Selenoprotein M Protects Intestinal Health in Nickel-Exposed Mice: Implications for Animal Welfare Under Heavy Metal Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment of Experimental Animals
2.2. In Vitro Cell Culture
2.3. Histological Observation of Colonic Tissue
2.4. Antioxidant Enzyme Activity and Content Assays
2.5. MDC and ROS Staining of MCEC
2.6. Determination of Protein Content by Western Blot
2.7. Determination of mRNA Content by qRT-PCR
2.8. Statistical Analysis
3. Results
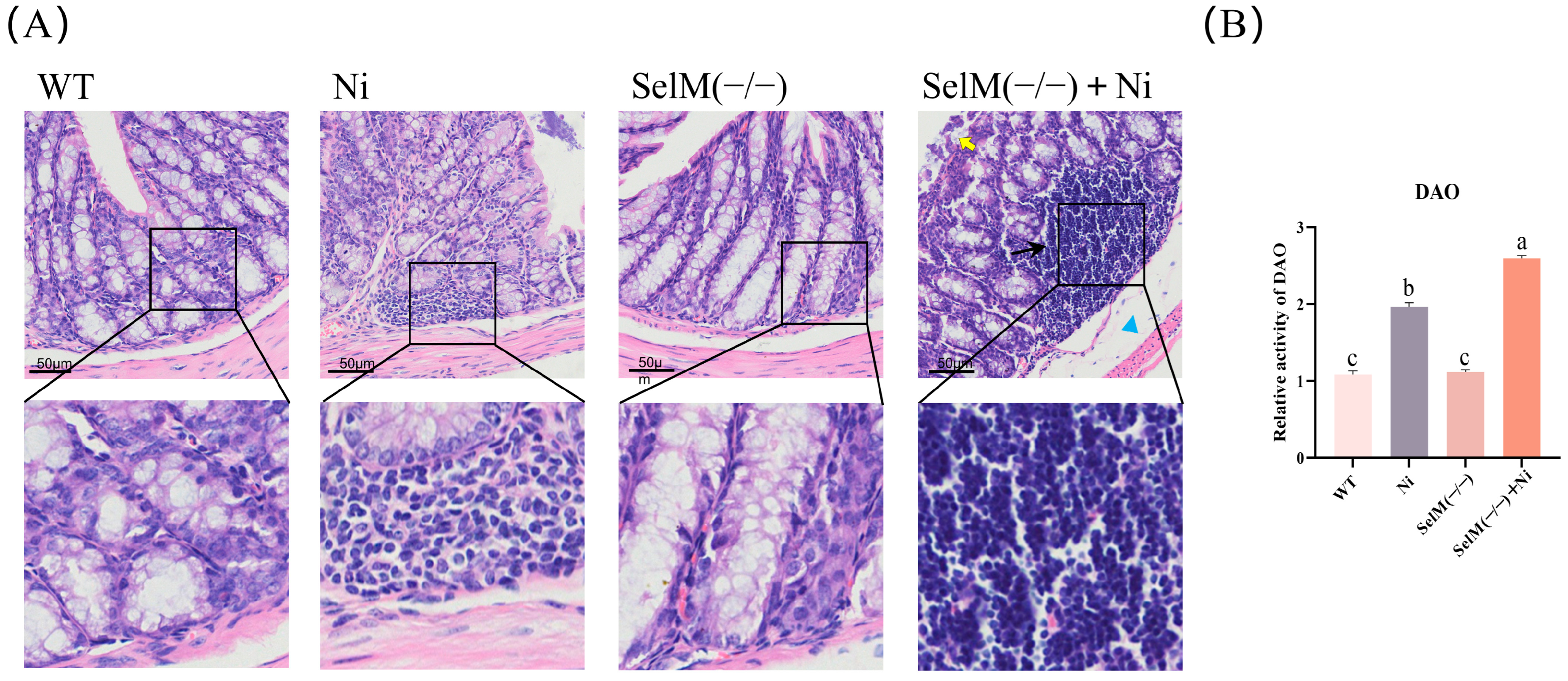
3.1. SelM Knockout Exacerbates Ni-Induced Colonic Tissue Pathology
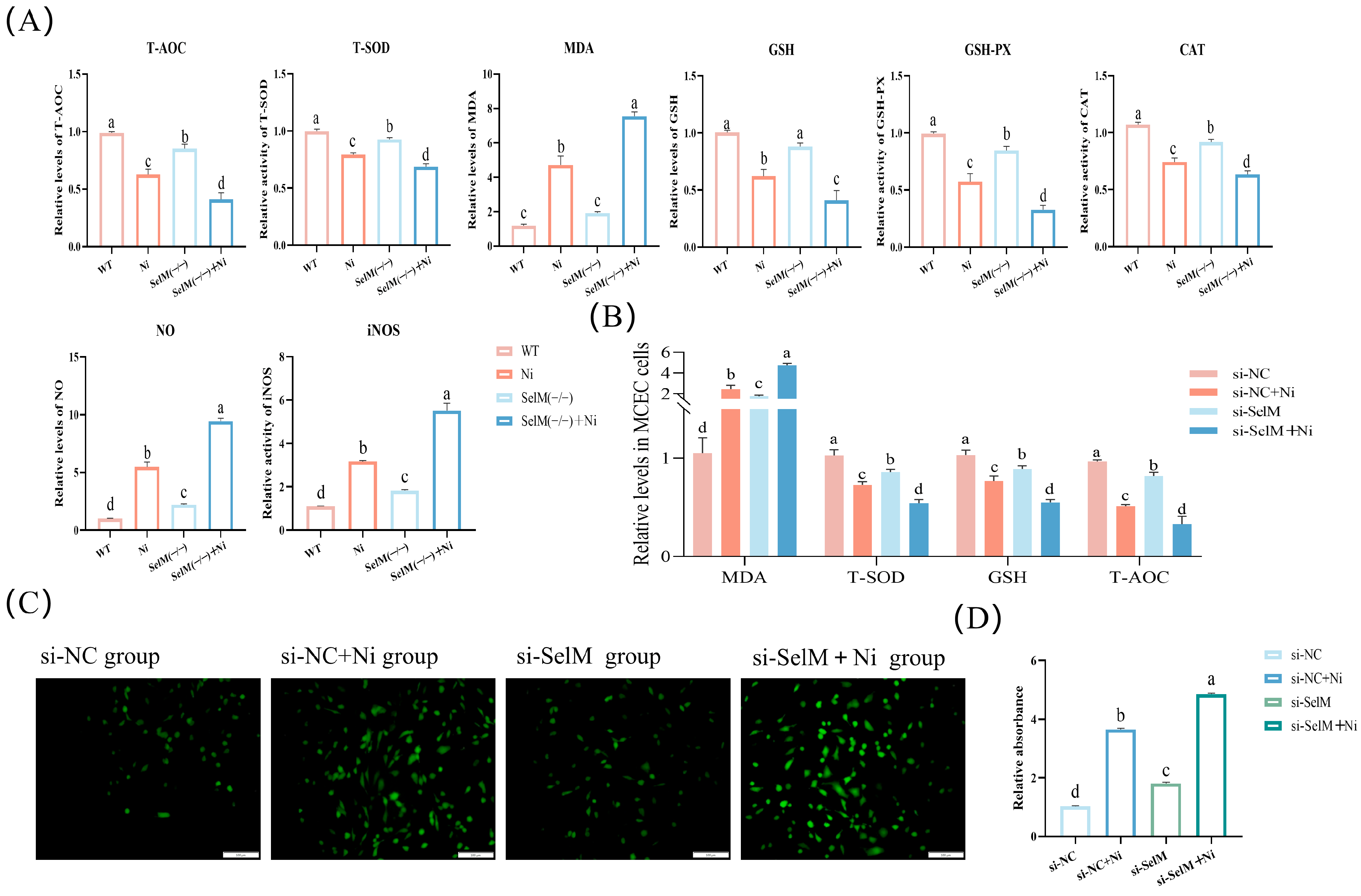
3.2. SelM Knockout Exacerbates Ni-Induced Oxidative Stress Parameters in Colonic Tissue and MCEC
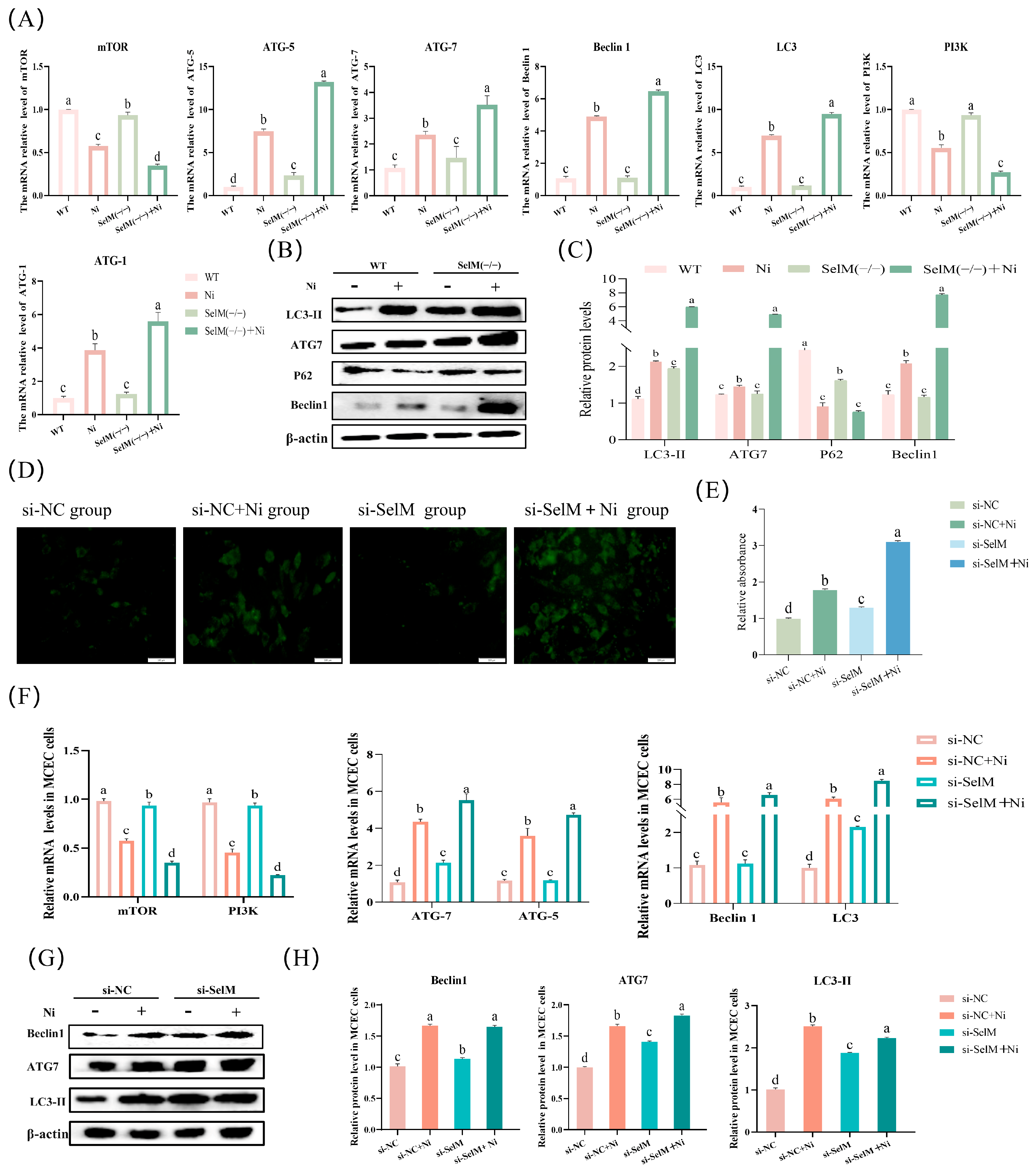
3.3. SelM Knockout Exacerbates Ni-Induced Autophagy Parameters in Colonic Tissue and MCEC
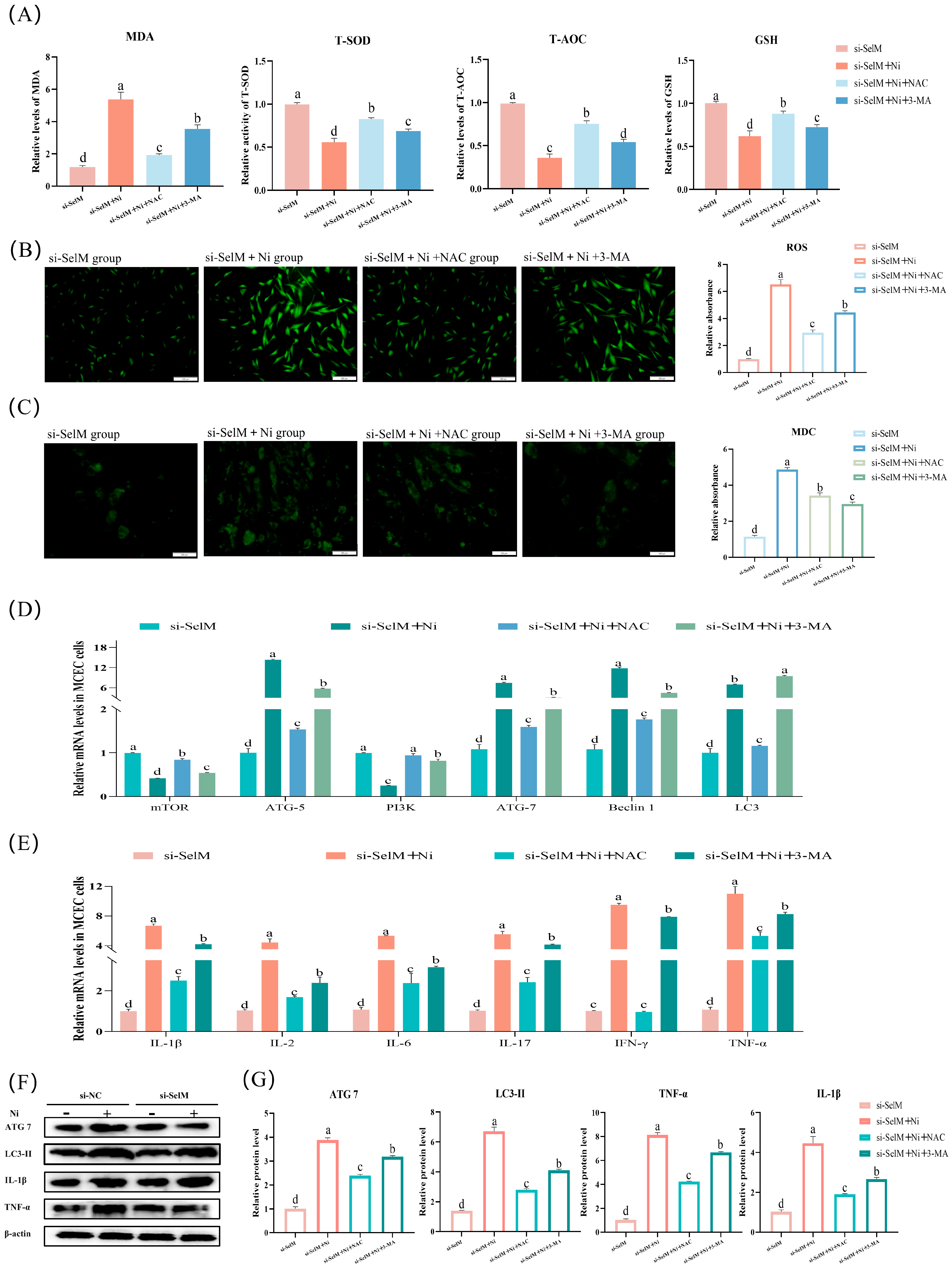
3.4. SelM Knockout Exacerbates Ni-Induced Inflammatory Response Parameters in Colonic Tissue and MCEC
3.5. Effects of NAC and 3-MA on Ni-Exposed MCEC with SelM Knockdown
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Jigyasu, D.K.; Kumar, A.; Subrahmanyam, G.; Mondal, R.; Shabnam, A.A.; Cabral-Pinto, M.M.S.; Malyan, S.K.; Chaturvedi, A.K.; Gupta, D.K.; et al. Nickel in terrestrial biota: Comprehensive review on contamination, toxicity, tolerance and its remediation approaches. Chemosphere 2021, 275, 129996. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Use of zeolite to neutralise nickel in a soil environment. Environ. Monit. Assess. 2018, 190, 54. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Sun, Y.; Jiang, Y.; Chen, X.; Cai, J.; Liu, Q.; Zhang, Z. Melatonin ameliorates nickel induced autophagy in mouse brain: Diminution of oxidative stress. Toxicology 2022, 473, 153207. