Powerflow Doppler Ultrasonography in the Evaluation of Mares with and Without Endometritis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location and Duration
2.2. Animals and Management
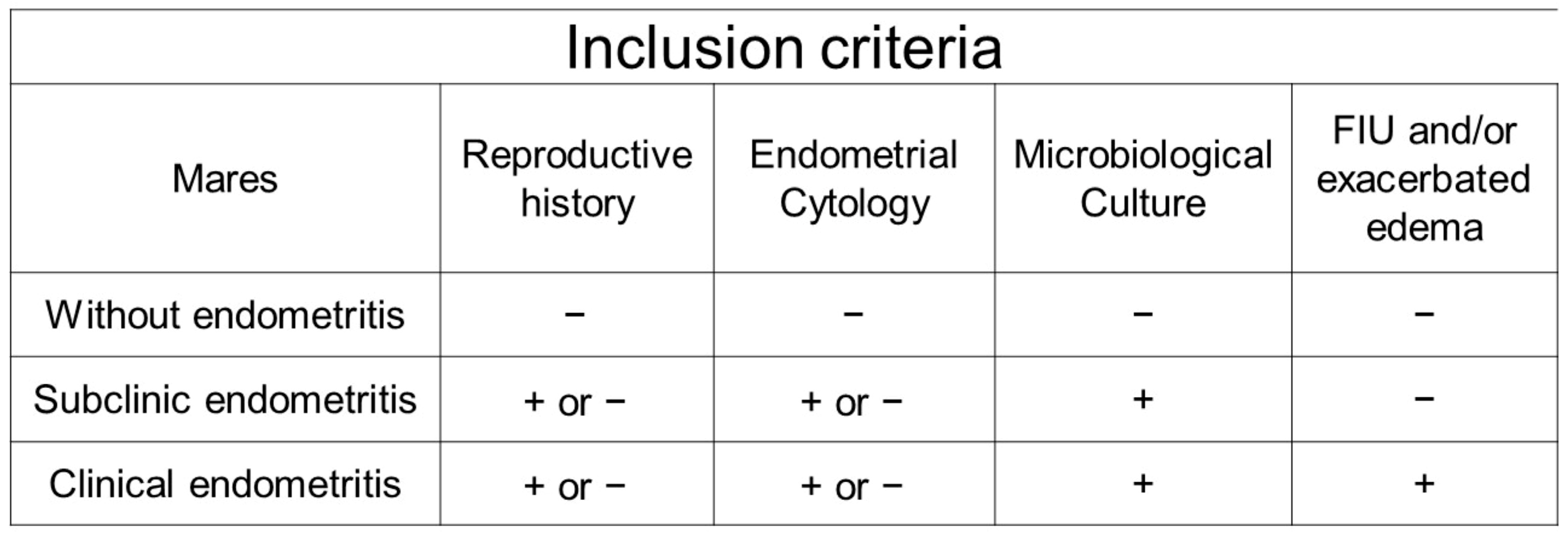
2.3. Criteria for Distributing the Mares into Experimental Groups
2.4. Experiment
2.4.1. Complementary Tests
Fungal Culture
Bacterial Culture
Endometrial Cytology
Uterine Biopsy
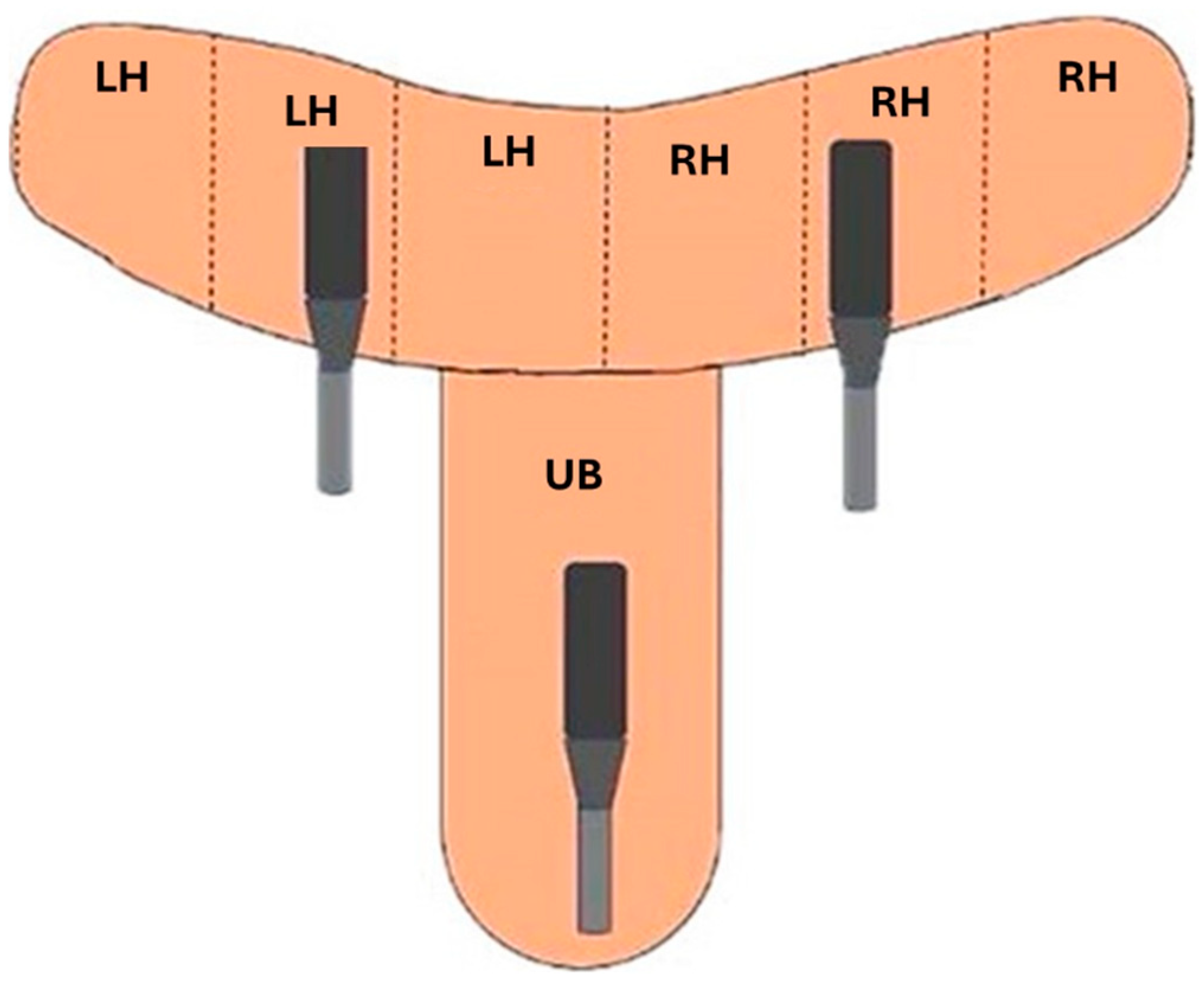
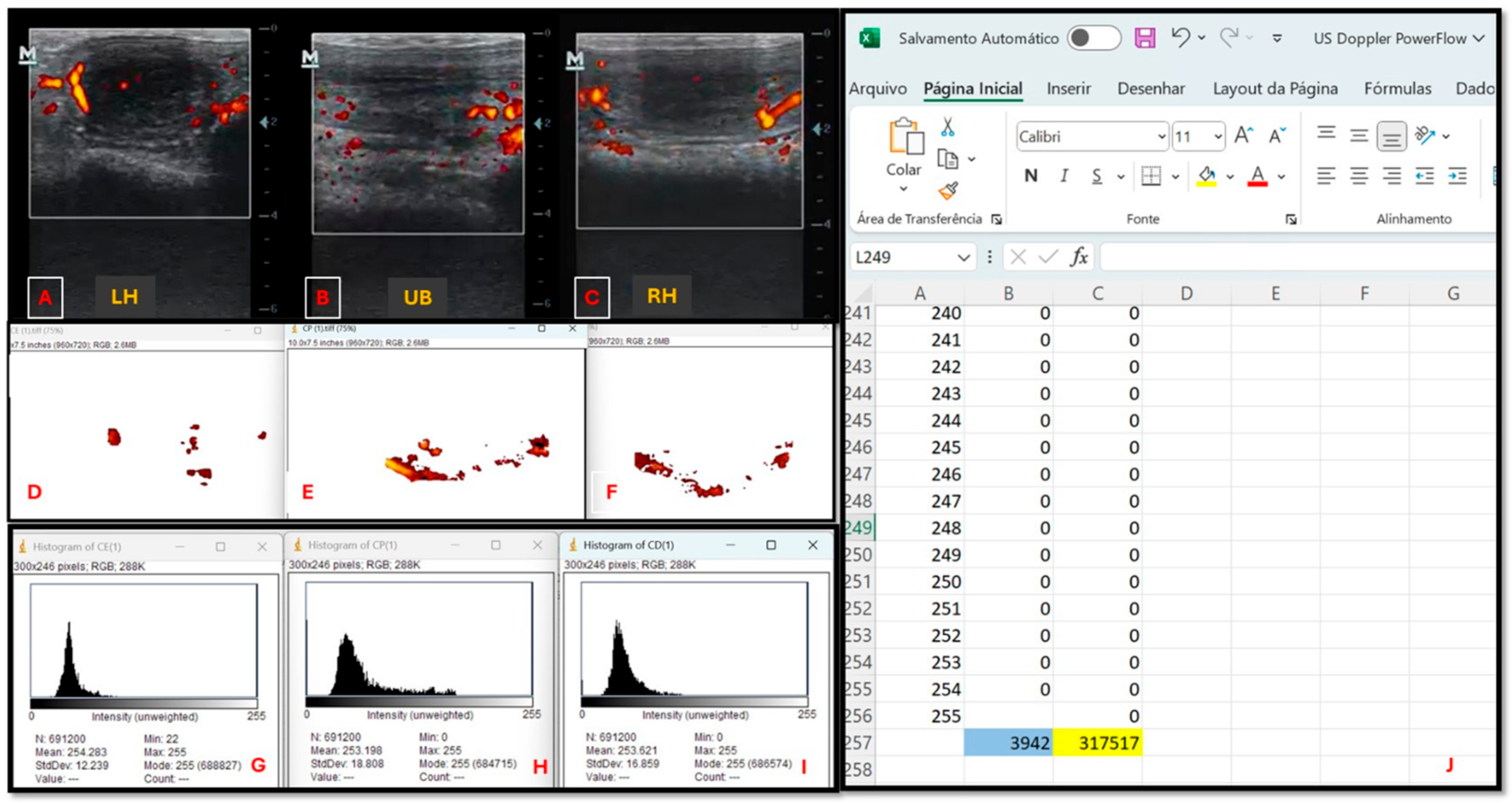
2.4.2. Ultrasound Evaluation
2.5. Statistical Analysis
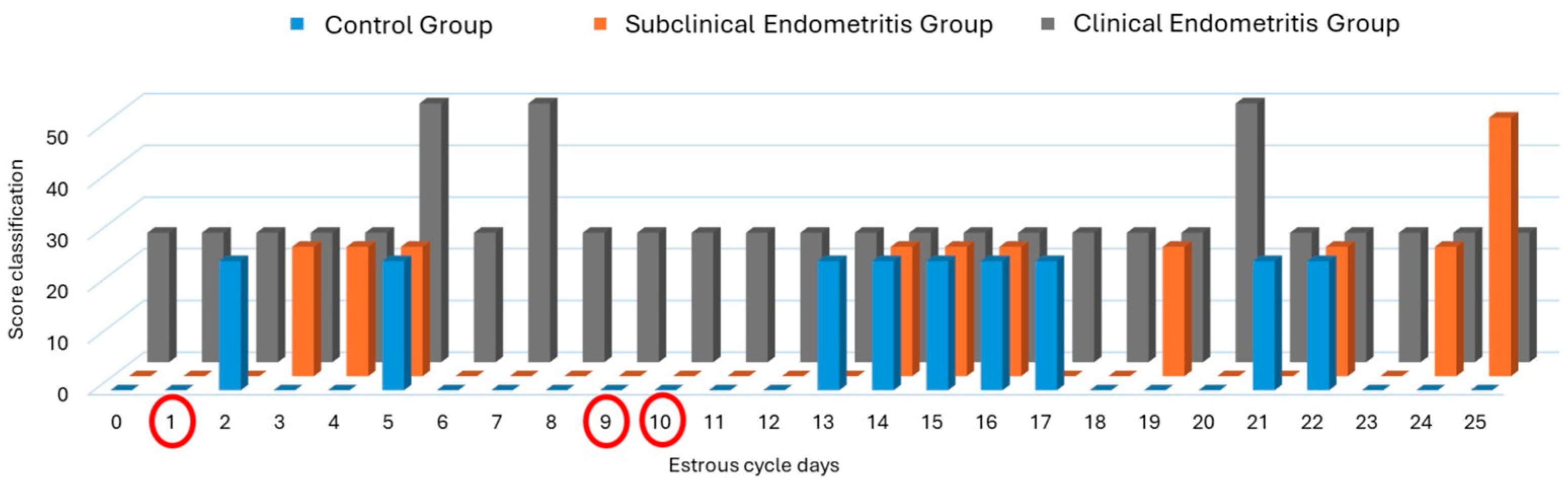
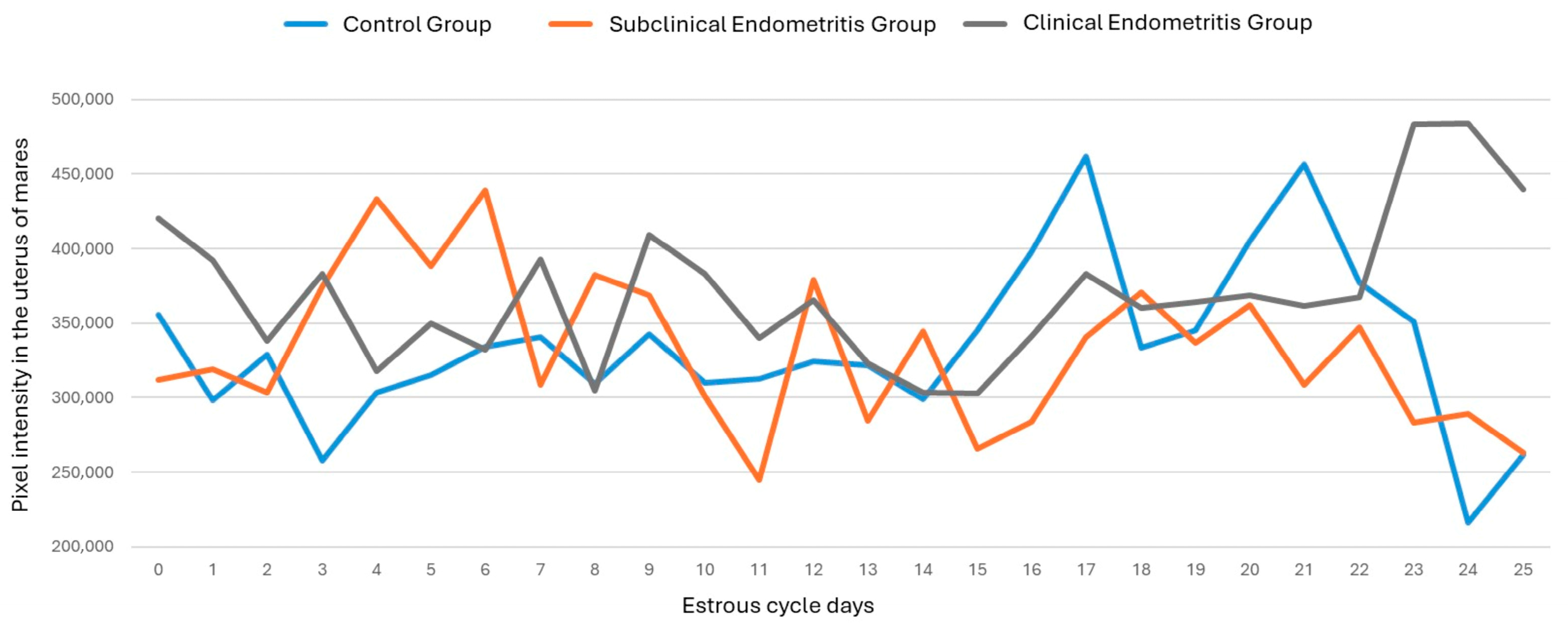
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, I.K.M.; Troedsson, M.H.T. The Diagnosis and Treatment of Endometritis in the Mare: Yesterday and Today. Theriogenology 2008, 70, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Canisso, I.F.; Segabinazzi, L.G.T.M.; Fedorka, C.E. Persistent Breeding-Induced Endometritis in Mares—A Multifaceted Challenge: From Clinical Aspects to Immunopathogenesis and Pathobiology. Int. J. Mol. Sci. 2020, 21, 1432. [Google Scholar] [CrossRef]
- Morris, L.H.; McCue, P.M.; Aurich, C. Equine Endometritis: A Review of Challenges and New Approaches. Reproduction 2020, 160, R95–R110. [Google Scholar] [CrossRef]
- Satué, K.; Gardon, J.C. Infection and Infertility in Mares. In Genital Infections and Infertility; Darwish, A.M., Ed.; InTech: London, UK, 2016; ISBN 978-953-51-2488-7. [Google Scholar]
