Critical Analysis of Protocols for Good Veterinary Practices in Monitoring, Prevention and Treatment of Ketosis in Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Purpose and Objectives
- (1)
- to study the good veterinary practices established as such for monitoring and controlling ketosis in the scientific literature;
- (2)
- to conduct a critical analysis of the effectiveness of such established practices;
- (3)
- to offer future perspectives for the development of good practices for ketosis control.
3. Materials and Methods
- (1)
- Sources and databases used for search:
- ScienceDirect (Elsevier)
- PubMed (NCBI)
- SpringerLink
- Google Scholar (if you need access to extended texts)
- Veterinary conferences and proceedings: World Buiatrics Congress, Vet. Clin. North Am., J. Dairy Sci.
- (2)
- Method of defining a thematic framework
- Metabolism and etiology of ketosis (type I, II, silage ketosis).
- Diagnostic methods based on the detection of BHBA, NEFA, citrate, fat/protein (F/P) ratio in milk.
- Therapeutic approaches based on the application of proven products such as propylene glycol, glucose, glucocorticoids, and monensin.
- Prevention and monitoring at the herd/farm level.
- (3)
- Selection process:
- Keywords and search—used combinations such as:
- “subclinical ketosis in dairy cows”, “BHBA monitoring”, “monensin prevention dairy cattle”, “milk fat to protein ratio—ketosis”
- Review of titles and abstracts—primary selection by keywords
- Analysis and evaluation of the full text
- Data extraction and citation in standard format (APA)
- (4)
- Survey of Veterinary Practitioners and Companies
- (5)
- Categorization and integration:
- Etiology and pathogenesis
- Diagnostic methods and values
- Treatment strategies
- Prevention/feeding/monitoring
- (6)
4. Monitoring
- Notes:
- Preventive measures must be combined with good transition-cow management and nutritional consistency.
- Monensin usage depends on regional regulatory approval; veterinary oversight is mandatory.
- Practical application is strongest when prevention is integrated into herd-level metabolic monitoring.
4.1. Fat/Protein Ratio (F/P) in Milk as a Practical Indicator of the Presence of Ketosis
- Methods for diagnosing ketosis
- Direct methods include:
Determination of Ketone Bodies in Milk or Urine
- Combined diagnostic approach recommended:
- Screening—by milk F/P ratio or milk BHBA;
- Confirmation—by blood BHBA measurement;
- Risk monitoring—by prepartum NEFA sampling.
5. Ketosis Treatment
- Propylene glycol remains the gold-standard therapy for subclinical ketosis.
- Combining glucose infusion with glucocorticoids may be beneficial in severe clinical cases but should be administered under veterinary control.
- Avoid repeated corticosteroid use in lactating cows due to risk of immunosuppression.
- Integration of therapy with herd-level metabolic monitoring ensures sustained recovery and reduces relapse rate.
6. Discussion
- Purpose of the Protocol:
- On-time identification of animals at risk
- Early diagnosis of subclinical ketosis
- Prevention of clinical complications
- Optimizing productivity and reproduction
- Monitoring: Indicators, limit values, and measurement methods;
- Preventive strategies: best nutritional and management strategies;
- Prevention and treatment by stage of ketosis.
- Table 8 presents the integrated GVP protocol for monitoring, prevention, and treatment of ketosis in dairy cows.
- BHBA ≥ 1.2 mmol/L indicates subclinical ketosis (SCK); ≥3.0 mmol/L indicates clinical ketosis (CK).
- Integration of monitoring, prevention, and treatment ensures effective herd-level control.
- Protocols should be adjusted to local regulations and farm management conditions.