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Zhu, Y.; Qiao, S.; Cai, J.; Zhang, Z. Melatonin relieves liver fibrosis induced by Txnrd3 knockdown and nickel exposure via IRE1/NF-kB/NLRP3 and PERK/TGF-β1 axis activation. Life Sci. 2022, 301, 120622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gai, X.; Xu, L.; Ma, W.; Liu, Q.; Shi, B.; Fang, C.; Cai, J.; Zhang, Z. Role of selenoprotein M knockdown in the melatonin antagonism of nickel-induced apoptosis and endoplasmic reticulum stress in mouse heart. J. Zhejiang Univ.-Sci. B 2023, 24, 406–417. [Google Scholar] [CrossRef]
- Wu, B.; Cui, H.; Peng, X.; Pan, K.; Fang, J.; Zuo, Z.; Deng, J.; Wang, X.; Huang, J. Toxicological effects of dietary nickel chloride on intestinal microbiota. Ecotoxicol. Environ. Saf. 2014, 109, 70–76. [Google Scholar] [CrossRef]
- Huang, L.; He, F.; Wu, B. Mechanism of effects of nickel or nickel compounds on intestinal mucosal barrier. Chemosphere 2022, 305, 135429. [Google Scholar] [CrossRef]
- Wu, B.; Guo, H.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Deng, J.; Wang, X.; Huang, J. Pathway underlying small intestine apoptosis by dietary nickel chloride in broiler chickens. Chem. Biol. Interact. 2016, 243, 91–106. [Google Scholar] [CrossRef]
- Matsuda, H.; Nibe-Shirakihara, Y.; Tamura, A.; Aonuma, E.; Arakawa, S.; Otsubo, K.; Nemoto, Y.; Nagaishi, T.; Tsuchiya, K.; Shimizu, S.; et al. Nickel particles are present in Crohn’s disease tissue and exacerbate intestinal inflammation in IBD susceptible mice. Biochem. Biophys. Res. Commun. 2022, 592, 74–80. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, J.; Miao, Y.; Lei, P.; Zhang, Q. ROS and Autophagy: Interactions and Molecular Regulatory Mechanisms. Cell. Mol. Neurobiol. 2015, 35, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Tang, Z.; Bai, Y.; Wan, M.; Yu, M.; Chen, J.; Li, G.; Zhang, R.; Ge, M. Astragalus Polysaccharide Protects Against Cadmium-Induced Autophagy Injury Through Reactive Oxygen Species (ROS) Pathway in Chicken Embryo Fibroblast. Biol. Trace Elem. Res. 2022, 200, 318–329. [Google Scholar] [CrossRef]