- Morrell, J.M.; Rocha, A. A Novel Approach to Minimising Acute Equine Endometritis That May Help to Prevent the Development of the Chronic State. Front. Vet. Sci. 2022, 8, 799619. [Google Scholar] [CrossRef]
- LeBlanc, M.; Causey, R. Clinical and Subclinical Endometritis in the Mare: Both Threats to Fertility. Reprod. Domest. Anim. 2009, 44, 10–22. [Google Scholar] [CrossRef]
- LeBlanc, M. Advances in the Diagnosis and Treatment of Chronic Infectious and Post–Mating-Induced Endometritis in the Mare. Reprod. Domest. Anim. 2010, 45, 21–27. [Google Scholar] [CrossRef]
- Woodward, E.M.; Troedsson, M.H.T. Inflammatory Mechanisms of Endometritis. Equine Vet. J. 2015, 47, 384–389. [Google Scholar] [CrossRef]
- Ferris, R.A. Current Understanding of Bacterial Biofilms and Latent Infections: A Clinical Perspective. Rev. Bras. Reprod. Anim. 2017, 41, 74–80. [Google Scholar]
- Rua, M.A.; Quirino, C.R.; Bartholazzi Junior, A.; Barreto, M.A.P. Métodos diagnósticos de endometrite em éguas. Pubvet 2016, 10, 873–945. [Google Scholar] [CrossRef]
- Schöniger, S.; Schoon, H.-A. The Healthy and Diseased Equine Endometrium: A Review of Morphological Features and Molecular Analyses. Animals 2020, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Katila, T. Evaluation of Diagnostic Methods in Equine Endometritis. Reprod. Biol. 