Breed- and Line-Dependent Severity of Inflammation and Necrosis Syndrome in AI Boars, and the Related Risk of Inflammation and Necrosis in Their Progeny
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and the Tested Animals
2.2. The Maintenance and Feeding of the AI Boars
2.3. The Maintenance and Feeding of Sows
2.4. Paternity Testing
2.5. Clinical Evaluation of AI Boars and Piglets
2.6. Statistics
3. Results
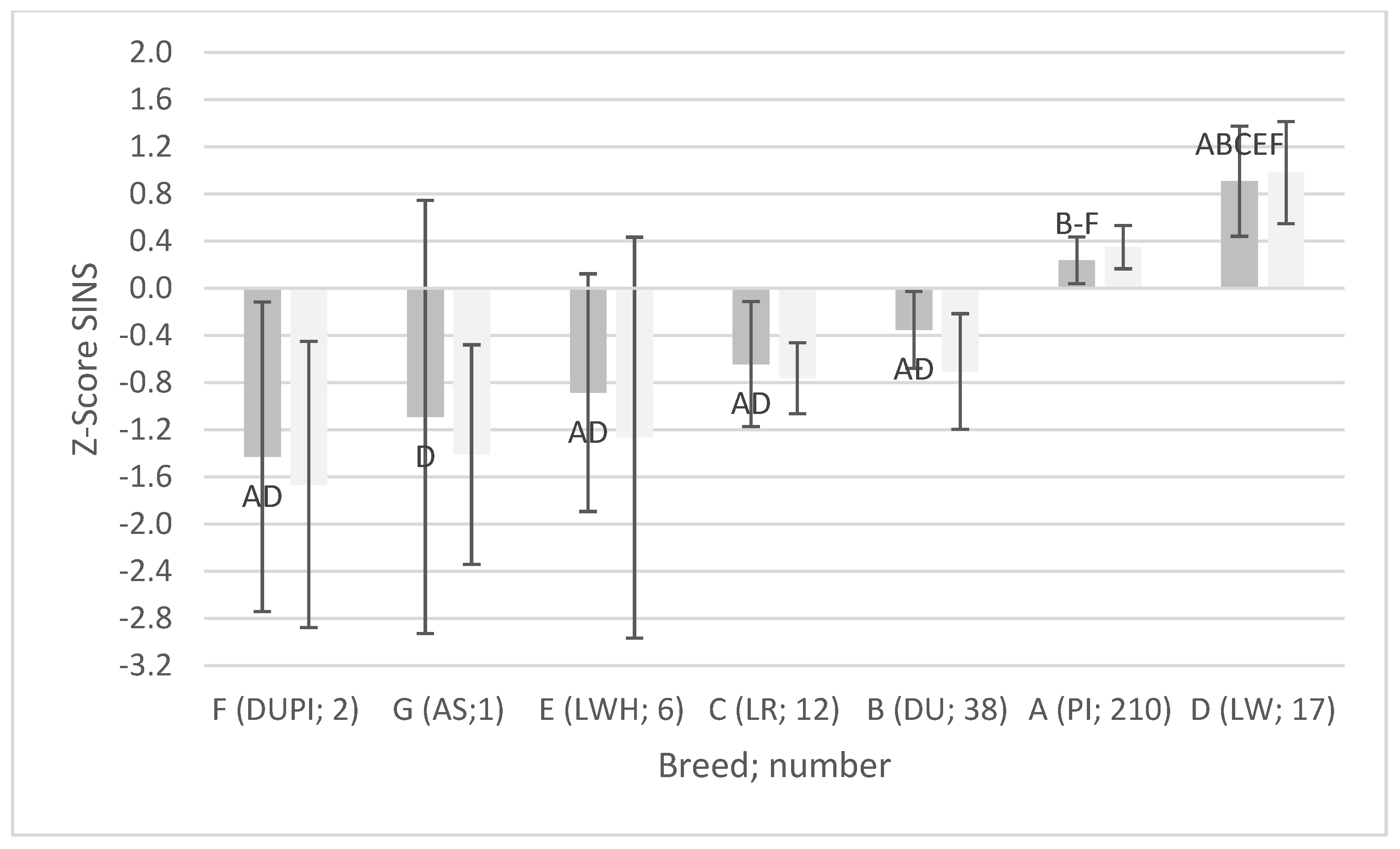
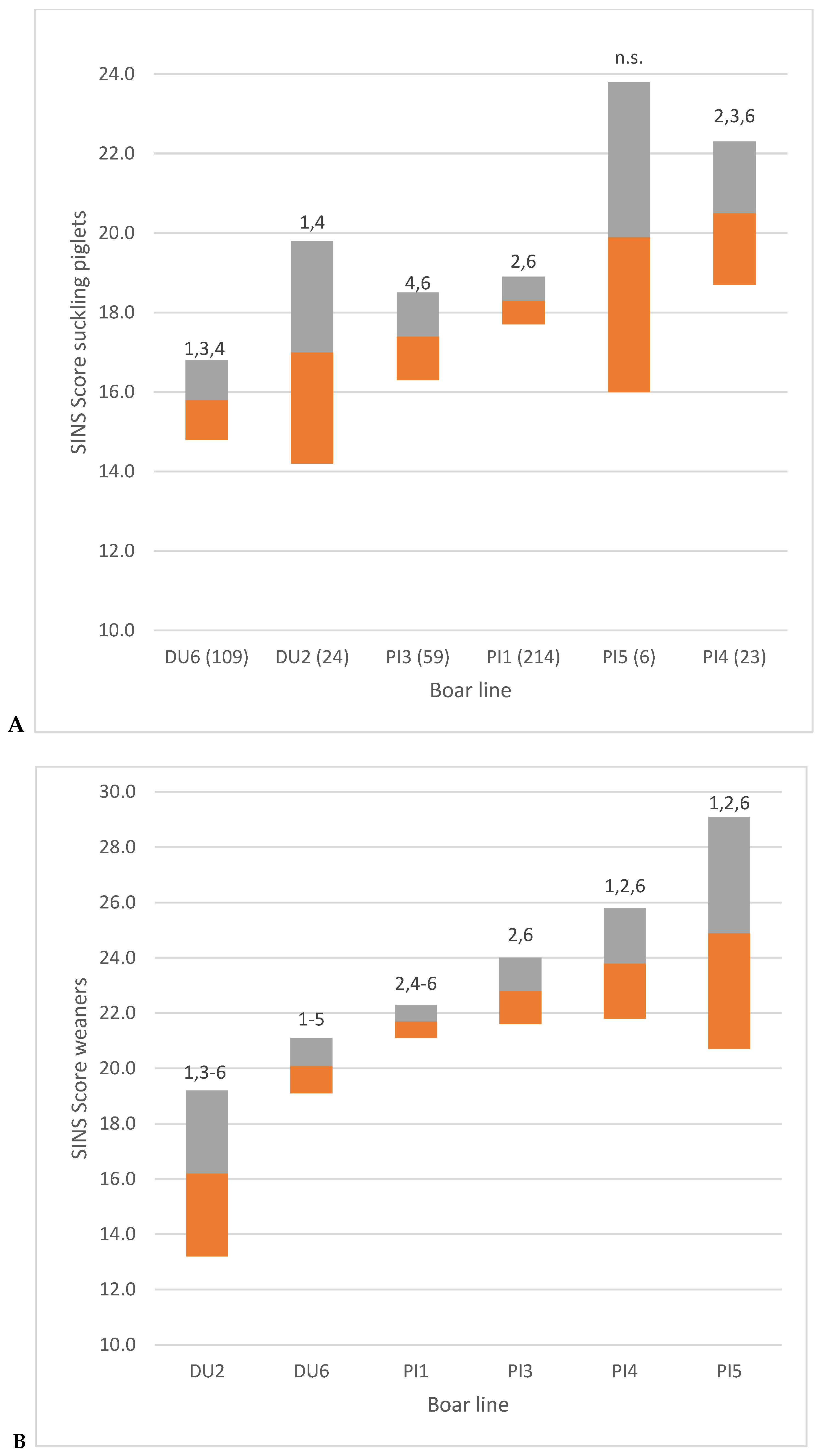
3.1. AI Boar Phenotypes
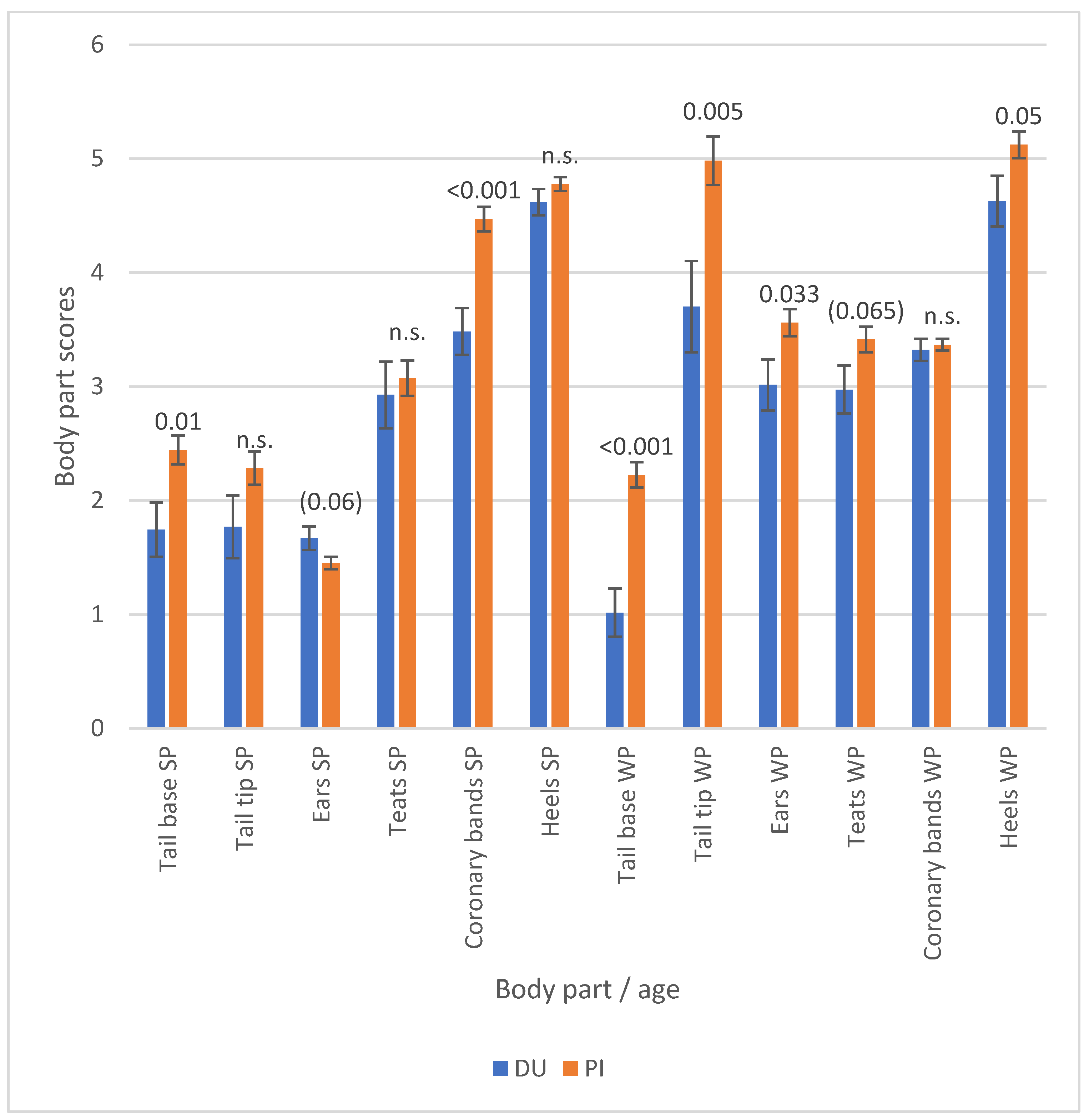
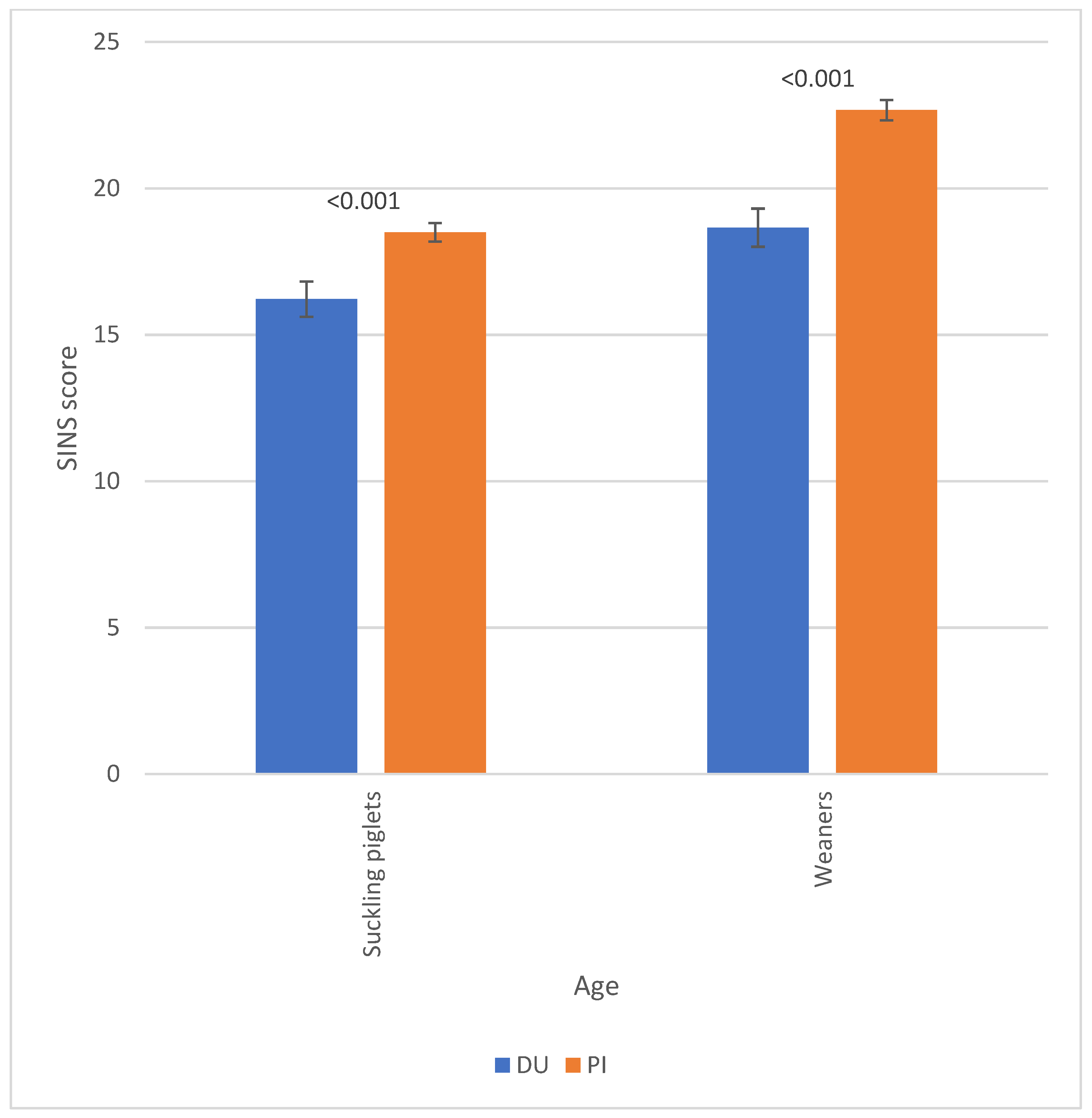
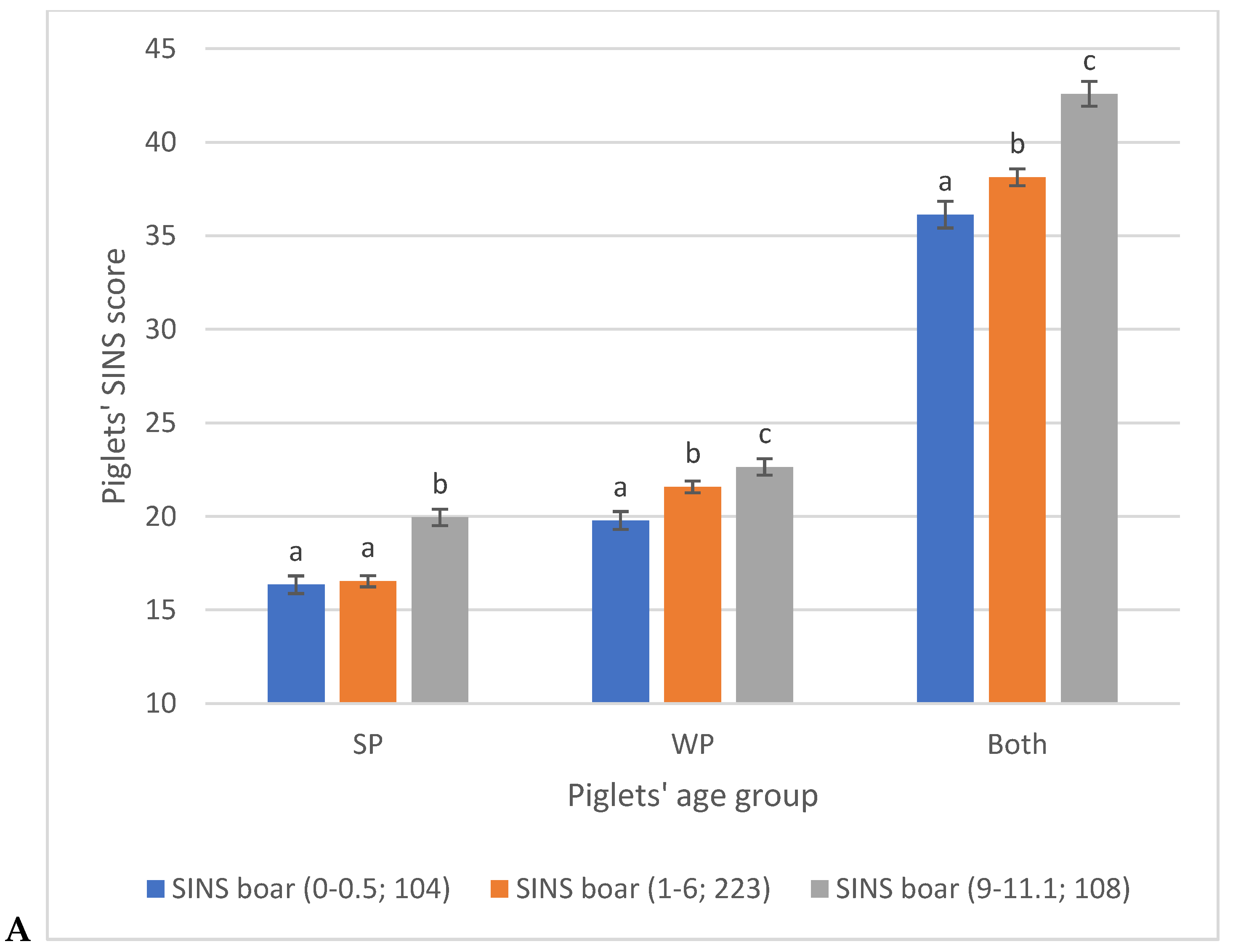
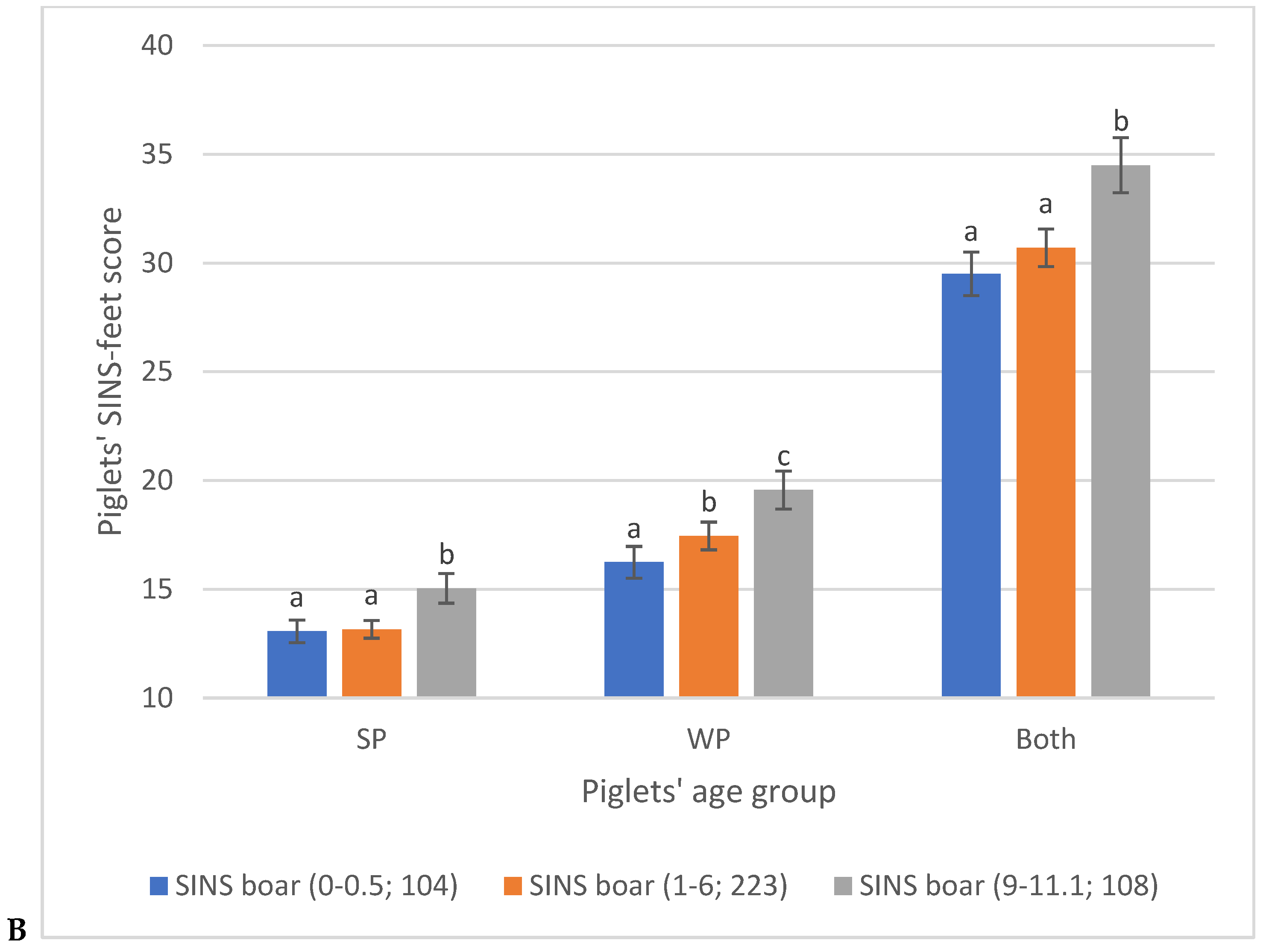
3.2. Piglet Phenotypes
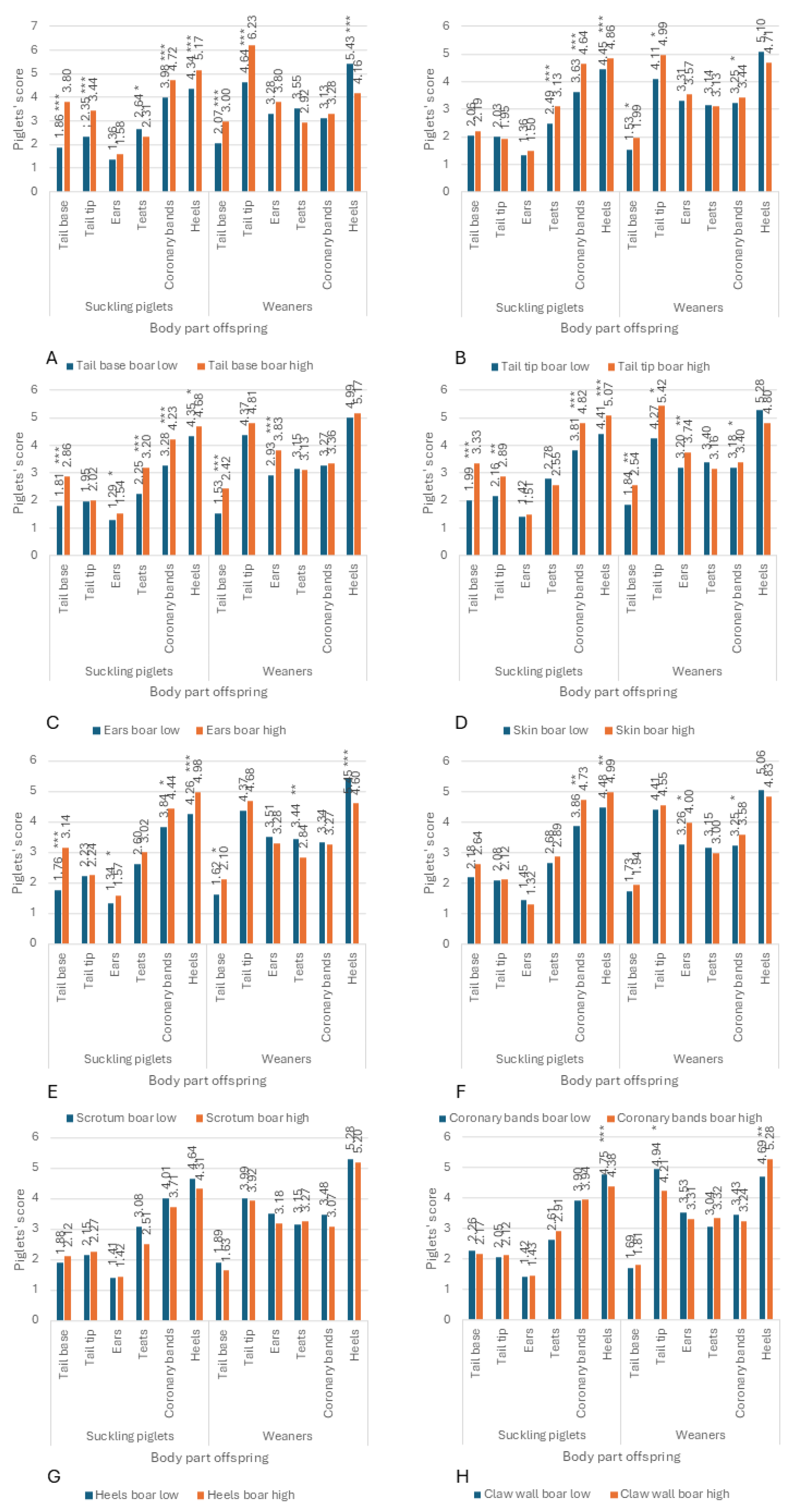
3.3. Associations Between SINS Scores in Duroc and Pietrain AI Boars and Their Offspring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SINS | Swine inflammation and necrosis syndrome |
| PI | Pietrain |
| DU | Duroc |
| LW | Large White, commercial lines |
| LWH | Large White, herdbook |
| LR | Landrace |
| AS | Angler Saddleback |
| SP | Suckling piglets |
| WP | Weaned piglets |
References
- Mellor, D.J. Updating Animal Welfare Thinking: Moving beyond the “Five Freedoms” towards “A Life Worth Living”. Animals 2016, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Animal Health and Welfare (AHAW); Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Rojas, J.L.G.; Schmidt, G.; et al. Welfare of pigs on farm. EFSA J. 2022, 20, e07421. [Google Scholar] [CrossRef]
- Zuliani, A.; Romanzin, A.; Corazzin, M.; Salvador, S.; Abrahantes, J.; Bovolenta, S. Welfare assessment in traditional mountain dairy farms: Above and beyond resource-based measures. Anim. Welf. 2017, 26, 203–211. [Google Scholar] [CrossRef]