- Gu, X.; Qi, Y.; Feng, Z.; Ma, L.; Gao, K.; Zhang, Y. Lead (Pb) induced ATM-dependent mitophagy via PINK1/Parkin pathway. Toxicol. Lett. 2018, 291, 92–100. [Google Scholar] [CrossRef]
- Zhao, S.-C.; Xu, Z.-R.; Xu, C.-L.; He, Q.-K.; Yang, G.-M.; Li, Y.-P.; Luo, Y.-S.; Wang, H.-L.; Qi, Z.-Q.; Liu, Y. Nickel sulfate exposure induces ovarian inflammation and fibrosis and decreases oocyte quality in mice. Ecotoxicol. Environ. Saf. 2021, 224, 112634. [Google Scholar] [CrossRef]
- Kinbara, M.; Bando, K.; Shiraishi, D.; Kuroishi, T.; Nagai, Y.; Ohtsu, H.; Takano-Yamamoto, T.; Sugawara, S.; Endo, Y. Mast cell histamine-mediated transient inflammation following exposure to nickel promotes nickel allergy in mice. Exp. Dermatol. 2016, 25, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Jiang, M.; Chen, W.; Zhao, T.; Wei, Y. Cancer and ER stress: Mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed. Pharmacother. 2019, 118, 109249. [Google Scholar] [CrossRef]
- Martinet, W.; De Meyer, G.R.Y. Autophagy in Atherosclerosis: A Cell Survival and Death Phenomenon with Therapeutic Potential. Circ. Res. 2009, 104, 304–317. [Google Scholar] [CrossRef]
- Racanelli, A.C.; Kikkers, S.A.; Choi, A.M.K.; Cloonan, S.M. Autophagy and inflammation in chronic respiratory disease. Autophagy 2018, 14, 221–232. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Huang, J.; Yang, J.; Chen, X.; Zhang, H.; Zhu, Y.; Liu, Q.; Zhang, Z. The protective effect of selenoprotein M on non-alcoholic fatty liver disease: The role of the AMPKα1–MFN2 pathway and Parkin mitophagy. Cell. Mol. Life Sci. 2022, 79, 354. [Google Scholar] [CrossRef]
- Gan, X.; Zhang, X.; E, Q.; Zhang, Q.; Ye, Y.; Cai, Y.; Han, A.; Tian, M.; Wang, C.; Su, Z.; et al. Nano-selenium attenuates nickel-induced testosterone synthesis disturbance through inhibition of MAPK pathways in Sprague-Dawley rats. Environ. Toxicol. 2019, 34, 968–978. [Google Scholar] [CrossRef]
- Gong, T.; Hashimoto, A.C.; Sasuclark, A.R.; Khadka, V.S.; Gurary, A.; Pitts, M.W. Selenoprotein M Promotes Hypothalamic Leptin Signaling and Thioredoxin Antioxidant Activity. Antioxid. Redox Signal. 2021, 35, 775–787. [Google Scholar] [CrossRef]