2016, 16, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Pascottini, O.B.; Aurich, C.; England, G.; Grahofer, A. General and Comparative Aspects of Endometritis in Domestic Species: A Review. Reprod. Domest. Anim. 2023, 58, 49–71. [Google Scholar] [CrossRef]
- Christoffersen, M.; Söderlind, M.; Rudefalk, S.R.; Pedersen, H.G.; Allen, J.; Krekeler, N. Risk Factors Associated with Uterine Fluid after Breeding Caused by Streptococcus Zooepidemicus. Theriogenology 2015, 84, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Katila, T.; Ferreira-Dias, G. Evolution of the Concepts of Endometrosis, Post Breeding Endometritis, and Susceptibility of Mares. Animals 2022, 12, 779. [Google Scholar] [CrossRef]
- Ginther, O.J. Color-Doppler Ultrasonography; Equiservices Publishing: Cross Plains, WI, USA, 2007. [Google Scholar]
- Camargo Ferreira, J.; de Meira, C. Aplicação da ultrassonografia colorida doppler em programas de transferência de embriões equinos. Ciência Rural 2011, 41, 1063–1069. [Google Scholar] [CrossRef]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between Condition Score, Physical Measurements and Body Fat Percentage in Mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef]
- Manual of Equine Reproduction, 3rd ed.; Brinsko, S.P., Ed.; Mosby Elsevier: Maryland Heights, MO, USA, 2011; ISBN 978-0-323-06482-8. [Google Scholar]
