Unraveling Spermatogenesis in Molly Fish (Poecilia sphenops): An Integrative Study of Testicular Ultrastructure and Immunohistochemistry
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Histological and Histochemical Analyses
2.3. Semithin Sections and TEM
2.4. Localization of Calretinin and Vimentin Using Indirect Peroxidase Immunohistochemistry Staining
2.5. Digitally Colored TEM Images
2.6. Quantification of Spermatogenic Stages and Morphometric Analysis of Ductal Epithelium
3. Results
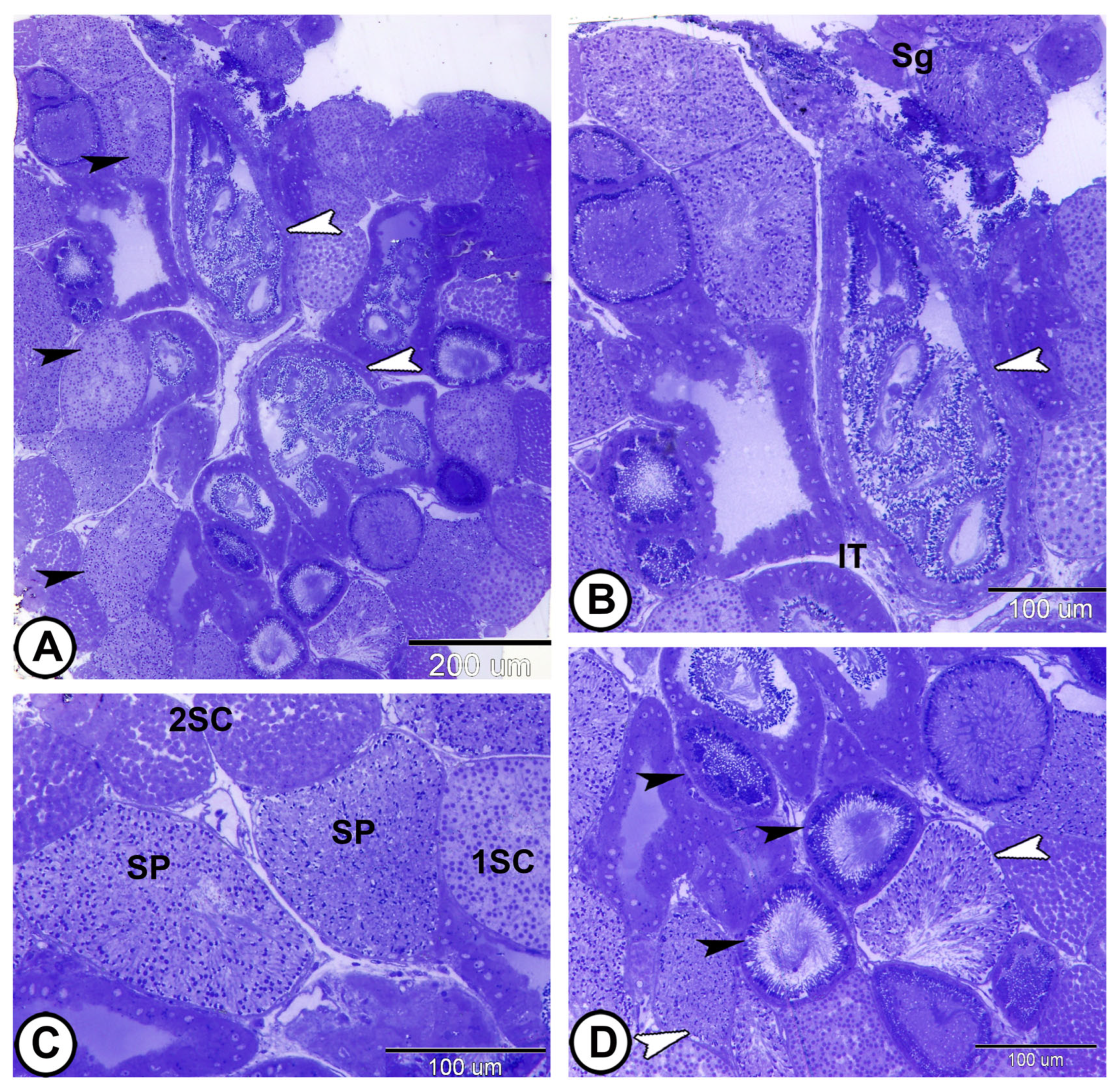
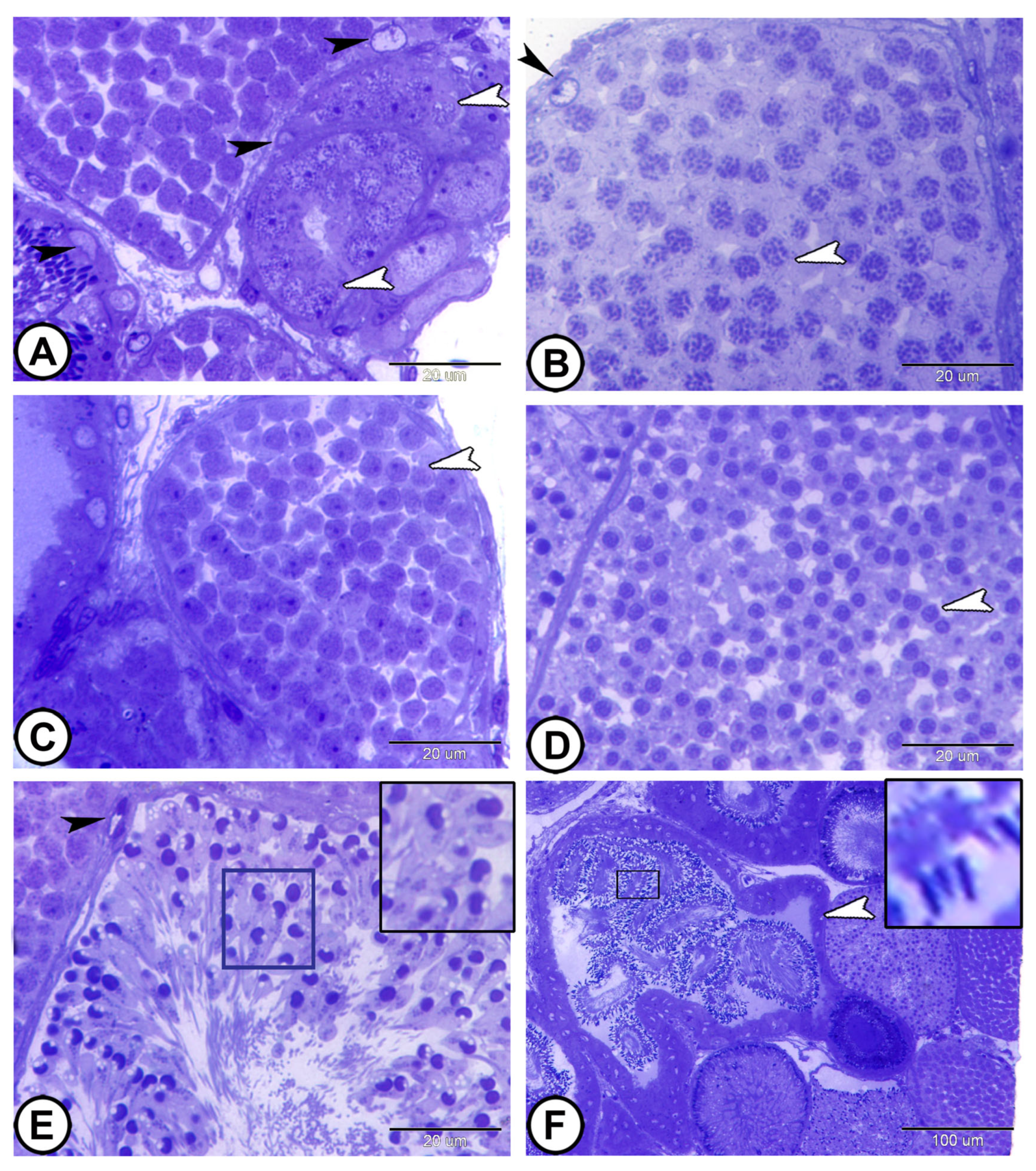
3.1. Light Microscopy
3.2. Spermatogenic Stage Distribution
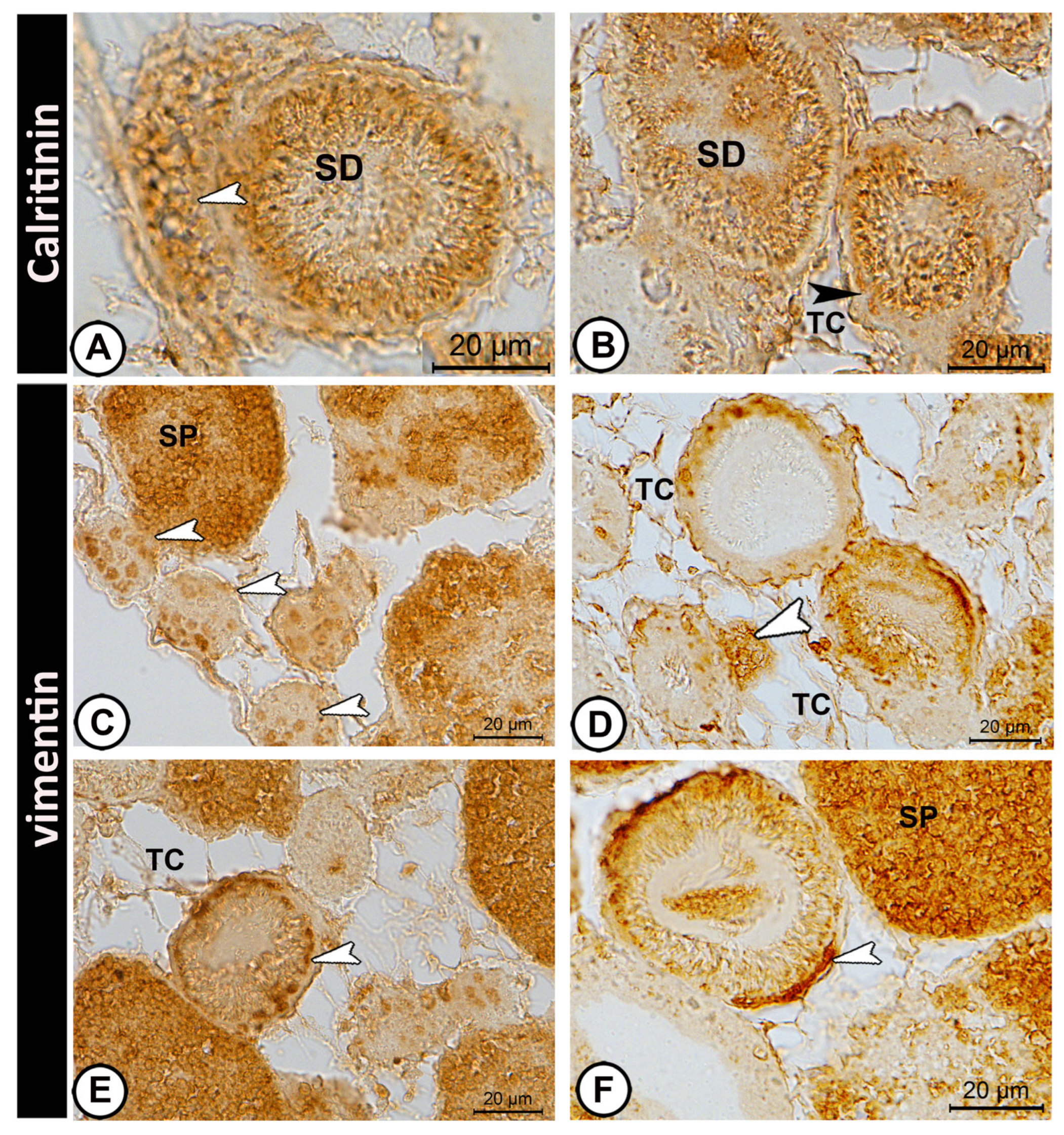
3.3. Immunohistochemistry
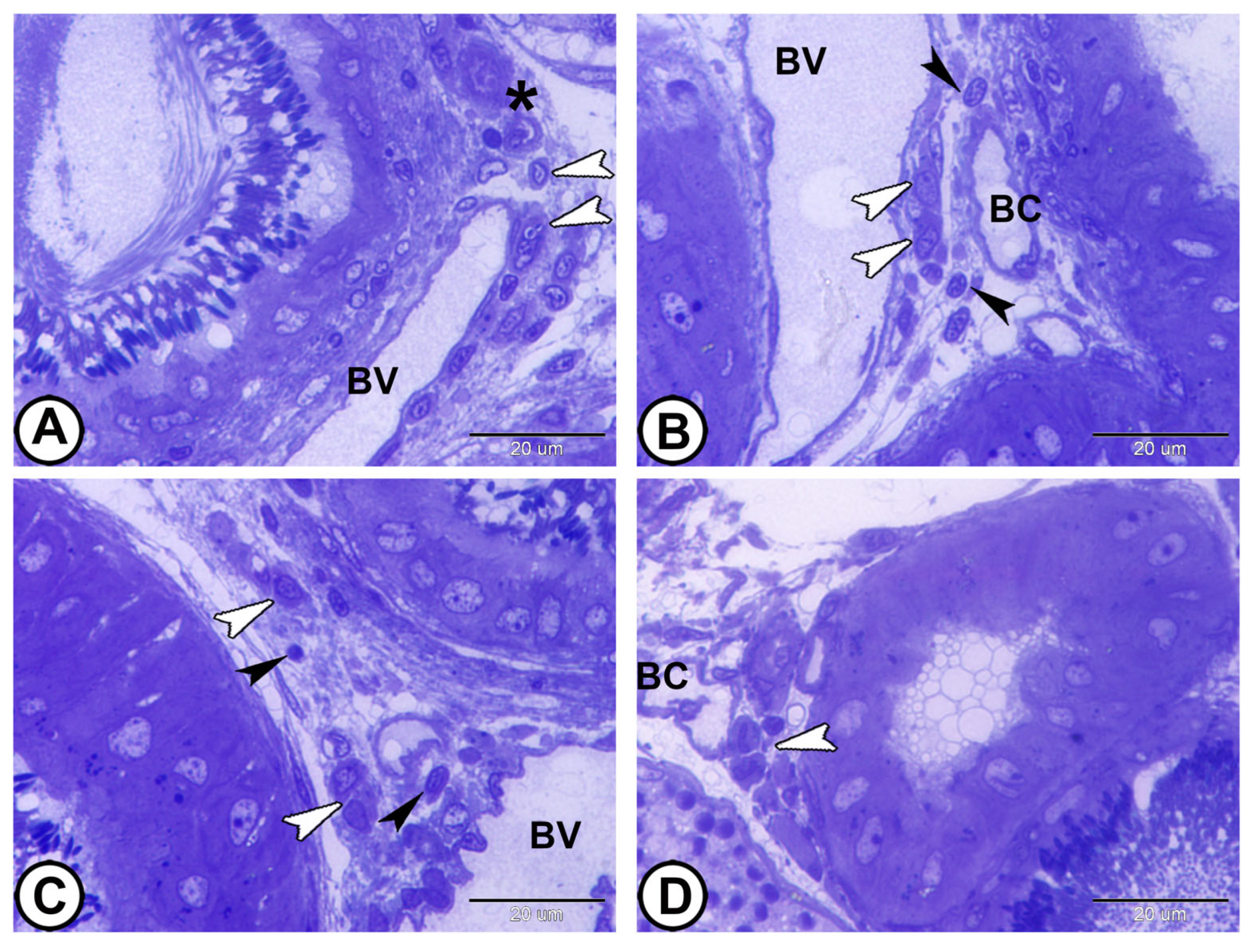
3.4. Ultrastructural Characteristics
- (1)
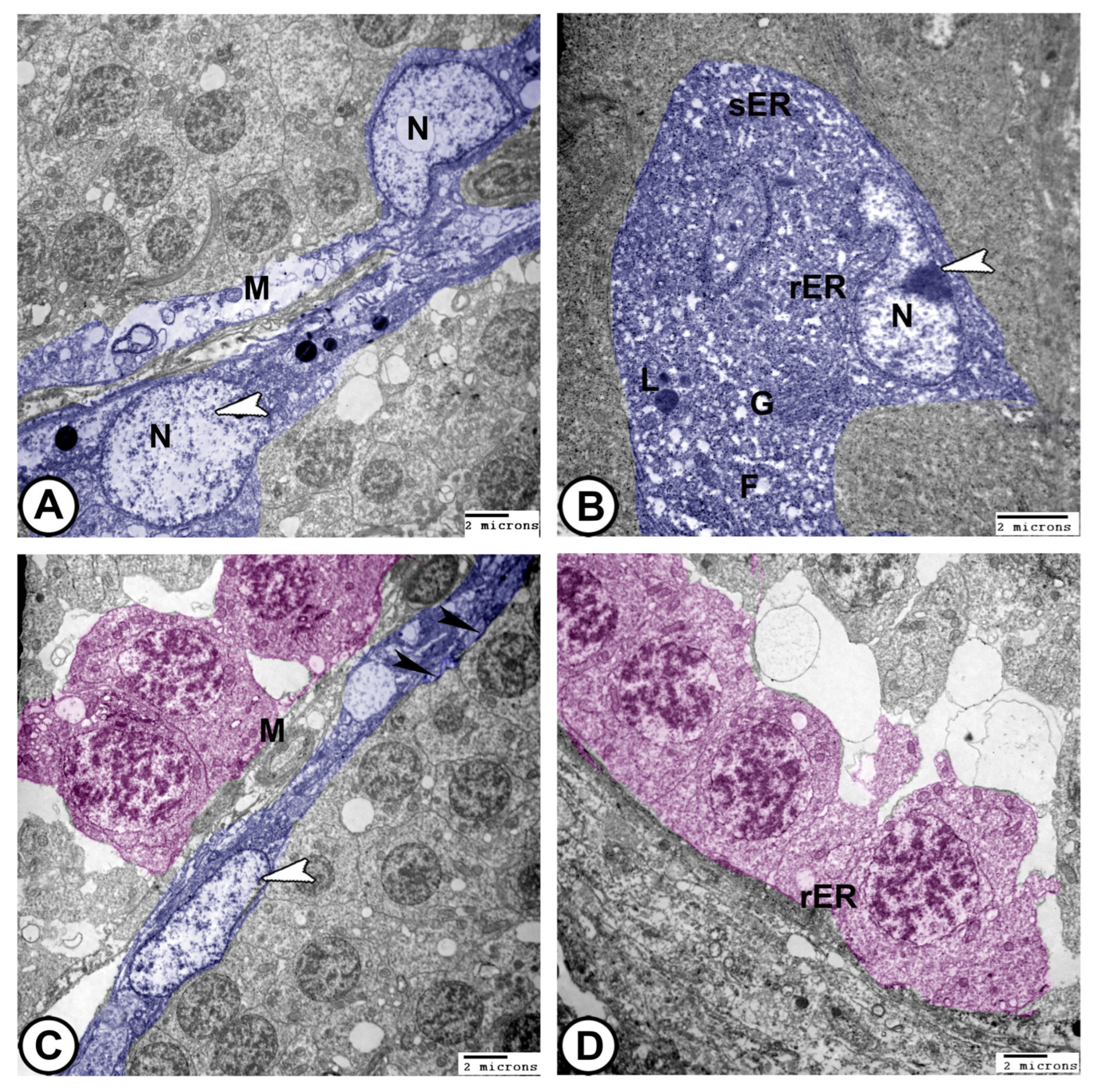
- Sertoli cells were completely encysted, developing spermatogenic cells (Figure 8A). They displayed large euchromatic nuclei with a prominent nucleolus (Figure 8B). The cytoplasm contained abundant rough endoplasmic reticulum (rER), smooth endoplasmic reticulum (SER), lysosomes, mitochondria, Golgi apparatus, and many fat droplets. Sertoli cells were connected to germ cells and cysts by tight junctional complexes (Figure 8A–C).
- (2)
- (3)
- Spermatids: These typically have a spherical, electron-dense nucleus with unevenly dispersed chromatin was observed in the cells. Numerous mitochondria were arranged in the cytoplasm close to the nucleus, while the endoplasmic reticulum′s cisternae were dispersed throughout the cytoplasm. The flagellum was composed of microtubules arranged in a distinctive “9 + 2” pattern, with nine microtubule doublets surrounding a central pair that were encased in the plasma membrane (Figure 9C,D and Figure 10A).
- (4)
- Spermatozoa: They typically have an elongated head, a midpiece with a mitochondrial sheath, and a flagellum (Figure 10B–D).