- Chen, P.; Wang, R.-R.; Ma, X.-J.; Liu, Q.; Ni, J.-Z. Different Forms of Selenoprotein M Differentially Affect Aβ Aggregation and ROS Generation. Int. J. Mol. Sci. 2013, 14, 4385–4399. [Google Scholar] [CrossRef]
- Yim, S.Y.; Chae, K.R.; Shim, S.B.; Hong, J.T.; Park, J.Y.; Lee, C.Y.; Son, H.J.; Sheen, Y.Y.; Hwang, D.Y. Hwang ERK activation induced by selenium treatment significantly downregulates β/γ-secretase activity and Tau phosphorylation in the transgenic rat overexpressing human selenoprotein M. Int. J. Mol. Med. 2009, 24, 91–96. [Google Scholar] [CrossRef][Green Version]
- Santesmasses, D.; Mariotti, M.; Gladyshev, V.N. Tolerance to Selenoprotein Loss Differs between Human and Mouse. Mol. Biol. Evol. 2020, 37, 341–354. [Google Scholar] [CrossRef]
- Hofstee, P.; Perkins, A.V.; Cuffe, J.S.M. Selenium Deficiency during Pregnancy in Mice Impairs Exercise Performance and Metabolic Function in Adult Offspring. Nutrients 2022, 14, 1125. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Liu, Y.; Xu, L.; Gai, X.; Sun, Y.; Qiao, S.; Liu, P.; Liu, Q.; Zhang, Z. The role of selenoprotein M in nickel-induced pyroptosis in mice spleen tissue via oxidative stress. Environ. Sci. Pollut. Res. 2022, 30, 34270–34281. [Google Scholar] [CrossRef]
- Šulinskienė, J.; Bernotienė, R.; Baranauskienė, D.; Naginienė, R.; Stanevičienė, I.; Kašauskas, A.; Ivanov, L. Effect of Zinc on the Oxidative Stress Biomarkers in the Brain of Nickel-Treated Mice. Oxid. Med. Cell. Longev. 2019, 2019, 8549727. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, C.; Li, X.; Liu, J. The mechanism of nickel-induced autophagy and its role in nephrotoxicity. Ecotoxicol. Environ. Saf. 2024, 273, 116150. [Google Scholar] [CrossRef]
- Zambelli, B.; Uversky, V.N.; Ciurli, S. Nickel impact on human health: An intrinsic disorder perspective. Biochim. Biophys. Acta BBA—Proteins Proteom. 2016, 1864, 1714–1731. [Google Scholar] [CrossRef]