- Whay, H.R.; Main, D.C.J.; Green, L.E.; Webster, A.J.F. Animal-Based Measures for the Assessment of Welfare State of Dairy Cattle, Pigs and Laying Hens: Consensus of Expert Opinion. Anim. Welf. 2003, 12, 205–217. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare (AHAW). Statement on the use of animal-based measures to assess the welfare of animals. EFSA J. 2012, 10, 2767. [Google Scholar] [CrossRef]
- Koenders-van Gog, K.; Wijnands, T.; Lechner, M.; Reiner, G.; Fink-Gremmels, J. Screening of Piglets for Signs of Inflammation and Necrosis as Early Life Indicators of Animal Health and Welfare Hazards. Animals 2025, 15, 378. [Google Scholar] [CrossRef]
- Kuehling, J.; Loewenstein, F.; Wenisch, S.; Kressin, M.; Herden, C.; Lechner, M.; Reiner, G. An in-depth diagnostic exploration of an inflammation and necrosis syndrome in a population of newborn piglets. Animal 2021, 15, 100078. [Google Scholar] [CrossRef]
- Reiner, G.; Kuehling, J.; Lechner, M.; Schrade, H.J.; Saltzmann, J.; Muelling, C.; Daenicke, S.; Loewenstein, F. Swine Inflammation and Necrosis Syndrome is influenced by husbandry and quality of sow in suckling piglets, weaners and fattening pigs. Porc. Health Manag. 2020, 6, 32. [Google Scholar] [CrossRef]
- Reiner, G.; Kuehling, J.; Loewenstein, F.; Lechner, M.; Becker, S. Swine Inflammation and Necrosis Syndrome (SINS). Animals 2021, 11, 1670. [Google Scholar] [CrossRef]
- Reiner, G.; Lechner, M. Inflammation and necrosis syndrome (SINS) in swine. CAB Rev. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Leite, N.G.; Knol, E.F.; Nuphaus, S.; Vogelzang, R.; Tsuruta, S.; Wittmann, M.; Lourenco, D. The genetic basis of swine inflammation and necrosis syndrome and its genetic association with post-weaning skin damage and production traits. J. Anim. Sci. 2023, 101, skad067. [Google Scholar] [CrossRef] [PubMed]