7. Inferences
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duffield, T.F.; Lissemore, K.D.; McBride, B.W.; Leslie, K.E. Impact of hyperketonemia in early lactation dairy cows on health and production. J. Dairy Sci. 2009, 92, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Duffield, T.F. Subclinical ketosis in lactating dairy cattle. In Metabolic Disorders of Ruminants; Herdt, T.H., Ed.; W. B. Saunders: Philadelphia, PA, USA, 2000; pp. 231–253. [Google Scholar]
- Andersson, L. Subclinical ketosis in dairy cows. Vet. Clin. N. Am. Food Anim. Pract. 1988, 4, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Rajala-Schultz, P.J.; Gröhn, Y.T.; McCulloch, C.E. Effects of milk fever, ketosis, and lameness on milk yield in dairy cows. J. Dairy Sci. 1999, 82, 288–294. [Google Scholar] [CrossRef]
- Geishauser, T.; Leslie, K.E.; Duffield, T.F.; Edge, V.L. Evaluation of AST activity and β-hydroxybutyrate concentration in blood as predictors of left displaced abomasum in dairy cows. Am. J. Vet. Res. 1997, 58, 1216–1220. [Google Scholar] [CrossRef]
- Walsh, R.B.; Walton, J.S.; Kelton, D.F.; LeBlanc, S.J.; Leslie, K.E.; Duffield, T.F. The effect of subclinical ketosis in early lactation on reproductive performance of postpartum dairy cows. J. Dairy Sci. 2007, 90, 2788–2796. [Google Scholar] [CrossRef]
- Tveit, B.; Lingaas, F.; Svendsen, M.; Sjaastad, O.V. Etiology of acetonemia in Norwegian cattle. 1. Effect of ketogenic silage, season, energy level, and genetic factors. J. Dairy Sci. 1992, 75, 2421–2432. [Google Scholar] [CrossRef]
- Melendez, P. Ketosis. In Five-Minute Veterinary Consult: Ruminant, 2nd ed.; Anderson, D.E., Rings, M.J., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 425–427. [Google Scholar]
- Melendez, P.; Risco, C.A. Reproduction, events and pregnancy management: Periparturient disorders. In Encyclopedia of Dairy Sciences, 3rd ed.; Fuquay, J.W., van Soest, P.J., Fahey, G.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1031–1037. [Google Scholar]
- Suthar, V.S.; Canelas-Raposo, J.; Deniz, A.; Heuwieser, W. Prevalence of subclinical ketosis and relationships with postpartum diseases in European dairy cows. J. Dairy Sci. 2013, 96, 2925–2938. [Google Scholar] [CrossRef]
- Gordon, J.L.; LeBlanc, S.J.; Duffield, T.F. Ketosis treatment in lactating dairy cattle. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 433–445. [Google Scholar] [CrossRef]
- Oetzel, G.R. Monitoring and testing dairy herds for metabolic diseases. Proc. World Buiatrics Congr. 2004, 20, 651–674. [Google Scholar]
- Holtenius, P.; Holtenius, K. New aspects of ketone bodies in energy metabolism of dairy cows: A review. J. Vet. Med. A 1996, 43, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Grummer, R.R. Nutritional and management strategies for the prevention of fatty liver in dairy cattle. Vet. J. 2008, 176, 10–20. [Google Scholar] [CrossRef]
- McArt, J.A.A. Hyperketonemia in Cattle (Ketosis, Acetonemia); Cornell University, College of Veterinary Medicine: Ithaca, NY, USA, 2024. [Google Scholar]
- Guliński, P. Ketone bodies—Causes and effects of their increased presence in cows’ body fluids: A review. Vet. World 2021, 14, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; McArt, J.A.A. Hyperketonemia: A marker of disease, a sign of a high-producing dairy cow, or both? Vet. Clin. Food Anim. Pract. 2023, 39, 307–324. [Google Scholar] [CrossRef]
- Meléndez, P.; Pinedo, P. Update on fatty liver in dairy cattle with major emphasis on epidemiological patterns, pathophysiology in relationship to abdominal adiposity, and early diagnosis. Dairy 2024, 5, 672–687. [Google Scholar] [CrossRef]
- Rezaei Ahvanooei, M.R.; Norouzian, M.A.; Piray, A.H.; Vahmani, P.; Ghaffari, M.H. Effects of monensin supplementation on lactation performance of dairy cows: A systematic review and dose-response meta-analysis. Sci. Rep. 2023, 13, 568. [Google Scholar] [CrossRef]
- Mammi, L.M.E.; Guadagnini, M.; Mechor, G.; Cainzos, J.M.; Cavallini, D.; Fustini, M.; Fusaro, I.; Giammarco, M.; Formigoni, A.; Palmonari, A. The use of monensin for ketosis prevention in dairy cows during the transition period: A systematic review. Animals 2021, 11, 1988. [Google Scholar] [CrossRef]
- Guliński, P.; Salamończyk, E.; Młynek, K. Possibilities of modifying the chemical composition of cow’s milk. Wyd. Nauk. UPH w Siedlcach. 2018. Available online: https://agris.fao.org/search/en/providers/122651/records/64776168a3fd11e4303bead9 (accessed on 8 May 2025).