- Christoffersen, M.; Brandis, L.; Samuelsson, J.; Bojesen, A.M.; Troedsson, M.H.T.; Petersen, M.R. Diagnostic Double-Guarded Low-Volume Uterine Lavage in Mares. Theriogenology 2015, 83, 222–227. [Google Scholar] [CrossRef]
- Riddle, W.T.; LeBlanc, M.M.; Stromberg, A.J. Relationships Between Uterine Culture, Cytology and Pregnancy Rates in a Thoroughbred Practice. Theriogenology 2007, 68, 395–402. [Google Scholar] [CrossRef]
- Kenney, R.M.; Doig, P.A. Equine Endometrial Biopsy. Curr. Ther. Theriogenology 1986, 2, 723–729. [Google Scholar]
- Sieme, H.; Lüttgenau, J.; Sielhorst, J.; Martinsson, G.; Bollwein, H.; Thomas, S.; Burger, D. Melhorando a formação e função do corpo lúteo na égua. Rev. Bras. Reprodução Anim. 2015, 39, 117–120. [Google Scholar]
- Troedsson, M.H.T.; Woodward, E.M. Our Current Understanding of the Pathophysiology of Equine Endometritis with an Emphasis on Breeding-Induced Endometritis. Reprod. Biol. 2016, 16, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, M.; Troedsson, M. Inflammation and Fertility in the Mare. Reprod. Domest. Anim. 2017, 52, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bollwein, H.; Heppelmann, M.; Lüttgenau, J. Ultrasonographic Doppler Use for Female Reproduction Management. Vet. Clin. Food Anim. Pract. 2016, 32, 149–164. [Google Scholar] [CrossRef] [PubMed]


| n | Subjective Assessment | Number of Pixels | Intensity of Pixels | |
|---|---|---|---|---|
