Portillo, A.; Hills, C.; Compres, G.; Friedman, R.A.; Cheng, B.; Poneros, J.; Lightdale, C.J.; De La Rue, R.; Di Pietro, M.; et al. Randomized Controlled Trial of the Gastrin/CCK2 Receptor Antagonist Netazepide in Patients with Barrett’s Esophagus. Cancer Prev. Res. 2021, 14, 675–682. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Athelogou, M.; Lupp, A.; Lenhardt, I.; Schulz, S.; Luisa, P.; Hommann, M.; Prasad, V.; Binnig, G.; Baum, R.P. Somatostatin Receptor Immunohistochemistry in Neuroendocrine Tumors: Comparison Between Manual and Automated Evaluation. Int. J. Clin. Exp. Pathol. 2014, 7, 4971–4980. [Google Scholar]
- Klöppel, G.; Anlauf, M. Epidemiology, Tumour Biology and Histopathological Classification of Neuroendocrine Tumours of the Gastrointestinal Tract. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kulkarni, H.R. THERANOSTICS: From Molecular Imaging Using Ga-68 Labeled Tracers and PET/CT to Personalized Radionuclide Therapy—The Bad Berka Experience. Theranostics 2012, 2, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; De Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A Common Classification Framework for Neuroendocrine Neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) Expert Consensus Proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; De Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Batts, T.L.; Sasaki, E.; Miller, M.; Sparago, J.; Bauer, R.W.; Paulsen, D.; Boudreaux, B.; Liu, C.-C.; Byrum, S.D.; Johnston, A.N. Neoplastic Signatures: Comparative Proteomics of Canine Hepatobiliary Neuroendocrine Tumors to Normal Niche Tissue. PLoS ONE 2023, 18, e0280928. [Google Scholar] [CrossRef]
- Lessels, N.; Woolford, L.; Hayward, D. Gastric Gastrinoma in a Dog: A Case Report, Application of Current WHO Criteria for Prognostication and Validation of a Local Gastrin Immunohistochemistry Assay. Aust. Vet. J. 2025; early view. [Google Scholar] [CrossRef]
- Cruz Cardona, J.A.; Wamsley, H.L.; Farina, L.L.; Kiupel, M. CASE REPORT: Metastatic Pancreatic Polypeptide-Secreting Islet Cell Tumor in a Dog: Pancreatic Polypeptidoma in a Dog. Vet. Clin. Pathol. 2010, 39, 371–376. [Google Scholar] [CrossRef]
- Ritter, J.M.; Garner, M.M.; Chilton, J.A.; Jacobson, E.R.; Kiupel, M. Gastric Neuroendocrine Carcinomas in Bearded Dragons (Pogona vitticeps). Vet. Pathol. 2009, 46, 1109–1116. [Google Scholar] [CrossRef]
- Struthers, J.D.; Robl, N.; Wong, V.M.; Kiupel, M. Gastrinoma and Zollinger-Ellison Syndrome in Canids: A Literature Review and a Case in a Mexican Gray Wolf. J. Vet. Diagn. Investig. 2018, 30, 584–588. [Google Scholar] [CrossRef]
- Malatos, J.M.; Kurpios, N.A.; Duhamel, G.E. Small Intestinal Lymphatic Hypoplasia in Three Dogs with Clinical Signs of Protein-Losing Enteropathy. J. Comp. Pathol. 2018, 160, 39–49. [Google Scholar] [CrossRef]
- Champion, C.P.; Miller, A.D.; Parry, S.; Demeter, E.A. Immunohistochemical and Histomorphologic Characterization of Canine Neoplasms of the Disseminated Neuroendocrine System. Vet. Pathol. 2025, 62, 490–501. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Lieberman, P.H.; Hurvitz, A.I.; Johnson, G.F. Canine Hepatic Carcinoids. Vet. Pathol. 1981, 18, 445–453. [Google Scholar] [CrossRef]
- Kulaksiz, H. Identification of Somatostatin Receptor Subtypes 1, 2A, 3, and 5 in Neuroendocrine Tumours with Subtype Specific Antibodies. Gut 2002, 50, 52–60. [Google Scholar] [CrossRef]
- Schmid, H.A.; Lambertini, C.; Van Vugt, H.H.; Barzaghi-Rinaudo, P.; Schäfer, J.; Hillenbrand, R.; Sailer, A.W.; Kaufmann, M.; Nuciforo, P. Monoclonal Antibodies Against the Human Somatostatin Receptor Subtypes 1–5: Development and Immunohistochemical Application in Neuroendocrine Tumors. Neuroendocrinology 2012, 95, 232–247. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Al-Toubah, T.; El-Haddad, G.; Reidy Lagunes, D.; Bodei, L. Sequencing of Somatostatin-Receptor–Based Therapies in Neuroendocrine Tumor Patients. J. Nucl. Med. 2024, 65, 340–348. [Google Scholar] [CrossRef]
- Jablonski, S.A.; Mazepa, A.S.W.; Tolbert, M.K. Use of Octreotide for the Treatment of Protein-Losing Enteropathy in Dogs: Retrospective Study of 18 Cases. J. Vet. Intern. Med. 2024, 38, 145–151. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-T.; Kelly, N.; Langohr, I.M.; Sokol, S.; Gerdin, J.; Liu, C.-C.; Butsch, T.J.; Johnston, A.N. Proliferation-Based WHO Grading and Heterogeneous Gastrin Expression in Canine Gallbladder Neuroendocrine Tumors. Vet. Sci. 2025, 12, 989. https://doi.org/10.3390/vetsci12100989
Wu Y-T, Kelly N, Langohr IM, Sokol S, Gerdin J, Liu C-C, Butsch TJ, Johnston AN. Proliferation-Based WHO Grading and Heterogeneous Gastrin Expression in Canine Gallbladder Neuroendocrine Tumors. Veterinary Sciences. 2025; 12(10):989. https://doi.org/10.3390/vetsci12100989
Chicago/Turabian StyleWu, Yen-Tse, Nadia Kelly, Ingeborg M. Langohr, Set Sokol, Jodie Gerdin, Chin-Chi Liu, Tyler J. Butsch, and Andrea N. Johnston. 2025. "Proliferation-Based WHO Grading and Heterogeneous Gastrin Expression in Canine Gallbladder Neuroendocrine Tumors" Veterinary Sciences 12, no. 10: 989. https://doi.org/10.3390/vetsci12100989
APA StyleWu, Y.-T., Kelly, N., Langohr, I. M., Sokol, S., Gerdin, J., Liu, C.-C., Butsch, T. J., & Johnston, A. N. (2025). Proliferation-Based WHO Grading and Heterogeneous Gastrin Expression in Canine Gallbladder Neuroendocrine Tumors. Veterinary Sciences, 12(10), 989. https://doi.org/10.3390/vetsci12100989