- Fortune, H.; Micout, S.; Monjouste, A. Évaluation de la prévalence du syndrome inflammatoire et nécrotique porcin dans les troupeaux français. Journées Rech. Porc. 2024, 56, 301–302. [Google Scholar]
- Betz, P. Histological and enzyme histochemical parameters for the age estimation of human skin wounds. Int. J.-Nal. Leg. Med. 1994, 107, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, F.; Becker, S.; Kuehling, J.; Schrade, H.; Lechner, M.; Ringseis, R.; Eder, K.; Moritz, A.; Reiner, G. Inflammation and necrosis syndrome is associated with alterations in blood and metabolism in pigs. BMC Vet. Res. 2022, 18, 50. [Google Scholar] [CrossRef]
- Ringseis, R.; Gessner, D.K.; Loewenstein, F.; Kuehling, J.; Becker, S.; Willems, H.; Lechner, M.; Eder, K.; Reiner, G. Swine Inflammation and Necrosis Syndrome Is Associated with Plasma Metabolites and Liver Transcriptome in Affected Piglets. Animals 2021, 11, 772. [Google Scholar] [CrossRef]
- Gerhards, K.; Becker, S.; Kuehling, J.; Lechner, M.; Willems, H.; Ringseis, R.; Reiner, G. Screening for transcriptomic associations with Swine Inflammation and Necrosis Syndrome. BMC Vet. Res. 2025, 21, 26. [Google Scholar] [CrossRef]
- Reiner, G.; Lechner, M.; Eisenack, A.; Kallenbach, K.; Rau, K.; Müller, S.; Fink-Gremmels, J. Prevalence of an inflammation and necrosis syndrome in suckling piglets. Animal 2019, 13, 2007–2017. [Google Scholar] [CrossRef]
- Kuehling, J.; Eisenhofer, K.; Lechner, M.; Becker, S.; Willems, H.; Reiner, G. The effects of boar on susceptibility to swine inflammation and necrosis syndrome in piglets. Porc. Health Managem. 2021, 7, 15. [Google Scholar] [CrossRef]