- (5)
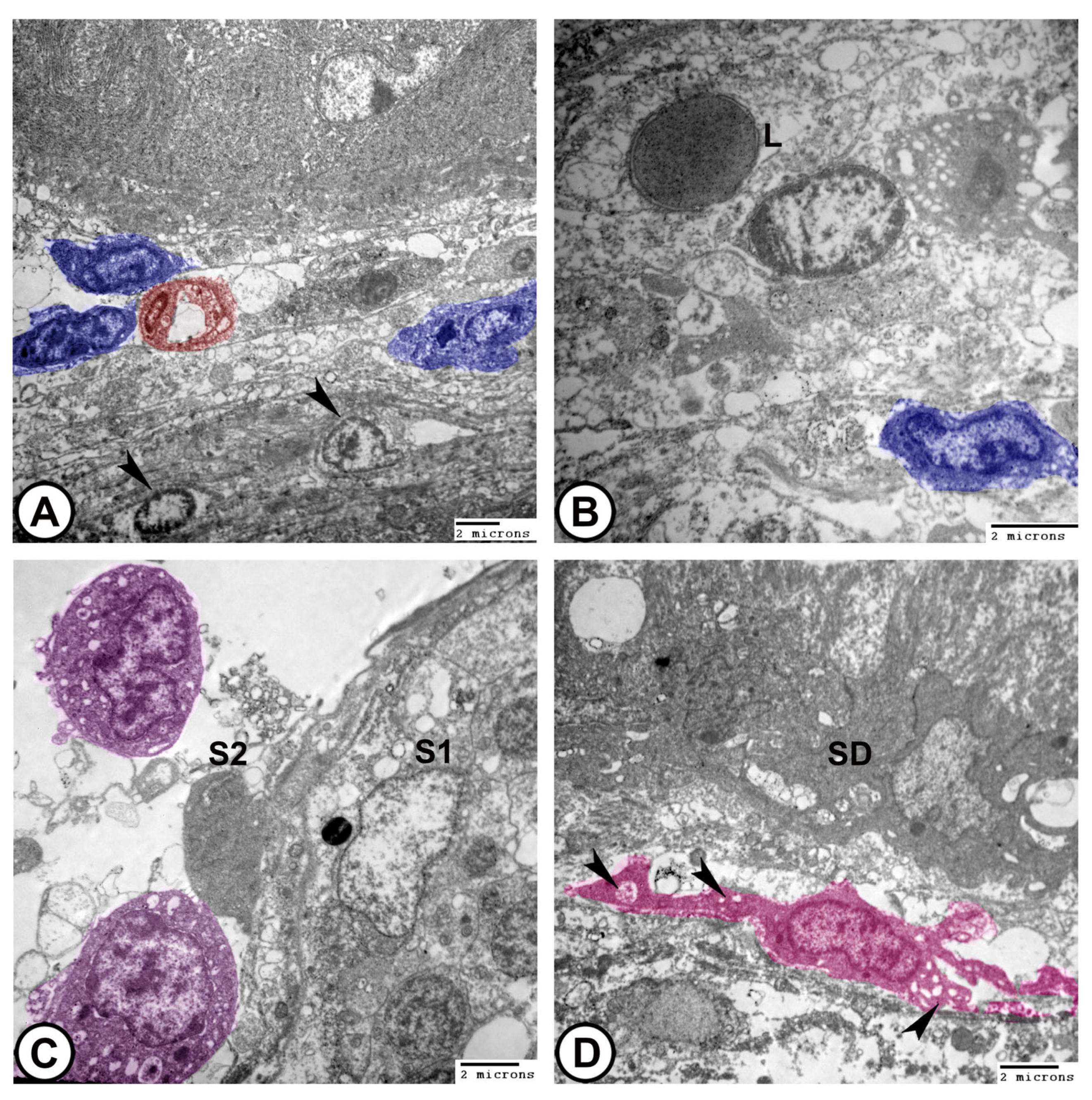
- The sperm duct wall was lined with simple columnar epithelium covered by short cilia. The cytoplasm contained numerous electron-lucent vesicles, rER, mitochondria, and lysosomes. Tight junctions (zonula occludens) and desmosomes formed a barrier between adjacent epithelial cells. Sertoli cells were found beneath the epithelium (Figure 10C,D).
- (6)
- Interstitial tissues contained Leydig cells around the blood capillaries that were characterized by abundant smooth endoplasmic reticulum, lipid droplets, few lysosomes, and mitochondria, in addition to fibroblasts (Figure 11A,B). Macrophages appeared as irregular-shaped cells with large lysosomes, phagosomes, and pseudopodia (Figure 11C). Telocytes were present in the interstitum and were characterized by a spindle body and telopodes containing secretory vesicles (Figure 11D).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulz, R.W.; de França, L.R.; Lareyre, J.J.; Le Gac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. ComEndocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef] [PubMed]
- Pudney, J. Spermatogenesis in nonmammalian vertebrates. Microsc. Res. Tech. 1995, 32, 459–497. [Google Scholar] [CrossRef]
- Blackburn, D.G.; Hughes, D.F. Phylogenetic analysis of viviparity, matrotrophy, and other reproductive patterns in chondrichthyan fishes. Biol. Rev. 2024, 99, 1314–1356. [Google Scholar] [CrossRef]
- Orr, T.J.; Brennan, P.L. Sperm storage: Distinguishing selective processes and evaluating criteria. Trends Ecol. Evol. 2015, 30, 261–272. [Google Scholar] [CrossRef]
- Martínez, V.H.; Monasterio de Gonzo, G.; Uribe, M.C.; Grier, H.J. Testicular structure in three viviparous species of teleosts in the genus Jenynsia (Anablepidae). Spermatogenesis 2014, 4, e983399. [Google Scholar] [CrossRef][Green Version]
- Uribe, M.C.; Grier, H.J.; Mejía-Roa, V. Comparative testicular structure and spermatogenesis in bony fishes. Spermatogenesis 2014, 4, e983400. [Google Scholar] [CrossRef]
- Billard, R. Spermatogenesis and spermatology of some teleost fish species. Reprod. Nutr. Dev. 1986, 26, 877–920. [Google Scholar] [CrossRef]
- Grier, H.J.; Aranzabal, M.C.U. The testis and spermatogenesis in teleosts. In Reproductive Biology and Phylogeny of Fishes (Agnathans and Bony Fishes); CRC Press: Boca Raton, FL, USA, 2009; pp. 119–142. [Google Scholar]
- Torres-Martínez, A.; de Dios, L.R.; Hernández-Franyutti, A.; Uribe, M.C.; Sánchez, W.C. Structure of the testis and spermatogenesis of the viviparous teleost Poecilia mexicana (Poeciliidae) from an active sulfur spring cave in Southern Mexico. J. Morphol. 2019, 280, 1537–1547. [Google Scholar] [CrossRef]
- Costa, F.; Adolfi, M.; Gomes, C.; Jesus, L.; Batlouni, S.; Borella, M. Testes of Astyanax altiparanae: The Sertoli cell functions in a semicystic spermatogenesis. Micron 2014, 61, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, R.H.; Lareyre, J.J. Testicular function and hormonal regulation in fishes. In Hormones and Reproduction of Vertebrates; Elsevier: Amsterdam, The Netherlands, 2024; Volume 1, pp. 63–90. [Google Scholar]
- Augusto, D.; Leteurtre, E.; De La Taille, A.; Gosselin, B.; Leroy, X. Calretinin: A valuable marker of normal and neoplastic Leydig cells of the testis. Appl. Immunohistochem. Mol. Morphol. 2002, 10, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Yokota, S.; Kitajima, S. Immunohistochemical analysis of the vimentin filaments in Sertoli cells is a powerful tool for the prediction of spermatogenic dysfunction. Acta Histochem. 2023, 125, 152046. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Abdel-Ghani, M.A.; Abdelhafez, E.A.; Albano, M.; Alkhodair, K.M.; Zaccone, G. Histological, Immunohistochemical, and Ultrastructural Characterization of Cartilage in Molly Fish (Poecilia sphenops): Insights into Skeletal Adaptations in Teleosts. Fishes 2025, 10, 202. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Attaai, A.; Zaccone, G.; Alesci, A.; Alonaizan, R.; Hussein, M.T. Morphological distribution patterns and neuroimmune communication of ganglia in Molly fish (Poecilia Sphenops, Valenciennes 1846). Fishes 2023, 8, 289. [Google Scholar] [CrossRef]