- Buttchereit, N.; Heuwieser, W.; Suthar, V.S. Evaluation of a new test for the detection of subclinical ketosis in dairy cows. J. Dairy Sci. 2010, 93, 2693–2700. [Google Scholar] [CrossRef]
- Eicher, R. Evaluation of the metabolic and nutritional situation in dairy herds: Diagnostic use of milk components. In Proceedings of the 23rd World Buiatrics Congress, Quebec City, QC, Canada, 11–16 July 2004. [Google Scholar]
- Jeppesen, R.; Enemark, J.M.D.; Enevoldsen, C. Ketone body measurement in dairy cows. In Proceedings of the 24th World Buiatrics Congress, Nice, France, 15–19 October 2006. [Google Scholar]
- Guliński, P.; Salamończyk, E.; Młynek, K. Improving nitrogen use efficiency of dairy cows in relation to urea in milk—A review. Anim. Sci. Pap. Rep. 2016, 34, 5–24. [Google Scholar]
- Yang, W.; Zhang, B.; Xu, C.; Zhang, H.; Xia, C. Effects of ketosis in dairy cows on blood biochemical parameters, milk yield and composition, and digestive capacity. J. Vet. Res. 2019, 63, 555–560. [Google Scholar] [CrossRef]
- Ranaraja, U.; Cho, K.H.; Park, M.N.; Choi, T.J.; Kim, S.D.; Lee, J.S.; Kim, H.S.; Do, C.H. Impact of environmental factors on milk β-hydroxybutyric acid and acetone levels in Holstein cattle associated with production traits. Korean J. Agric. Sci. 2016, 43, 394–400. [Google Scholar] [CrossRef][Green Version]
- Hendriks, S.J.; Roche, J.R.; McArt, J.A.A.; Grala, T.M.; Turner, S.-A.; Burke, C.R.; Kuhn-Sherlock, B.; Phyn, C.V.C. Investigating the epidemiology of hyperketonemia in grazing dairy cows in early lactation: Incidence, prevalence, and time to resolution of hyperketonemia. J. Dairy Sci. 2025, 108, 5257–5270. [Google Scholar] [CrossRef]
- Marczuk, J.; Kiczorowska, B.; Kurek, Ł.; Brodzki, P. Advances in the diagnosis, therapy and prophylaxis of ketosis in dairy cattle. Magazyn Weterynaryjny, September 2013; pp. 953–962. Available online: https://www.researchgate.net/publication/284510596_Postepy_w_diagnostyce_terapii_i_profilaktyce_ketozy_u_bydla_mlecznego (accessed on 8 May 2025).