- Gerhards, K.; Becker, S.; Kuehling, J.; Lechner, M.; Bathke, J.; Willems, H.; Reiner, G. GWAS reveals genomic associations with swine inflammation and necrosis syndrome. Mamm. Genome 2023, 34, 586–601. [Google Scholar] [CrossRef]
- Gerhards, K.; Becker, S.; Kühling, J.; Mickan, J.; Lechner, M.; Willems, H.; Reiner, G. Fine Mapping Identifies Candidate Genes Associated with Swine Inflammation and Necrosis Syndrome. Vet. Sci. 2025, 12, 508. [Google Scholar] [CrossRef]
- Becker, S.; Hindenlang, K.; Kuehling, J.; Lechner, M.; Reiner, G. Inflammation and Necrosis Syndrome in Young Piglets—A Longitudinal Study. Vet. Sci. 2025, 12, 752. [Google Scholar] [CrossRef]
- Mouttotou, N.; Hatchell, F.; Green, L. The prevalence and risk factors associated with forelimb skin abrasions and sole bruising in preweaning piglets. Prev. Vet. Med. 1999, 39, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Kilbride, A.L.; Gillman, C.E.; Ossent, P.; Green, L.E. A cross sectional study of prevalence, risk factors, population attributable fractions and pathology for foot and limb lesions in preweaning piglets on commercial farms in England. BMC Vet. Res. 2009, 5, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Lösel, D.; Dodenhoff, J.; Eisenreich, R.; Götz, K.U. Untersuchungen zur Erblichkeit des Schweine-Entzündungs- und Nekrose-syndroms—Erste Ergebnisse aus dem Projekt HeriSINS. In Proceedings of the Vortragstagung der DGfZ und GfT, Göttingen, Germany, 18–19 September 2024; Available online: https://www.lfl.bayern.de/mam/cms07/itz/dateien/herisins_ergebnisse_der_dgfz-vortragsveranstaltung_2024.pdf (accessed on 9 September 2025).