| Treatment Groups | ||||
| GC | 16 | Low perfusion a | 5212 ± 2452 a | 33,187 ± 170,984 a |
| GES | 15 | Low perfusion a | 5069 ± 2534 a | 436,207 ± 15,772,230 a |
| GEC | 14 | Mild perfusion b | 5475 ± 2856 a | 362,201 ± 208,170 a |
| Microbiological culture | ||||
| 0 | 16 | Low perfusion a | 5221 ± 2456 ac | 333,600 ± 171,370 ab |
| F | 4 | Low perfusion ac | 4187 ± 1441 bd | 276,727 ± 109,125 b |
| G+ | 6 | Mild perfusion b | 5480 ± 2101 ac | 357,362 ± 155,629 ab |
| G− | 9 | Mild perfusion bc | 5837 ± 3207 c | 379,045 ± 235,528 a |
| M | 10 | Low perfusion a | 5015 ± 2763 ad | 373,168 ± 452,380 a |
| Endometrial cytology | ||||
| Negative | 33 | Low perfusion a | 5048 ± 2257 a | 331,502 ± 187,156 a |
| Moderate | 10 | Mild perfusion b | 6013 ± 3565 b | 429,380 ± 452,930 b |
| Severe | 2 | Mild perfusion b | 4673 ± 1633 a | 297,283 ± 122,428 a |
| Endometrial biopsy | ||||
| I | 2 | Low perfusion a | 4326 ± 1237 a | 288,050 ± 103,864 a |
| IIA | 13 | Mild perfusion b | 6013 ± 3420 b | 415,687 ± 413,469 b |
| IIB | 18 | Low perfusion a | 4885 ± 2078 a | 330,696 ± 198,668 a |
| III | 12 | Low perfusion ab | 4885 ± 2079 a | 325,753 ± 159,811 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, C.S.C.; Pinna, A.E.; dos Santos, I.P.F.; Dias, M.C.R.; dos Santos, N.S.L.; Bragueroli, S.d.S.; Quintino, P.M.; Almeida, G.B.; Penna, B.d.A.; da Rocha, E.M.d.S.; et al. Powerflow Doppler Ultrasonography in the Evaluation of Mares with and Without Endometritis. Vet. Sci. 2025, 12, 941. https://doi.org/10.3390/vetsci12100941