- Awad, M.; Sayed, R.K.A.; Mohammadin, D.; Hussein, M.M.; Mokhtar, D.M. Structural characteristics and regenerative potential: Insights from the molly fish spinal cord. Microsc. Res. Tech. 2024, 87, 2643–2653. [Google Scholar] [CrossRef]
- Hussein, M.T.; Sayed, R.K.A.; Mokhtar, D.M. Neuron mapping in the Molly fish optic tectum: An emphasis on the adult neurogenesis process. Microsc. Res. Tech. 2024, 87, 2336–2354. [Google Scholar] [CrossRef]
- Posner, L.P.; Scott, G.N.; Law, J.M. Repeated exposure of goldfish (Carassius auratus) to tricaine methanesulfonate (MS-222). J. Zoo Wildl. Med. 2013, 44, 340–347. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Porcino, C.; Briglia, M.; Aragona, M.; Mhalhel, K.; Laurà, R.; Levanti, M.; Abbate, F.; Montalbano, G.; Germanà, G.; Lauriano, E.R.; et al. Potential Neuroprotective Role of Calretinin-N18 and Calbindin-D28k in the Retina of Adult Zebrafish Exposed to Different Wavelength Lights. Int. J. Mol. Sci. 2023, 24, 1087. [Google Scholar] [CrossRef]
- Aragona, M.; Porcino, C.; Briglia, M.; Mhalhel, K.; Abbate, F.; Levanti, M.; Montalbano, G.; Laurà, R.; Lauriano, E.R.; Germanà, A.; et al. Vimentin Localization in the Zebrafish Oral Cavity: A Potential Role in Taste Buds Regeneration. Int. J. Mol. Sci. 2023, 24, 15619. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.L.; Campos-Junior, P.H.A.; Costa, G.M.J.; de França, L.R. Spermatogenic cycle length and sperm production in the freshwater turtle Kinosternon scorpioides. Biol. Reprod. 2014, 90, 35. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Q.; Ma, D.; Xiao, Y.; Xu, S.; Wang, X.; Song, Z.; You, F.; Li, J. Spermatogonial stem cells differentiation and testicular lobules formation in a seasonal breeding teleost: The evidence from the heat-induced masculinization of genetically female Japanese flounder (Paralichthys olivaceus). Theriogenology 2018, 120, 68–78. [Google Scholar] [CrossRef]
- Koulish; Kramer, C.R.; Grier, H.J. Organization of the male gonad in a protogynous fish, Thalassoma bifasciatum (Teleostei: Labridae). J. Morphol. 2002, 254, 292–311. [Google Scholar] [CrossRef]
- Parenti, L.R.; Grier, H.J. Evolution and phylogeny of gonad morphology in bony fishes. Integr. Comp. Biol. 2004, 44, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Fraile, B.; Sáez, F.J.; Vicentini, C.A.; Miguel, M.D.; Paniagua, R. The testicular cycle of Gambusia affinis holbrooki (Teleostei: Poeciliidae). J. Zool. 1992, 228, 115–126. [Google Scholar] [CrossRef]
- Grier, H.J.; Horner, J.; Mahesh, V.B. The morphology of enclosed testicular tubules in a teleost fish, Poecilia latipinna. Trans. Am. Microsc. Soc. 1980, 99, 268–276. [Google Scholar] [CrossRef]
- Uribe, M.C.; Grier, H.J.; De la Rosa-Cruz, G.; García-Alarcón, A. Modifications in ovarian and testicular morphology associated with viviparity in teleost. In Reproductive Biology and Phylogeny of Fishes (Agnathans and Bony Fishes); Jamieson, B., Ed.; Science Publishers: Enfield, NH, USA, 2009; pp. 85–117. [Google Scholar]
- Greven, H. Structural and behavioral traits associated with sperm transfer in Poeciliinae. Viviparous Fishes 2005, 147–165. [Google Scholar]
- Burns, J.; Weitzman, S. Sperm adaptations for internal fertilization in ostariophysan fishes. Viviparous Fishes 2005, 105–132. [Google Scholar]
- Pecio, A. Morfologiczne Modyfikacje Związane z Inseminacją w Układzie Rozrodczym Samców ryb Kąsaczowatych z Podrodzin Glandulocaudinae i Stevardiinae (Teleostei: Characiformes: Characidae); Wydawnictwo UJ: Kraków, Poland, 2010. [Google Scholar]
- Greven, H. Gonads, genitals, and reproductive biology. In Ecology and Evolution of Poeciliid Fishes; Evans, J.P., Pilastro, A., Schlupp, I., Eds.; University of Chicago Press: Chicago, IL, USA, 2011; pp. 193–217. [Google Scholar]
- Grier, H.J.; Uribe, M.; Parenti, L.R.; De la Rose-Cruz, G. Fecundity, the Germinal Epithelium, and Folliculogenesis in Viviparous Fishes; New Life Publications: Hayward, CA, USA, 2005. [Google Scholar]
- Grier; Fitzsimons, J.; Linton, J. Structure and ultrastructure of the testis and sperm formation in goodeid teleosts. J. Morphol. 1978, 156, 419–437. [Google Scholar]