- Duffield, T.F.; Lissemore, K.D.; McBride, B.W.; Leslie, K.E. A meta-analysis of the impact of monensin in lactating dairy cattle. Part 1: Metabolic effects. J. Dairy Sci. 2008, 91, 1334–1346. [Google Scholar] [CrossRef]
- Losand, B.; Blum, E.; Flor, J. Efficacy of Kexxtone in practice. Tierärztliche Umsch. 2015, 70, 156–161. [Google Scholar]
| Criterion | Description |
|---|---|
| Scientific validity | Only peer-reviewed publications in journals with an impact factor/impact rank. |
| Current affairs | Mostly publications from the last 10–15 years, with a focus on 2012–2024. |
| Relevance | They relate directly to ketosis, diagnostics, therapy or metabolism. |
| Authority | Authors and publications with proven contributions in the field of veterinary and animal science. |
| Source type | Reviews, clinical trials, official guidelines. |
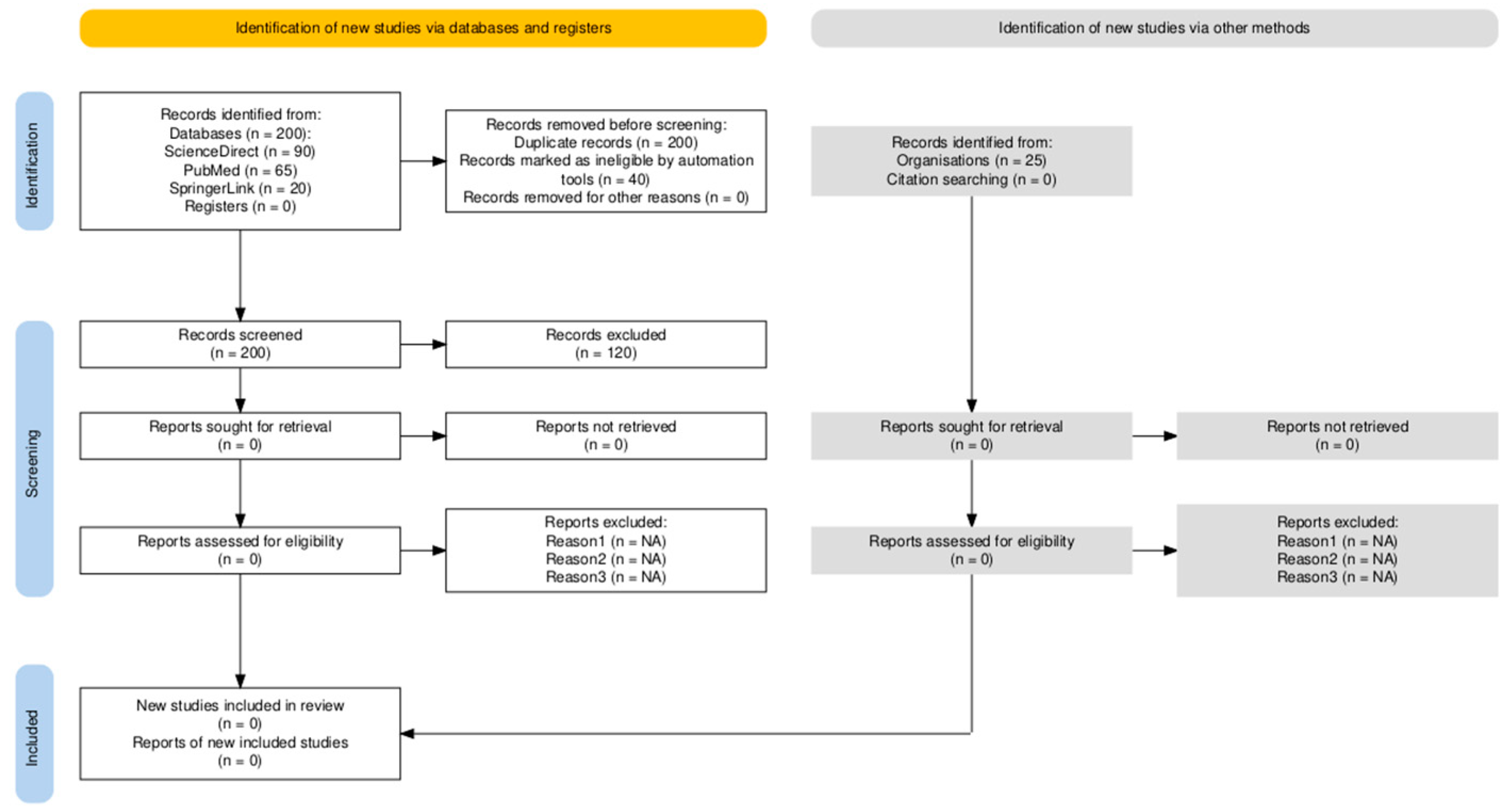
| Stage | Description | Number of Records |
|---|---|---|
| Identification | Records identified through database searching (ScienceDirect, PubMed, SpringerLink, Google Scholar) | 185 |
| Additional records identified through other sources (conference proceedings, veterinary websites, expert suggestions) | 25 | |
| Screening | Records after duplicates removed | 200 |
| Records screened (titles and abstracts) | 200 | |
| Records excluded (irrelevant topic, non-dairy cattle, non-ketosis) | 120 | |
| Eligibility | Full-text articles assessed for eligibility | 80 |
| Full-text articles excluded, with reasons (unrelated study design, incomplete data) | 40 | |
| Included | Studies included in qualitative synthesis | 40 |
| Studies included in quantitative synthesis (if applicable) | 0 |
| BHBA Concentration (µmol/L) | Interpretation | Animal Condition | Source |
|---|---|---|---|
| <1000 | Normal | No ketosis | [11,12] |
| 1000–1200 | Border zone | Potential onset of ketosis | |
| ≥1200 | Subclinical ketosis | No visible clinical signs | |
| ≥1400–1600 | Established subclinical ketosis | High risk of productive losses | |
| ≥3000 | Clinical ketosis | Signs: decreased appetite, Body Condition Score (BCS) |
| Indicator | Type I Ketosis | Type II Ketosis | Silage Ketosis (Butyric) |
|---|---|---|---|
| Time of appearance | 3–6 weeks after calving | 1–2 weeks after calving | At any time when receiving fermented silage |
| Etiology | Negative energy balance (high milk yield, energy needs) | Insulin resistance, pre-calving obesity | High levels of butyrate in silage |
| Main reason | Lipolysis → ↑ NEFA → hepatic ketogenesis | Hepatic steatosis (fatty liver) | Increased absorption of ketone bodies from the gastrointestinal tract |
| Typical animal profile | A cow losing weight, but with a good appetite | Fat cow, with reduced appetite | A normal-looking cow that consumed bad silage |
| BHBA in the blood | ≥1.2–3.0 mmol/L | ≥1.2–3.0 mmol/L | Moderately elevated—from intake, not synthesis |
| Blood glucose | low | very high | Normal to slightly |
| NEFA (non-esterified fatty acids) | high | Strong | They do not increase significantly |
| Liver changes | Ketogenesis | Lipid infiltration (fatty liver) | Usually without steatosis |
| Propylene glycol response | Good | Bad | Average to good |
| Strategy/Focus Area | Implementation/Practical Recommendations | Expected Outcome/Notes |
|---|---|---|