- Fairbairn, L.; Kapetanovic, R.; Sester, D.P.; Hume, D.A. The mononuclear phagocyte system of the pig as a model for understanding humaninnate immunity and disease. J. Leukoc. Biol. 2011, 89, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samuvel, D.J.; Sundararaj, K.P.; Nareika, A.; Lopes-Virella, M.F.; Huang, Y. Lactate boosts TLR4 signaling and NF-kappaB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. J. Immunol. 2009, 182, 2476–2484. [Google Scholar] [CrossRef]
- Manosalva, C.; Quiroga, J.; Hidalgo, A.I.; Alarcón, P.; Anseoleaga, N.; Hidalgo, M.A.; Burgos, R.A. Role of Lactate in Inflammatory Processes: Friend or Foe. Front. Immunol. 2022, 12, 808799, Erratum in: Front. Immunol. 2025, 16, 1553925. https://doi.org/10.3389/fimmu.2025.1553925. PMID: 35095895; PMCID: PMC8795514. [Google Scholar] [CrossRef]
- Visan, I. Stress-induced inflammation. Nat. Immunol. 2023, 24, 1051. [Google Scholar] [CrossRef]
- Corbitt, N.; Kimura, S.; Isse, K.; Specht, S.; Chedwick, L.; Rosborough, B.R.; Lunz, J.G.; Murase, N.; Yokota, S.; Demetris, A.J. Gut bacteria drive Kupffer cell expansion via MAMP-mediated ICAM-1 induction on sinusoidal endothelium and influence preservation-reperfusion injury after orthotopic liver transplantation. Am. J. Pathol. 2013, 182, 180–191. [Google Scholar] [CrossRef]
- Klein, K.; Fuchs, G.J.; Kulanpongs, P.; Mertz, G.; Suskind, R.M.; Olson, R.E. Endotoxemia in protein-energy malnutrition. J. Pediatr. Gastroent. Nutr. 1988, 7, 225–228. [Google Scholar]
- Ortega, A.D.S.V.; Szabó, C. Adverse Effects of Heat Stress on the Intestinal Integrity and Function of Pigs and the Mitigation Capacity of Dietary Antioxidants: A Review. Animals 2021, 11, 1135. [Google Scholar] [CrossRef]
- Ayman, M.; Mahmoud, A.M.; Wilkinson, F.L.; Sandhu, M.A.; Lightfoot, A.P. The Interplay of Oxidative Stress and Inflammation: Mechanistic Insights and Therapeutic Potential of Antioxidants. Oxid. Med. Cell. Longev. 2021, 2021, 9851914. [Google Scholar] [CrossRef]
- Pearce, C.S.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS ONE 2013, 8, e70215. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs. J. Anim. Sci. 2012, 90, 257–259. [Google Scholar] [CrossRef]
- Pearce, S.C.; Sanz-Fernandez, M.V.; Hollis, J.H.; Baumgard, L.H.; Gabler, N.K. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. J. Anim. Sci. 2014, 92, 5444–5454. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, X.; Cui, Y.; Wang, W.; Liu, H.; Li, Z.; Guo, Z.; Ma, S.; Li, D.; Wang, C.; et al. Consumption of Dietary Fiber from Different Sources during Pregnancy Alters Sow Gut Microbiota and Improves Performance and Reduces Inflammation in Sows and Piglets. mSystems 2021, 6, e00591-20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shang, Q.; Liu, H.; Liu, S.; He, T.; Piao, X. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets1. J. Anim. Sci. 2019, 97, 4922–4933. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Chen, J.; Liu, J.; Chen, F.; Guan, W.; Zhang, S. Dietary fiber and microbiota interaction regulates sow metabolism and reproductive performance. Anim. Nutr. 2020, 6, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Sanz Fernandez, M.V.; Stoakes, S.K.; Abuajamieh, M.; Seibert, J.T.; Johnson, J.S.; Horst, E.A.; Rhoads, R.P.; Baumgard, L.H. Heat stress increases insulin sensitivity in pigs. Physiol. Repetit. 2015, 3, e1247. [Google Scholar] [CrossRef]
- Sahasrabudhe, N.; Beukema, M.; Tian, L.; Troost, B.; Scholte, J.; Bruininx, E.; Bruggeman, G.; van den Berg, M.; Scheurink, A.; Schols, H.; et al. Dietary fiber pectin directly blocks Toll-like receptor 2-1 and prevents doxorubicin-induced ileitis. Front. Immunol. 2018, 9, 383. [Google Scholar] [CrossRef]
- Luu, M.; Monning, H.; Visekruna, A. Exploring the molecular mechanisms underlying the protective effects of microbial SCFAs on intestinal tolerance and food allergy. Front. Immunol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wei, H.; Xu, C.; Xie, X.; Jiang, S.; Peng, J. Maternal soluble fiber diet during pregnancy changes the intestinal microbiota, improves growth performance, and reduces intestinal permeability in piglets. Appl. Environ. Microbiol. 2018, 84, e01047-18. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Costermans, N.; Teerds, K.; Keijer, J.; Knol, E.; Koopmanschap, R.; Kemp, B.; Soede, N. Follicular development of sows at weaning in relation to estimated breeding value for within-litter variation in piglet birth weight. Animal 2019, 13, 554–563. [Google Scholar] [CrossRef]
- Jabbour, H.N.; Sales, K.J.; Catalano, R.D.; Norman, J.E. Inflammatory pathways in female reproductive health and disease. Reproduction 2009, 138, 903–919. [Google Scholar] [CrossRef]
- Sawicki, C.M.; Livingston, K.A.; Obin, M.; Roberts, S.B.; Chung, M.; McKeown, N.M. Dietary fiber and the human gut microbiota: Application of evidence mapping methodology. Nutrients 2017, 9, 125. [Google Scholar] [CrossRef]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef]
- Martens, E.; Neumann, M.; Desai, M. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat. Rev. Microbiol. 2018, 16, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Rietschel, E. Invited review: Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001, 7, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, A.S.; Krabbe, K.S.; Krogh-Madsen, R.; Taudorf, S.; Pedersen, B.K.; Møller, K. Human endotoxemia as a model of systemic inflammation. Curr. Med. Chem. 2008, 15, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.P.; Texeira, T.F.; Ferreira, A.B.; Peluzio Mdo, C.; Alfenas Rde, C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Rainone, V.; Schneider, L.; Saulle, I.; Ricci, C.; Biasin, M.; Al-Daghri, N.M.; Giani, E.; Zuccotti, G.V.; Clerici, M.; Trabattoni, D. Upregulation of inflammasome activity and increased gut permeability are associated with obesity in children and adolescents. Int. J. Obes. 2016, 40, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Munsterhjelm, C.; Nordgreen, J.; Aae, F.; Heinonen, M.; Valros, A.; Janczak, A.M. Sick and grumpy: Changes in social behaviour after a controlled immune stimulation in group-housed gilts. Physiol. Behav. 2019, 198, 76–83. [Google Scholar] [CrossRef]
- Nordgreen, J.; Edwards, S.A.; Boyle, L.A.; Bolhuis, J.E.; Veit, C.; Sayyari, A.; Marin, D.E.; Dimitrov, I.; Janczak, A.M.; Valros, A. A Proposed Role for Pro-Inflammatory Cytokines in Damaging Behavior in Pigs. Front. Vet. Sci. 2020, 7, 646. [Google Scholar] [CrossRef]
- Dänicke, S.; Valenta, H.; Ganter, M.; Brosig, B.; Kersten, S.; Diesing, A.K.; Kahlert, S.; Kluess, J.; Rothkötter, H.J. Lipopolysaccharides (LPS) modulate the metabolism of deoxynivalenol (DON) in the pig. Mycotox. Res. 2014, 30, 161–170. [Google Scholar] [CrossRef]
- Ganey, P.E.; Roth, R.A. Concurrent inflammation as a determinant of susceptibility to toxicity from xenobiotic agents. Toxicology 2001, 169, 195–208. [Google Scholar] [CrossRef]
- Van Limbergen, T.; Devreese, M.; Croubels, S.; Broekaert, N.; Michiels, A.; DeSaeger, S.; Maes, D. Role of mycotoxins in herds with and without problems with tail necrosis in neonatal pigs. Vet. Rec. 2017, 181, 539. [Google Scholar] [CrossRef]
- Schrauwen, E.; Thoonen, H.; Hoorens, J.; Houvenaghel, A. Pathophysiological effects of endotoxin infusion in young piglets. Br. Vet. J. 1986, 142, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Jadamus, A.; Schneider, D. Long-term effect of fusariotoxins on the reproduction performance of sows testing the effectiveness of detoxifying feed additives 700. Feed Magzine 2002, 10, 396–405. [Google Scholar]