- Zheng, G.-H.; Liu, C.-M.; Sun, J.-M.; Feng, Z.-J.; Cheng, C. Nickel-induced oxidative stress and apoptosis in Carassius auratus liver by JNK pathway. Aquat. Toxicol. 2014, 147, 105–111. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.; Liu, Y. Nickel Sulfate Induces Autophagy in Human Thyroid Follicular Epithelial Cells. Biol. Trace Elem. Res. 2022, 200, 122–133. [Google Scholar] [CrossRef]
- Wu, B.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Deng, J.; Huang, J. Dietary Nickel Chloride Induces Oxidative Intestinal Damage in Broilers. Int. J. Environ. Res. Public Health 2013, 10, 2109–2119. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; et al. Update of the risk assessment of nickel in food and drinking water. EFSA J. 2020, 18, e06268. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Z.; Cao, X.; Wang, L.; Gao, X.; Shen, Y.; Du, J.; Liu, P.; Zhuang, Y.; Guo, X. Insights into the effects of chronic combined chromium-nickel exposure on colon damage in mice through transcriptomic analysis and in vitro gastrointestinal digestion assay. Ecotoxicol. Environ. Saf. 2024, 279, 116458. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Jian, Z.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Copper Induces Oxidative Stress and Apoptosis in the Mouse Liver. Oxid. Med. Cell. Longev. 2020, 2020, 1359164. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Gao, J.; Kuang, T.; Chen, J.; Li, J.; Huang, Y.; Xin, H.; Fang, Y.; Han, X.; Sun, L.; et al. DIAPH3 promotes pancreatic cancer progression by activating selenoprotein TrxR1-mediated antioxidant effects. J. Cell. Mol. Med. 2021, 25, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, M.; Yang, Z.; Wang, W.; Lin, H.; Xu, S. Selenoprotein S silencing triggers mouse hepatoma cells apoptosis and necrosis involving in intracellular calcium imbalance and ROS-mPTP-ATP. Biochim. Biophys. Acta—Gen. Subj. 2018, 1862, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Park, J.S.; Yoo, H.J.; Lee, H.M.; Lee, B.C.; Kim, J.H. The Selenoprotein MsrB1 Instructs Dendritic Cells to Induce T-Helper 1 Immune Responses. Antioxidants 2020, 9, 1021. [Google Scholar] [CrossRef]