| Body condition score (BCS) management | Maintain BCS 3.0–3.5 at calving; avoid overconditioning during dry period; monitor weekly prepartum | Reduces risk of type II ketosis and fatty liver; promotes optimal metabolic adaptation |
| Transition diet management | Provide consistent and balanced pre- and postpartum rations; increase dietary propionate precursors; avoid abrupt diet changes | Ensures smoother transition and stabilizes energy balance; reduces NEFA surge |
| Feed intake and housing | Provide ≥75 cm feeding space per cow; ensure feed availability 24 h/day; minimize overcrowding and heat stress | Supports higher DMI and energy intake during early lactation |
| Propylene glycol (PG) and propionate salts | Administer PG 250–350 g/day orally or via TMR for 3–5 days post-calving in high-risk cows; use Ca-propionate 50–100 g/day in TMR | Effective prevention of SCK; improves hepatic glucose output |
| Glycerin supplementation | Include 200–400 g/day in TMR or as oral drench during early lactation | Safe energy source; improves glucose availability; supports appetite |
| Controlled-release monensin capsules (CRC) | Single intraruminal bolus 3–4 weeks before calving, only in permitted regions | Decreases incidence of hyperketonemia; use under veterinary supervision |
| Mineral and vitamin support | Ensure adequate supply of cobalt, niacin, and vitamin B complex in close-up diets | Supports rumen metabolism and hepatic function |
| Herd-level metabolic monitoring | Regular milk F/P ratio monitoring and periodic BHBA/NEFA testing | Early identification of at-risk cows; enables timely intervention |
| Method (Matrix) | Diagnostic Threshold/Key Indicator | Reliability and Limitations | Recommended Use/Application |
|---|---|---|---|
| Blood BHBA | ≥1.2 mmol/L (SCK threshold); ≥3.0 mmol/L (clinical ketosis) | High—gold standard for confirmation; portable meters validated in field studies | Individual cow diagnosis; confirmation of positive herd-screening results |
| Milk BHBA | Correlates with blood BHBA; threshold ≈ 0.10–0.15 mmol/L (varies by method) | Moderate—affected by milk yield and stage of lactation | Useful for herd-level screening where milk tests (Ketotest, FTIR) are available |
| Milk fat-to-protein ratio (F/P) | F/P > 1.4 indicates increased risk; >1.6–1.8 suggests SCK or energy deficit | Moderate—influenced by diet composition and stage of lactation | Suitable for herd monitoring through automated milk recording systems |
| Urine ketone strips (acetoacetate) | Colorimetric change indicates the presence of ketones | Low–moderate—subjective and less sensitive for early SCK | Rapid field test for clinical cases; adjunctive method |
| Blood NEFA | >0.4 mmol/L (prepartum risk threshold) | High—predictive for postpartum ketosis and displaced abomasum | Preventive screening in prepartum cows (−7 to 0 days before calving) |
| Milk citrate | <8.5 mmol/L associated with energy deficit | Low—influenced by multiple factors | Supportive indicator for metabolic monitoring at the herd level |
| Therapeutic Agent/Approach | Dosage and Route of Administration | Mechanism of Action/Expected Effect | Comments/Recommendations |
|---|---|---|---|
| Propylene glycol (PG) | 250–500 g per day orally for 3–5 consecutive days | Gluconeogenic precursor; increases plasma glucose and decreases BHBA | First-line therapy for SCK and supportive treatment for clinical ketosis |