- Busch, M.E.; Jensen, I.M.; Korsgaard, J. Development and consequences of ear necrosis in a weaner herd and two growingfinishing herds. In Proceedings of the 21st International Pig Veterinary Society Congress, Vancouver, BC, Canada, 18–21 July 2010; p. 45. [Google Scholar]
- Guillou, D.; Demey, V.; Chacheyras-Durand, F.; Le Treut, Y. Mise en evidence du transfer des endotoxines de la truie vers sa portée dans le context du syndrome de dysgalactie post-partum. J. Rech. Porc. 2013, 45, 269–270. [Google Scholar]
- Studzinski, T. The post-natal changes in minimal metabolic rate in the pig. J. Physiol. 1972, 224, 305–316. [Google Scholar] [CrossRef]
- Wellock, I.J.; Emmans, G.C.; Kyriazakis, I. Describing and predicting potential growth in the pig. Anim. Sci. 2004, 78, 379–388. [Google Scholar] [CrossRef]
- Kil, D.Y.; Kim, B.G.; Stein, H.H. Feed energy evaluation for growing pigs. Asian-Australas. J. Anim. Sci. 2013, 26, 1205–1217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, G.F.; Liu, D.W.; Wang, F.L.; Li, D.F. Estimation of the net energy requirements for maintenance in growing and finishing pigs. J. Anim. Sci. 2014, 92, 2987–2995. [Google Scholar] [CrossRef] [PubMed]
- Ask, B.; Pedersen, L.V.; Christensen, O.F.; Nielsen, H.M.; Turner, S.P.; Nielsen, B. Selection for social genetic effects in purebreds increases growth in crossbreds. Genet. Sel. Evol. 2021, 53, 15. [Google Scholar] [CrossRef]
- Hayward, A.; Gillooly, J.F. The cost of sex: Quantifying energetic investment in gamete production by males and females. PLoS ONE 2011, 6, e16557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henneberg, S.; Kleve-Feld, M.; Schröter, F.; Jung, M.; Schulze, M. Lifetime and removal reasons for Pietrain boars in European AI centers: A retrospective analysis. J. Anim. Sci. 2023, 101, skac408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hermesch, S. Genetic improvement of lean meat growth and feed efficiency in pigs. Aust. J. Exp. Agric. 2004, 44, 383–391. [Google Scholar] [CrossRef]
- van Marle-Köster, E.; Visser, C. Unintended consequences of selection for increased production on the health and welfare of livestock. Arch. Anim. Breed. 2021, 64, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Rauw, W.M.; Johnson, A.K.; Gomez-Raya, L.; Dekkers, J.C.M. Hypothesis and Review of the Relationship between Selection for Improved Production Efficiency, Coping Behavior, and Domestication. Front. Genet. 2017, 8, 00134. [Google Scholar] [CrossRef]
- Mathur, P.K.; Vogelzang, R.; Mulder, H.A.; Knol, E.F. Genetic Selection to Enhance Animal Welfare Using Meat Inspection Data from Slaughter Plants. Animals 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilbert, H.; Billon, Y.; Brossard, L.; Faure, J.; Gatellier, P.; Gondret, F.; Labussière, E.; Lebret, B.; Lefaucheur, L.; Le Floch, N.; et al. Review: Divergent selection for residual feed intake in the growing pig. Animal 2017, 11, 1427–1439. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, K.-S.; Cheng, J.; Tuggle, C.; Dyck, M.; Canada, P.; Fortin, F.; Harding, J.; Plastow, G.; Dekkers, J. Genetic analysis of the blood transcriptome of young healthy pigs to improve disease resilience. Genet. Sel. Evol. 2023, 55, 90. [Google Scholar] [CrossRef]
- Papatsiros, V.G.; Maragkakis, G.; Papakonstantinou, G.I. Stress Biomarkers in Pigs: Current Insights and Clinical Application. Vet. Sci. 2024, 11, 640. [Google Scholar] [CrossRef]
- Brooks, G.A.; Osmond, A.D.; Arevalo, J.A.; Duong, J.J.; Curl, C.C.; Moreno-Santillan, D.D.; Leija, R.G. Lactate as a myokine and exerkine: Drivers and signals of physiology and metabolism. J. Appl. Physiol. 2023, 134, 529–548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Essén-Gustavsson, B.; Fjelkner-Modig, S. Skeletal muscle characteristics in different breeds of pigs in relation to sensory properties of meat. Meat Sci. 1985, 13, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Gondret, F.; Lefaucheur, L.; Juin, H.; Louveau, I. Low birth weight is associated with enlarged muscle fiber area and impaired meat tenderness of the longissimus muscle in pigs. J. Anim. Sci. 2006, 84, 93–103. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition InfluenceMeat and Flesh Quality. Sci. World J. 2016, 2016, 3182746. [Google Scholar] [CrossRef]
- Rosenvold, K.; Andersen, H.J. Factors of Significance For Pork Quality—A Review. Meat Sci. 2003, 64, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhu, L.; He, C.; Xiang, R.; Liu, J.; Cai, J.; Wang, D. Lactate induces oxidative stress by HIF1α stabilization and circadian clock disturbance in mammary gland of dairy cows. J. Anim. Sci. Biotechnol. 2025, 16, 62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, W.D.; Weilandt, D.R.; Liang, L.; MacArthur, M.R.; Jaiswal, N.; Ong, O.; Mann, C.G.; Chu, Q.; Hunter, C.J.; Ryseck, R.P.; et al. Lactate homeostasis is maintained through regulation of glycolysis and lipolysis. Cell Metab. 2025, 37, 758–771.e8. [Google Scholar] [CrossRef] [PubMed]
- Gladden, L.B. Lactate metabolism: A new paradigm for the third millennium. J. Physiol. 2004, 558 Pt 1, 5–30. [Google Scholar] [CrossRef]
- Sheikh, A.Y.; Gibson, J.J.; Rollins, M.D.; Hopf, H.W.; Hussain, Z.; Hunt, T.K. Effect of hyperoxia on vascular endothelial growth factor levels in a wound model. Arch. Surg. 2000, 135, 1293–1297. [Google Scholar] [CrossRef]
- Ghani, Q.P.; Wagner, S.; Hussain, M.Z. Role of ADP-ribosylation in wound repair. The contributions of Thomas K. Hunt, MD. Wound Repair. Regen. 2003, 11, 439–444. [Google Scholar] [CrossRef]
- Constant, J.S.; Feng, J.J.; Zabel, D.D.; Yuan, H.; Suh, D.Y.; Scheuenstuhl, H.; Hunt, T.K.; Hussain, M.Z. Lactate elicits vascular endothelial growth factor from macrophages: A possible alternative to hypoxia. Wound Repair. Regen. 2000, 8, 353–360. [Google Scholar] [CrossRef]
- Trabold, O.; Wagner, S.; Wicke, C.; Scheuenstuhl, H.; Hussain, M.Z.; Rosen, N.; Seremetiev, A.; Becker, H.D.; Hunt, T.K. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair. Regen. 2003, 11, 504–509. [Google Scholar] [CrossRef]
- Eliceiri, B.P.; Paul, R.; Schwartzberg, P.L.; Hood, J.D.; Leng, J.; Cheresh, D.A. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell. 1999, 4, 915–924. [Google Scholar] [CrossRef]
- Hu, G.; Place, A.T.; Minshall, R.D. Regulation of endothelial permeability by Src kinase signaling: Vascular leakage versus transcellular transport of drugs and macromolecules. Chem. Biol. Interact. 2008, 171, 177–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tolstanova, G.; Khomenko, T.; Deng, X.; Szabo, S.; Sandor, Z. New molecular mechanisms of the unexpectedly complex role of VEGF in ulcerative colitis. Biochem. Biophys. Res. Commun. 2010, 399, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Dokmanovic, M.; Baltic, M.Z.; Duric, J.; Ivanovic, J.; Popovic, L.; Todorovic, M.; Markovic, R.; Pantic, S. Correlations among Stress Parameters, Meat and Carcass Quality Parameters in Pigs. Asian-Australas. J. Anim. Sci. 2015, 28, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.; Steinmann, C.; Scholl, E. Belastung durch die Samenentnahme am Phantom: Effekte auf Herz-Kreislauf-System, Säure-Basen-Haushalt und Plasmaenzyme bei Eber der Rassen Landrasse und Pietrain. Tierarztl. Prax. 1988, 3, 93–100. Available online: https://europepmc.org/article/med/3368904#abstract (accessed on 1 August 2025).