- Bao, R.; Zheng, S.; Wang, X. Selenium protects against cadmium-induced kidney apoptosis in chickens by activating the PI3K/AKT/Bcl-2 signaling pathway. Environ. Sci. Pollut. Res. 2017, 24, 20342–20353. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ouyang, Y.; Yin, H.; Cui, H.; Deng, H.; Liu, H.; Jian, Z.; Fang, J.; Zuo, Z.; Wang, X.; et al. Induction of autophagy via the ROS-dependent AMPK-mTOR pathway protects copper-induced spermatogenesis disorder. Redox Biol. 2022, 49, 102227. [Google Scholar] [CrossRef]
- Liang, Q.; Xiao, Y.; Liu, K.; Zhong, C.; Zeng, M.; Xiao, F. Cr(VI)-Induced Autophagy Protects L-02 Hepatocytes from Apoptosis Through the ROS-AKT-mTOR Pathway. Cell. Physiol. Biochem. 2018, 51, 1863–1878. [Google Scholar] [CrossRef]
- Chen, X.; Bi, M.; Yang, J.; Cai, J.; Zhang, H.; Zhu, Y.; Zheng, Y.; Liu, Q.; Shi, G.; Zhang, Z. Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. J. Hazard. Mater. 2022, 421, 126704. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, X.; Xu, S. Selenium Prevents Lead-Induced Necroptosis by Restoring Antioxidant Functions and Blocking MAPK/NF-κB Pathway in Chicken Lymphocytes. Biol. Trace Elem. Res. 2020, 198, 644–653. [Google Scholar] [CrossRef]
- Bjørklund, G. Selenium as an antidote in the treatment of mercury intoxication. BioMetals 2015, 28, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Murai, H.; Okazaki, S.; Hayashi, H.; Kawakita, A.; Hosoki, K.; Yasutomi, M.; Sur, S.; Ohshima, Y. Alternaria extract activates autophagy that induces IL-18 release from airway epithelial cells. Biochem. Biophys. Res. Commun. 2015, 464, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-N.; Suh, D.-H.; Trinh, H.K.T.; Chwae, Y.-J.; Park, H.-S.; Shin, Y.S. The role of autophagy in allergic inflammation: A new target for severe asthma. Exp. Mol. Med. 2016, 48, e243. [Google Scholar] [CrossRef]