| Intravenous glucose (dextrose 50%) | 500 mL IV once daily for 1–3 days | Rapid elevation of blood glucose; transient reduction in ketone bodies | Short-term effect; combine with PG for sustained response |
| Glucocorticoids (dexamethasone, isoflupredone acetate) | Dexamethasone 10–20 mg IM once; Isoflupredone 5–10 mg IM once | Induces gluconeogenesis and appetite stimulation | Use with caution; may cause immunosuppression or induce calving if overdosed |
| Glycerin (glycerol) | 200–400 g/day orally or in TMR for 5–7 days | Alternative glucogenic substrate; stimulates insulin secretion | Safe and well tolerated; slower effect compared to PG |
| Calcium propionate | 50–100 g/day orally or in TMR for 5 days | Provides propionate for gluconeogenesis and a calcium source | Effective as adjunct preventive and supportive treatment |
| Insulin (regular insulin) | 0.25–0.5 IU/kg SC or IM, combined with glucose infusion | Enhances glucose utilization and decreases lipolysis | Used in severe clinical ketosis; requires veterinary supervision |
| Monensin (rumen modulator) | 300 mg/day via controlled-release capsule (CRC) | Alters rumen fermentation to increase propionate production | Preventive use; not recommended as a stand-alone treatment |
| B-complex vitamins (B12, niacin) | As per label dosage (commonly 10–20 mg niacin/day) | Improves hepatic metabolism and reduces fatty infiltration | Supportive therapy during the recovery phase |
| Stage/Focus | Recommended Actions (Good Veterinary Practice Protocol) | Key Parameters to Monitor | Responsible Personnel/Frequency |
|---|---|---|---|
| Prepartum (−3 to 0 weeks before calving) | -Evaluate BCS (target 3.0–3.5). -Introduce a transition diet with balanced energy density. -Administer controlled-release monensin capsule (where approved). -Monitor NEFA levels (>0.4 mmol/L = risk). | BCS, NEFA, diet composition | Veterinarian/farm nutritionist; weekly |
| Early lactation (0–21 days postpartum) | -Observe DMI and feeding behavior daily. -Screen milk F/P ratio (>1.4 = risk). -Test BHBA in blood (≥1.2 mmol/L = SCK; ≥3.0 mmol/L = CK). -Administer propylene glycol 250–350 g/day for 3–5 days in high-risk cows. | BHBA, milk F/P ratio, DMI | Herd manager/veterinarian; 2–3 times weekly |
| Clinical case management | -Confirm diagnosis with blood BHBA or NEFA. -Administer IV glucose (500 mL 50%) + PG orally for 3–5 days. -Add dexamethasone (10–20 mg IM) in severe cases. - Support with vitamin B complex. | BHBA response, clinical signs, appetite | Veterinarian; daily until recovery |
| Prevention and nutritional follow-up | -Adjust ration to ensure adequate propionate precursors (PG, glycerin, Ca-propionate). -Monitor milk production and feed intake trends. -Continue PG supplementation for at-risk multiparous cows. | Milk yield, F/P ratio, body weight | Nutritionist/herd manager; weekly |
| Herd-level monitoring and evaluation | -Record SCK prevalence and treatment outcomes. -Review metabolic test data quarterly. -Update herd protocols annually based on results and new evidence. | SCK rate, treatment success, economic losses | Veterinarian/herd consultant; quarterly |
| Category | Best Choice | Reason |
|---|---|---|
| Monitoring | BHBA in blood + F/P in milk | Highest sensitivity and practicality |