- Barb, C.R.; Hausman, G.J.; Houseknecht, K.L. Biology of leptin in the pig. Domest. Anim. Endocrinol. 2001, 21, 297–317. [Google Scholar] [CrossRef]
- Choi, I.; Steibel, J.P.; Bates, R.O.; Raney, N.E.; Rumph, J.M.; Ernst, C.W. Application of alternative models to identify QTL for growth traits in an F2 Duroc x Pietrain pig resource population. BMC Genet. 2010, 11, 97. [Google Scholar] [CrossRef]
- La Cava, A. Leptin in inflammation and autoimmunity. Cytokine 2017, 98, 51–58. [Google Scholar] [CrossRef]
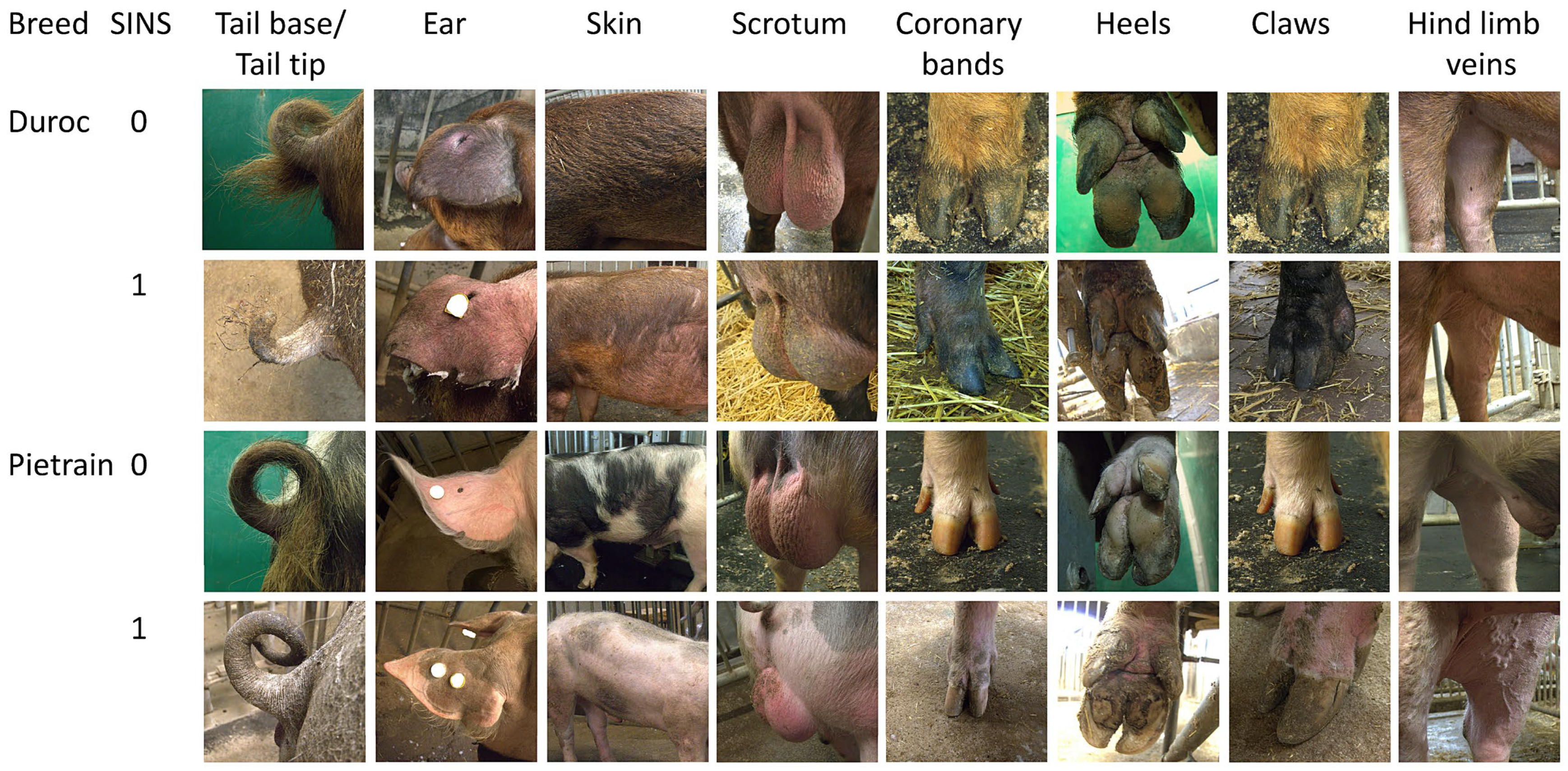

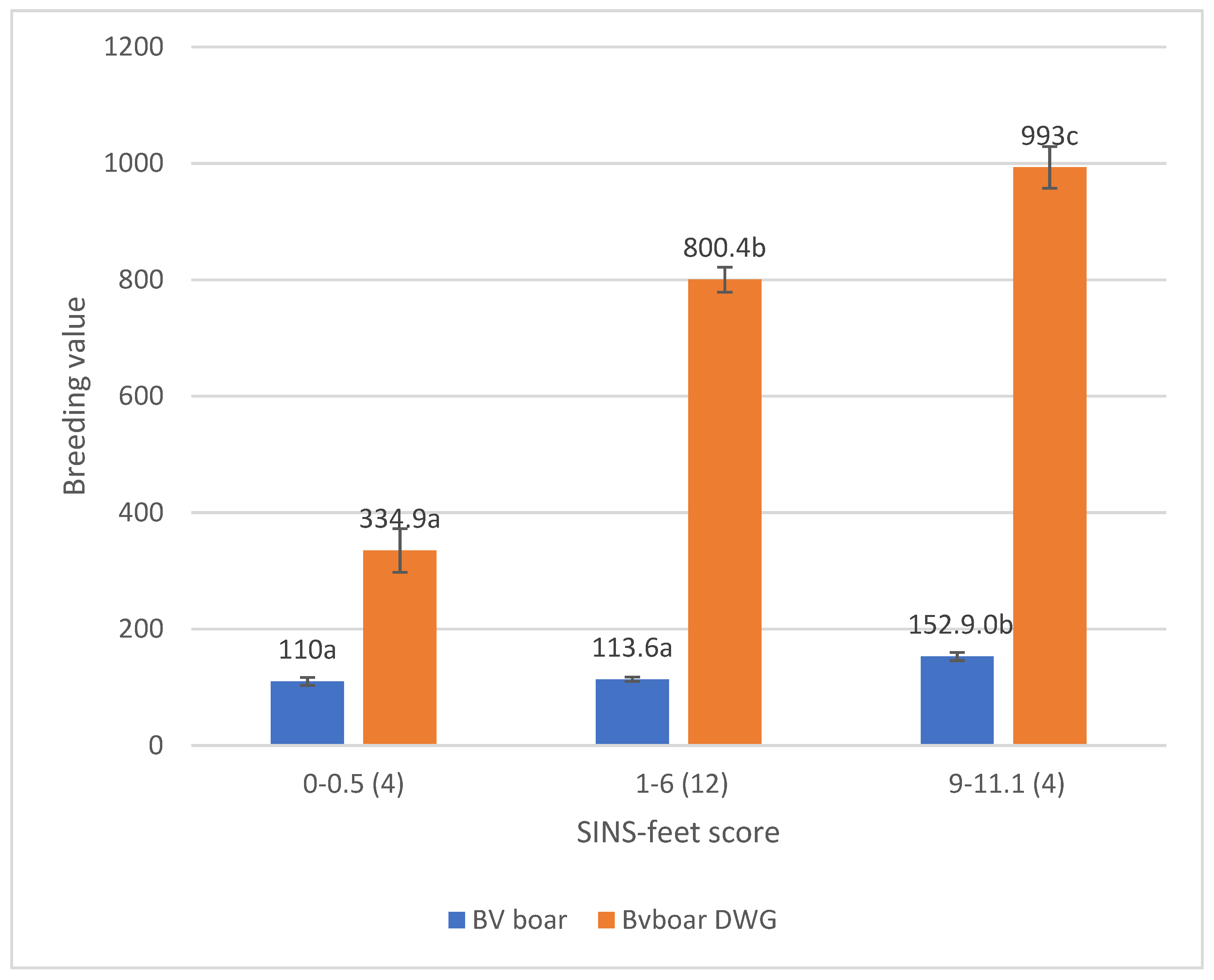
| Large White Commercial | Pietrain | Duroc | Landrace | Large White Herdbook | PIDU | Angler Saddle-Back | Overall | ||
|---|---|---|---|---|---|---|---|---|---|
| Body Part | Characteristic | a 1 (17) 2 | b (210) | c (38) | d (12) | e (6) | f (2) | g (1) | |
| Tail base | no bristles | 94.7 (b–f) 3 | 75.2 (a,d–f) | 68.0 (a,d–f) | 6.6 (a–c,g) | 9.1 (a–c,g) | 3.2 (a–d,g) | 94.6 (d–f) | 47.4 ± 0.269 |
| Swelling | 7.3 | 4.1 | 4.2 | 2.6 | 2.4 | 3.1 | 1.8 | 3.3 ± 0.036 | |
| Redness | 10.8 | 1.1 | 1.6 | 0 | 0 | 0 | 0 | 0.0 ± 0.012 | |
| Tail tip | no bristles | 89.2 (b–g) | 27.1 (e) | 17.6 (e) | 21.5 | 2.4 | 2.9 | 2.2 | 14.0 ± 0.130 |
| Swelling | 7.1 | 4.2 | 2.6 | 2.4 | 2.1 | 3 | 1.7 | 3.0 ± 0.033 | |
| Redness | 10.6 | 6.8 | 0 | 0 | 0 | 0 | 0 | 0.0 ± 0.003 | |
| Exsudate | 6.8 | 3.1 | 3 | 2.8 | 2.8 | 3 | 2.5 | 3.7 ± 0.036 | |
| Necrosis | 3.2 | 3 | 2.9 | 2.7 | 2.6 | 3 | 2.1 | 2.8 ± 0.031 | |
| Ears | no bristles (low) | 21.5 (b,g) | 38.6 (c,g) | 62.9 (g) | 49.3 (g) | 30 (g) | 50.9 | 99.8 (c–e) | 64.8 ± 0.872 |
| no bristles (high) | 78.3 (c,e–g) | 60.5 (c,f,g) | 31 (a,b,f,g) | 46.1 (f,g) | 29.3 (a,f,g) | 0 (a–e,g) | 100 (a–f) | 51.2 ± 8.53 | |
| ear margin no bristles (low) | 59.3 (b,e–g) | 31.7 (a,e–g) | 23.4 (f,g) | 26.7 (f,g) | 9.2 (a,b,f) | 0 (a–d,g) | 100 (a–f) | 33.3 ± 4.63 | |
| ear margin no bristles (high) | 16.1 | 10.7 (c–g) | 2.3 | 0 | 0 | 0 | 0 | 0.0 ± 0.022 | |
| ear base redness | 26.1 | 18.9 (e) | 10 | 21.5 | 3.5 | 3.2 | 2.4 | 8.7 ± 0.087 | |
| venous congestion (low) | 59 | 67.3 (c,e–g) | 16.8 (a,b,d) | 63.8 (c,f,g) | 30.9 (bc,f,g) | 2.7 (a,b,d) | 3.1 (a,b,d) | 24.8 ± 0.198 | |
| venous congestion (high) | 23.7 | 16.2 | 2.3 (a,b) | 17.4 | 7.3 | 3 | 1.4 | 6.7 ± 0.068 | |
| Skin | no bristles | 72.1 (c–g) | 67.7 (c–g) | 26.9 (a,b,d–g) | 5.6 (a,b) | 0 (a–c) | 0 (a–c) | 0 (a–c) | 0.5 ± 0.267 |
| Redness | 2.9 | 2.9 | 4.1 | 2.9 | 2.8 | 2.9 | 2.9 | 3.0 ± 0.034 | |
| rhaghades | 3.8 | 6.9 | 15.5 | 6.3 | 2.2 | 3.4 | 1.2 | 4.2 ± 0.046 | |
| Scrotum | Swelling | 35.3 | 21.3 | 6.3 | 17 | 7.8 | 0 | 0.2 | 3.4 ± 0.250 |
| Coronary bands | Swelling | 3.3 | 5.6 | 10.1 | 3 | 3.7 | 3.1 | 2.6 | 4.0 ± 0.044 |
| Redness | 7.3 | 12.2 (f,g) | 4.3 | 5.4 | 6.8 | 0 (b) | 0 (b) | 0.5 ± 0.268 | |
| exudation | 12.8 | 3.2 | 3.2 | 2.7 | 2.7 | 3.1 | 2 | 3.5 ± 0.038 | |
| Heels | Swelling | 11.7 | 11.5 (d) | 6.3 | 2.3 (b) | 37 | 2.9 | 1.5 | 6.4 ± 0.066 |
| Bleeding | 10.1 | 6.7 | 2.1 | 2.6 | 13.4 | 2.7 | 2.6 | 4.5 ± 0.048 | |
| Rupture | 66.3 (f,g) | 43.2 (f,g) | 49.3 (f,g) | 41.1 (f,g) | 35.2 (f,g) | 0.1 (a–g,g) | 99.5 (a–f) | 40.7 ± 1.775 | |
| detachment | 74.7 (b–e,g) | 21.7 (a,g) | 35.1 (a,e,g) | 10.2 (a,c) | 18.4 | 50.7 | 0.6 (a,b) | 20.4 ± 0.149 | |
| Claw wall | Cleft | 28.2 | 9.1 | 18.5 | 8.4 | 18.8 | 0 | 0 | 1.6 ± 0.329 |
| Bleeding | 2.8 | 3.2 | 3 | 3 | 3.1 | 2.8 | 3.3 | 3.0 ± 0.034 | |
| Hind limb | vein congestion | 78 (c–g) | 61.8 (c–g) | 7.8 (a,b) | 21.4 (a,b) | 2.9 (a,b) | 2.9 (a,b) | 2.8 (a,b) | 14.2 ± 0.134 |