- Burns, J.R.; Pecio, A.; Weitzman, S.H. Sperm and spermatozeugma structure in Xenurobrycon (Teleostei: Characidae: Stevardiinae: Xenurobryconini). Ichthyol. Herpetol. 2008, 2008, 656–660. [Google Scholar] [CrossRef]
- Zickler, D. From early homologue recognition to synaptonemal complex formation. Chromosoma 2006, 115, 158–174. [Google Scholar] [CrossRef]
- Jamieson, B.G. Ultrastructure of spermatozoa: Acanthopterygii: Mugilomorpha; Atherinomorpha. In Reproductive Biology and Phylogeny of Fishes (Agnathans and Bony Fishes); CRC Press: Boca Raton, FL, USA, 2009; pp. 447–501. [Google Scholar]
- Javonillo, R.; Malabarba, L.R.; Weitzman, S.H.; Burns, J.R. Relationships among major lineages of characid fishes (Teleostei: Ostariophysi: Characiformes), based on molecular sequence data. Mol. Phylogenetics Evol. 2010, 54, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Grier, H.J. Comparative organization of Sertoli cells, including the Sertoli cell barrier. In The Sertoli Cell; Russel, L.D., Griswald, M.D., Eds.; Cache River Press: Clearwater, FL, USA, 1993; pp. 704–739. [Google Scholar]
- Rupik, W.; Huszno, J.; Klag, J. Cellular organisation of the mature testes and stages of spermiogenesis in Danio rerio (Cyprinidae; Teleostei)-structural and ultrastructural studies. Micron 2011, 42, 833–839. [Google Scholar] [CrossRef]
- Dadhich, R.K.; Real, F.M.; Zurita, F.; Barrionuevo, F.J.; Burgos, M.; Jiménez, R. Role of apoptosis and cell proliferation in the testicular dynamics of seasonal breeding mammals: A study in the Iberian mole, Talpa occidentalis. Biol. Reprod. 2010, 83, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Grier, H.J. Testis structure and formation of spermatophores in the Atherinomorph teleost Horaichthys setnai. Copeia 1984, 4, 833–839. [Google Scholar] [CrossRef]
- Barinka, F.; Druga, R. Calretinin expression in the mammalian neocortex: A review. Physiol. Res. 2010, 59, 665. [Google Scholar] [CrossRef]
- Mojumder, D.K.; Wensel, T.G.; Frishman, L.J. Subcellular compartmentalization of two calcium binding proteins, calretinin and calbindin-28 kDa, in ganglion and amacrine cells of the rat retina. Mol. Vis. 2008, 14, 1600–1613. [Google Scholar] [PubMed]
- Germanà, A.; Paruta, S.; Germanà, G.P.; Ochoa-Erena, F.J.; Montalbano, G.; Cobo, J.; Vega, J.A. Differential distribution of S100 protein and calretinin in mechanosensory and chemosensory cells of adult zebrafish (Danio rerio). Brain Res. 2007, 1162, 48–55. [Google Scholar] [CrossRef]
- Castro, A.; Becerra, M.; Manso, M.J.; Anadón, R. Calretinin immunoreactivity in the brain of the zebrafish, Danio rerio: Distribution and comparison with some neuropeptides and neurotransmitter-synthesizing enzymes. I. Olfactory organ and forebrain. J. Comp. Neurol. 2006, 494, 435–459. [Google Scholar] [CrossRef]
- Schwaller, B. Calretinin: From a “simple” Ca2+ buffer to a multifunctional protein implicated in many biological processes. Front. Neuroanat. 2014, 8, 3. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Stevenson, L.; Allen, W.L.; Proutski, I.; Stewart, G.; Johnston, L.; McCloskey, K.; Wilson, P.M.; Longley, D.B.; Johnston, P.G.; Gartel, A.L. Calbindin 2 (CALB2) regulates 5-fluorouracil sensitivity in colorectal cancer by modulating the intrinsic apoptotic pathway. PLoS ONE 2011, 6, e20276. [Google Scholar] [CrossRef]
- Strauss, K.I.; Isaacs, K.R.; Ha, Q.N.; Jacobowitz, D.M. Calretinin is expressed in the Leydig cells of rat testis. Biochim. Et Biophys. Acta (BBA)-Gene Struct. Expr. 1994, 1219, 435–440. [Google Scholar] [CrossRef]
- Foresta, C.; Mioni, R. The role of calcium ions in rat Leydig cell steroidogenesis induced by atrial natriuretic peptide. Eur. J. Endocrinol. 1993, 128, 274–280. [Google Scholar] [CrossRef][Green Version]
- Xiang, Y.; Wu, Y.; Zhang, H.; Wu, J.; Zhang, J. Characterization and Localization of Calb 2 in Both the Testis and Ovary of the Japanese Flounder (Paralichthys olivaceus). Animals 2020, 10, 1503. [Google Scholar] [CrossRef] [PubMed]
- Dressen, C.; Schwaller, B.; Vegh, G.; Leleux, F.; Gall, D.; Lebrun, P.; Lybaert, P. Characterization and potential roles of calretinin in rodent spermatozoa. Cell Calcium 2018, 74, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Blois, S.M.; Freitag, N.; Bergmann, M.; Bhushan, S.; Wahle, E.; Huang, A.C.-C.; Chen, H.-L.; Hartmann, M.F.; Wudy, S.A.; et al. Targeted disruption of galectin 3 in mice delays the first wave of spermatogenesis and increases germ cell apoptosis. Cell. Mol. Life Sci. 2021, 78, 3621–3635. [Google Scholar] [CrossRef] [PubMed]