| Prevention (small herds) | Propylene glycol + BCS control | Economical and effective approach |
| Prevention (large herds) | Kexxtone + feed-grade glycerin | Long-term protection, lower labor resources |
| Treatment (mild ketosis) | Propylene glycol | Proven effect of early intervention |
| Treatment (severe ketosis) | IV glucose + insulin (veterinarian) | Rapid recovery in clinical form |
| Stage/Component | Method/Action | Purpose/Benefits | Critical Notes |
|---|---|---|---|
| Risk assessment | -BCS (3.0–3.5)- NEFA before calving (<0.3 mmol/L)- F/P <1.4 | Ketosis risk prediction | NEFA > 0.4 = risk; F/P > 1.5 = subclinical ketosis |
| Monitoring (incremental) | -BHBA in blood (≥1.2 mmol/L) -Ketone bodies in milk (ketotest) -F/P analysis -Observation: appetite, milkiness | Timely detection of subclinical and clinical ketosis | BHBA is the gold standard; F/P is easy but indirect |
| Prevention—nutrition | -Limited ration in dry period (75–80%) -Addition of straw -Gradual addition of concentrate before calving | Reducing NEB, preventing ketosis type I and II | Avoiding silage with butyric acid |
| Prevention—supplements | -Propylene glycol: 250–350 g/day -Calcium propionate: 450 g/day -Kexxtone: 3–4 weeks before calving (bolus) | Supporting gluconeogenesis, reducing βOHB | Kexxtone is effective but requires a veterinarian and precise application |
| Subclinical ketosis | -Propylene glycol (300 mL, 3–5 days) -Glycerin/propionates -Retest | Preventing progression to clinical ketosis | Without glucocorticoids |
| Clinical ketosis | -IV glucose (500 mL) -B-vitamins -Propylene glycol -Glucocorticoids (type I only) | Rapid correction of hypoglycemia, improvement of condition | Glucocorticoids are contraindicated in type II ketosis (with steatosis) |
| Tools and tests | -Precision Xtra -Ketotest -Tank milk analysis (F/P) -NEFA analysis | Precise monitoring, tracking of metabolic status | Portable devices allow field testing |
| Performance evaluation | -Increased appetite -Reduced βOHB values -Increased milk production -Documented treatment | Objective monitoring of response to treatment | Keeping individual cards and records |
| Staff training | -Recognizing symptoms -Administering a bolus -Working with a ketometer | Error reduction, early intervention | Key to successful prevention in large herds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stancheva, E.; Penev, T. Critical Analysis of Protocols for Good Veterinary Practices in Monitoring, Prevention and Treatment of Ketosis in Dairy Cows. Vet. Sci. 2025, 12, 1019. https://doi.org/10.3390/vetsci12101019
Stancheva E, Penev T. Critical Analysis of Protocols for Good Veterinary Practices in Monitoring, Prevention and Treatment of Ketosis in Dairy Cows. Veterinary Sciences. 2025; 12(10):1019. https://doi.org/10.3390/vetsci12101019
Chicago/Turabian StyleStancheva, Elena, and Toncho Penev. 2025. "Critical Analysis of Protocols for Good Veterinary Practices in Monitoring, Prevention and Treatment of Ketosis in Dairy Cows" Veterinary Sciences 12, no. 10: 1019. https://doi.org/10.3390/vetsci12101019
APA StyleStancheva, E., & Penev, T. (2025). Critical Analysis of Protocols for Good Veterinary Practices in Monitoring, Prevention and Treatment of Ketosis in Dairy Cows. Veterinary Sciences, 12(10), 1019. https://doi.org/10.3390/vetsci12101019