- Feng, W.; Su, S.; Song, C.; Yu, F.; Zhou, J.; Li, J.; Jia, R.; Xu, P.; Tang, Y. Effects of Copper Exposure on Oxidative Stress, Apoptosis, Endoplasmic Reticulum Stress, Autophagy and Immune Response in Different Tissues of Chinese Mitten Crab (Eriocheir sinensis). Antioxidants 2022, 11, 2029. [Google Scholar] [CrossRef]
- Chlubek, M.; Baranowska-Bosiacka, I. Selected Functions and Disorders of Mitochondrial Metabolism under Lead Exposure. Cells 2024, 13, 1182. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.S.; Parvez, M.A.K.; Kim, B. The Emerging Role of Autophagy as a Target of Environmental Pollutants: An Update on Mechanisms. Toxics 2023, 11, 135. [Google Scholar] [CrossRef]
- Unsal, V.; Dalkiran, T.; Çiçek, M.; Kölükçü, E. The Role of Natural Antioxidants Against Reactive Oxygen Species Produced by Cadmium Toxicity: A Review. Adv. Pharm. Bull. 2020, 10, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Kipp, A. Glutathione peroxidases in different stages of carcinogenesis. Biochim. Biophys. Acta BBA—Gen. Subj. 2009, 1790, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J. Focus on mammalian thioredoxin reductases—Important selenoproteins with versatile functions. Biochim. Biophys. Acta BBA—Gen. Subj. 2009, 1790, 495–526. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ding, G.; Gu, C.; Zhou, J.; Kuang, M.; Ji, Y.; He, Y.; Kondo, T.; Fan, J. Decreased Selenium-Binding Protein 1 Enhances Glutathione Peroxidase 1 Activity and Downregulates HIF-1α to Promote Hepatocellular Carcinoma Invasiveness. Clin. Cancer Res. 2012, 18, 3042–3053. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| β-actin | CCAGCCATGTATGTAGCCATCCAG | AACACCATCACCAGAGTCCATCAC |
| ATG5 | CAAGGATGCGGTTGAGGC | TGAGTTTCCGGTTGATGG |
| ATG7 | TCGAAAACCCCATGCTCCTC | AGGGCCTGGATCTGTTTTGG |
| Beclin1 | TGCAGGTGAGCTTCGTGTG | GCTCCTCTCCTGAGTTAGCCT |
| LC3 | CTTCGCCGACCGCTGTAA | GCCGGATGATCTTGACCAACT |