Ferreira CSC, Pinna AE, dos Santos IPF, Dias MCR, dos Santos NSL, Bragueroli SdS, Quintino PM, Almeida GB, Penna BdA, da Rocha EMdS, et al. Powerflow Doppler Ultrasonography in the Evaluation of Mares with and Without Endometritis. Veterinary Sciences. 2025; 12(10):941. https://doi.org/10.3390/vetsci12100941
Chicago/Turabian StyleFerreira, Camila Silva Costa, Aline Emerim Pinna, Isadora Pires Ferreira dos Santos, Maria Clara Rangel Dias, Natália Sales Leal dos Santos, Samanta da Silva Bragueroli, Petruska Montezuma Quintino, Giovanna Brito Almeida, Bruno de Araújo Penna, Elisabeth Martins da Silva da Rocha, and et al. 2025. "Powerflow Doppler Ultrasonography in the Evaluation of Mares with and Without Endometritis" Veterinary Sciences 12, no. 10: 941. https://doi.org/10.3390/vetsci12100941
APA StyleFerreira, C. S. C., Pinna, A. E., dos Santos, I. P. F., Dias, M. C. R., dos Santos, N. S. L., Bragueroli, S. d. S., Quintino, P. M., Almeida, G. B., Penna, B. d. A., da Rocha, E. M. d. S., de Souza, G. N., Barbosa, C. G., de Jesus, V. L. T., & Jacob, J. C. F. (2025). Powerflow Doppler Ultrasonography in the Evaluation of Mares with and Without Endometritis. Veterinary Sciences, 12(10), 941. https://doi.org/10.3390/vetsci12100941