- Tabar, N.N.; Azizi, H.; Karoii, D.H.; Skutella, T. Testicular localization and potential function of vimentin positive cells during spermatogonial differentiation stages. Animals 2022, 12, 268. [Google Scholar] [CrossRef]
- Wellard, S.R.; Schindler, K.; Jordan, P. Aurora B and C kinases regulate prophase exit and chromosome segregation during spermatogenesis. bioRxiv 2019, 868836. [Google Scholar] [CrossRef]
- Vogl, A.W.; Vaid, K.S.; Guttman, J.A. The sertoli cell cytoskeleton. Adv. Exp. Med. Biol. 2008, 636, 186–211. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Abdel-Ghani, M.A.; Khalaf, M.A.; Alkhodair, K.M.; Abdelhafez, E.A. “Histological and Immunohistochemical Char acterization of the Kidney and Adrenal Gland in Nile Monitor Lizards (Varanus niloticus)”. Microsc. Res. Tech. 2025, 88, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Arenas, M.I.; Fraile, B.; De Miguel, M.; Paniagua, R. Intermediate filaments in the testis of the teleost mosquito fish Gambusia affinis holbrooki: A light and electron microscope immunocytochemical study and Western blotting analysis. Histochem. J. 1995, 27, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, G.; Mokhtar, D.M.; Alesci, A.; Capillo, G.; Albano, M.; Hussein, M.T.; Aragona, M.; Germanà, A.; Lauriano, E.R.; Sayed, R.K.A. From proliferation to protection: Immunohistochemical profiling of cardiomyocytes and immune cells in molly fish hearts. Fishes 2024, 9, 283. [Google Scholar] [CrossRef]
- Mokhtar, D.M. Fish Histology: From Cells to Organs, 2nd ed; Apple Academic Press: Burlington, ON, Canada, 2021; ISBN 978177188452. [Google Scholar]
- Radu, B.M.; Banciu, A.; Banciu, D.D.; Radu, M.; Cretoiu, D.; Cretoiu, S.M. Calcium Signaling in Interstitial Cells: Focus on Telocytes. Int. J. Mol. Sci. 2017, 18, 397. [Google Scholar] [CrossRef]
- Sayed, R.K.; Mokhtar, D.M.; Fernández-Ortiz, M.; Escames, G.; Acuña-Castroviejo, D. Retinoid-related orphan nuclear receptor alpha (RORα)-deficient mice display morphological testicular defects. Lab. Investig. 2019, 99, 1835–1849. [Google Scholar] [CrossRef]





| Primary antibodies | Calretinin (N-18) | Supplier | Catalog Number | Source | Dilution | Antibody ID |
| Santa Cruz Biotechnology, Santa Cruz, CA, USA | sc-11644 | goat | 1:100 | AB_634545 | ||
| Vimentin (RV202) | Thermo Fisher Scientific, Carlsbad, CA, USA | OMA1-06001 | mouse | 1:100 | AB_325529 | |
| Secondary antibodies | Anti-goat IgG (H+L) HRP conjugate | Thermo Fisher Scientific, Carlsbad, CA, USA | #31402 | Rabbit | 1:300 | AB_228395 |
| Anti-mouse IgG (H+L) HRP conjugate | Thermo Fisher Scientific, Carlsbad, CA, USA | #31430 | Goat | 1:300 | AB_228307 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, D.M.; Zaccone, G.; Aragona, M.; Guerrera, M.C.; Alonizan, R.; Hussein, M.T. Unraveling Spermatogenesis in Molly Fish (Poecilia sphenops): An Integrative Study of Testicular Ultrastructure and Immunohistochemistry. Vet. Sci. 2025, 12, 930. https://doi.org/10.3390/vetsci12100930
Mokhtar DM, Zaccone G, Aragona M, Guerrera MC, Alonizan R, Hussein MT. Unraveling Spermatogenesis in Molly Fish (Poecilia sphenops): An Integrative Study of Testicular Ultrastructure and Immunohistochemistry. Veterinary Sciences. 2025; 12(10):930. https://doi.org/10.3390/vetsci12100930
Chicago/Turabian StyleMokhtar, Doaa M., Giacomo Zaccone, Marialuisa Aragona, Maria Cristina Guerrera, Rasha Alonizan, and Manal T. Hussein. 2025. "Unraveling Spermatogenesis in Molly Fish (Poecilia sphenops): An Integrative Study of Testicular Ultrastructure and Immunohistochemistry" Veterinary Sciences 12, no. 10: 930. https://doi.org/10.3390/vetsci12100930
APA StyleMokhtar, D. M., Zaccone, G., Aragona, M., Guerrera, M. C., Alonizan, R., & Hussein, M. T. (2025). Unraveling Spermatogenesis in Molly Fish (Poecilia sphenops): An Integrative Study of Testicular Ultrastructure and Immunohistochemistry. Veterinary Sciences, 12(10), 930. https://doi.org/10.3390/vetsci12100930