| TT | Ear | Skin | Scrotum | Veins | CB | Heel | Claw | |
|---|---|---|---|---|---|---|---|---|
| TB | 0.393 | 0.536 | 0.464 | 0.536 | 0.393 | 0.393 | 0.036 | 0.429 |
| TT | 0.821 * | 0.929 ** | 0.857 * | 1.000 ** | 0.893 ** | −0.321 | 0.857 * | |
| Ear | 0.857 * | 0.929 ** | 0.821 * | 0.821 * | −0.107 | 0.750 | ||
| Skin | 0.857 * | 0.929 ** | 0.964 ** | 0.000 | 0.964 ** | |||
| Scrotum | 0.857 * | 0.786 * | −0.179 | 0.821 * | ||||
| Veins | 0.893 ** | −0.321 | 0.857 * | |||||
| Coronary | −0.107 | 0.893 ** | ||||||
| Heel | 0.143 |
| Body Part | Characteristic | Suckling Piglets | Weaners |
|---|---|---|---|
| Tail base | Loss of bristles | 71.0 | 58.0 |
| Swelling | 62.2 | 32.0 | |
| Redness | 15.3 | 37.0 | |
| Exudation | 0.7 | 0.0 | |
| Necrosis | 0.0 | 0.0 | |
| Bleeding | 0.0 | 0.0 | |
| Tail tip | Loss of bristles | 27.8 | 79.3 |
| Swelling | 29.2 | 65.6 | |
| Redness | 59.9 | 72.2 | |
| Exudation | 2.1 | 30.4 | |
| Necrosis | 0.7 | 5.9 | |
| Ring constriction | 0.9 | 0.0 | |
| Bleeding | 0.2 | 0.0 | |
| Ears | No bristles, low | 19.1 | 48.0 |
| No bristles, high | 0.0 | 34.4 | |
| Margin, no bristles, low | 7.2 | 24.7 | |
| Margin, no bristles, high | 0.0 | 2.8 | |
| Ear base redness | 14.9 | 15.5 | |
| Ear base exudation | 0.0 | 0.0 | |
| Ear base necrosis | 0.0 | 0.7 | |
| Ear margin necrosis | 0.0 | 24.0 | |
| Ear tip necrosis | 0.0 | 59.8 | |
| Vein congestion low | 89.6 | 86.8 | |
| Vein congestion high | 11.3 | 26.8 | |
| Injuries | 60.8 | 90.8 | |
| Teats | Swelling | 86.0 | 61.1 |
| Redness | 31.9 | 90.1 | |
| Scabs | 9.3 | 3.8 | |
| Necrosis | 12.0 | 3.3 | |
| Coronary bands | Swelling | 99.8 | 100.0 |
| Redness | 97.3 | 98.6 | |
| Exudation | 55.9 | 12.7 | |
| Necrosis | 22.1 | 1.6 | |
| Heels | Swelling | 98.9 | 99.8 |
| Bleeding low | 57.2 | 80.5 | |
| Bleeding mean | 86.3 | 0.0 | |
| Blutung high | 60.6 | 0.0 | |
| Ablasions | 5.4 | 89.6 | |
| Claw wall | Bleeding | 59.7 | 100.0 |
| Ablasion | 0.7 | 1.4 | |
| Split | 1.4 | 0.0 | |
| Cleft | 0.7 | 34.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, S.; Kochendoerfer, E.; Kuehling, J.; Gerhards, K.; Lechner, M.; Zinner, S.; Lautner, M.; Reiner, G. Breed- and Line-Dependent Severity of Inflammation and Necrosis Syndrome in AI Boars, and the Related Risk of Inflammation and Necrosis in Their Progeny. Vet. Sci. 2025, 12, 967. https://doi.org/10.3390/vetsci12100967
Becker S, Kochendoerfer E, Kuehling J, Gerhards K, Lechner M, Zinner S, Lautner M, Reiner G. Breed- and Line-Dependent Severity of Inflammation and Necrosis Syndrome in AI Boars, and the Related Risk of Inflammation and Necrosis in Their Progeny. Veterinary Sciences. 2025; 12(10):967. https://doi.org/10.3390/vetsci12100967
Chicago/Turabian StyleBecker, Sabrina, Eva Kochendoerfer, Josef Kuehling, Katharina Gerhards, Mirjam Lechner, Silvia Zinner, Matthias Lautner, and Gerald Reiner. 2025. "Breed- and Line-Dependent Severity of Inflammation and Necrosis Syndrome in AI Boars, and the Related Risk of Inflammation and Necrosis in Their Progeny" Veterinary Sciences 12, no. 10: 967. https://doi.org/10.3390/vetsci12100967
APA StyleBecker, S., Kochendoerfer, E., Kuehling, J., Gerhards, K., Lechner, M., Zinner, S., Lautner, M., & Reiner, G. (2025). Breed- and Line-Dependent Severity of Inflammation and Necrosis Syndrome in AI Boars, and the Related Risk of Inflammation and Necrosis in Their Progeny. Veterinary Sciences, 12(10), 967. https://doi.org/10.3390/vetsci12100967