| ATG1 | GAAAGACAACGGACAAATCACC | GGGGGTGATATGTTTGAACTTG |
| mTOR | CAGACTGGCTCTTGCTCATAA | GCTGGAAGGCGTCAATC |
| PI3K | CTGGGGGACATACTGACTGT | GTTCCTGGAAAGTCTCCCCTC |
| NF-κB | ACTCTGTTTTGCACCTCGCT | TTCAGACCTTCACCGTTGGG |
| iNOS | ACTGGGTTGAATCTGGGTGA | GGAGTCTGGAGATTTCTTTGCTG |
| IL-18 | CAAAGTGCCAGTGAACCCCAGAC | ACAGAGAGGGTCACAGCCAGTC |
| IL-1β | CTTCATCTTCTACCGCCTGGACAG | CTGGTCGGGTTGGTTGGTGATG |
| COX-2 | CTGGCTCGGCACACTGATGATG | GCCCACGGACCAAATATCCACT |
| TNF-α | ACCACGCTCTTCTGCCTACT | GCTTTGACATTGGCTACAACG |
| IL-2 | GCAGAATGGCTACGACACCG | CACTATGCTGGACAGGCAG |
| IL-6 | CGGATGCTTCCAATCTGGGT | CAGGTGCCCCAGCTACATTA |
| IL-17 | CCCAGATAGAAAGCGACTGC | ACCTCCTTGACGATGATGCT |
| IFN-γ | ATCCCATCCTCCGTTGTCCT | ATCGTCGCCTTCTTCGAGTT |
| IL-10 | AGAGTGCCTTTAGCAAGCTCC | TAGAGTCGTCATCCTGGAAGGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Zhang, K.; Yang, H.; Jiang, X.; Fang, Y.; Cai, J.; Zhang, Z. Selenoprotein M Protects Intestinal Health in Nickel-Exposed Mice: Implications for Animal Welfare Under Heavy Metal Stress. Vet. Sci. 2025, 12, 955. https://doi.org/10.3390/vetsci12100955
Liu Q, Zhang K, Yang H, Jiang X, Fang Y, Cai J, Zhang Z. Selenoprotein M Protects Intestinal Health in Nickel-Exposed Mice: Implications for Animal Welfare Under Heavy Metal Stress. Veterinary Sciences. 2025; 12(10):955. https://doi.org/10.3390/vetsci12100955
Chicago/Turabian StyleLiu, Qiaohan, Kaixuan Zhang, Hongxue Yang, Xuehan Jiang, Yi Fang, Jingzeng Cai, and Ziwei Zhang. 2025. "Selenoprotein M Protects Intestinal Health in Nickel-Exposed Mice: Implications for Animal Welfare Under Heavy Metal Stress" Veterinary Sciences 12, no. 10: 955. https://doi.org/10.3390/vetsci12100955
APA StyleLiu, Q., Zhang, K., Yang, H., Jiang, X., Fang, Y., Cai, J., & Zhang, Z. (2025). Selenoprotein M Protects Intestinal Health in Nickel-Exposed Mice: Implications for Animal Welfare Under Heavy Metal Stress. Veterinary Sciences, 12(10), 955. https://doi.org/10.3390/vetsci12100955






