Combining Load–Close–Homogenize with Testing, Removal, and Rollover Strategies to Repopulate PRRSV Elimination Breeding Herds Using PRRSV-Positive Weaned Gilts
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Laboratory Tests
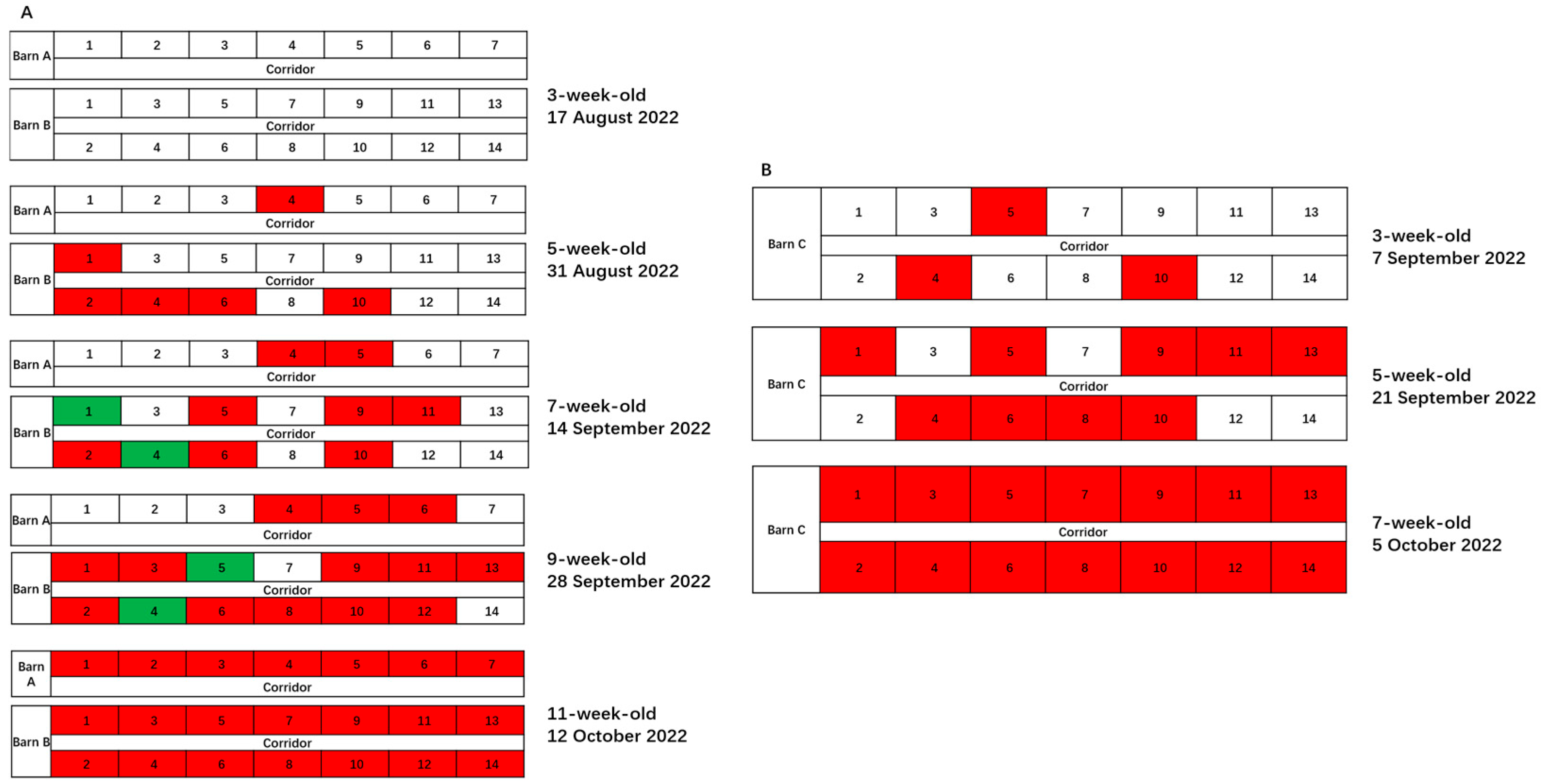
2.2. Basic Information, Roadmap, and Timeline of Our Strategy for PRRSV Elimination from Weaning Gilts
2.3. Roadmap and Timeline for PRRSV Elimination from Weaning Gilts
2.4. Pre-Elimination Evaluation
2.5. Evaluation of PRRSV Status and Genetic Diversity in Supplier Farms and Two Weaned Gilt Batches
2.6. Monitoring PRRSV Infection Dynamics in Two of Three Weaning Gilt Batches
2.7. LVI Material Preparation and Homogenized Infection
2.8. Monitoring Strategy from LVI to PRRSV Status 2 Achievement
2.9. Monitoring Strategy from PRRSV Herd Status 2 to Status 4 Achievement
2.10. Descriptive Statistics
3. Results
3.1. Pre-Elimination Evaluation at JS
3.2. PRRSV Status Evaluation and ORF 5 Sequencing Analysis at SG and FK
3.3. PRRSV Infection Dynamics in Two Weaning Gilt Batches Introduced from SS
3.4. Homogenized Infection and Herd PRRSV Shedding Status Evaluation
3.5. Achievement of PRRSV Stable State in Breeding Herd
3.6. Breeding Herd Status 4 Achievement After T&R and Rollover
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRRS | Porcine reproductive and respiratory syndrome |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| LVI | Live virus inoculation |
| MLV | Modified live vaccine |
| LCH | Load close and homogenize |
| T&R | Test and removal |
| ELISA | Enzyme-linked immunosorbent assay |
| PCR | Polymerase chain reaction |
| TTF | Tongue tip fluids |
| PF | Processing fluids |
| OF | Oral fluids |
| TTS | Time to stability |
| TTBP | Time to baseline productivity |
| TL | Total loss |
| AASV | American Association of Swine Veterinarians |
| ADWG | Average daily weight gain |
| ORF | Open reading frame |
| DPTRA | Days post T&R accomplished |
| DPI | Days post introduction |
| WPC | Weeks post challenged |
References
- Benfield, D.A.; Nelson, E.; Collins, J.E.; Harris, L.; Goyal, S.M.; Robison, D.; Christianson, W.T.; Morrison, R.B.; Gorcyca, D.; Chladek, D. Characterization of Swine Infertility and Respiratory Syndrome (Sirs) Virus (Isolate Atcc Vr-2332). J. Vet. Diagn. Investig. 1992, 4, 127–133. [Google Scholar] [CrossRef]
- Brinton, M.A.; Gulyaeva, A.A.; Balasuriya, U.B.R.; Dunowska, M.; Faaberg, K.S.; Goldberg, T.; Leung, F.C.C.; Nauwynck, H.J.; Snijder, E.J.; Stadejek, T.; et al. Ictv Virus Taxonomy Profile: Arteriviridae 2021. J. Gen. Virol. 2021, 102, 001632. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zheng, J.; Qiu, Y.; Chen, C.; Li, Q.; Wu, Q.; Lin, L.; Zhao, H.; Zhou, Q.; Gong, L.; et al. Isolation, Identification, and Pathogenicity of a Nadc30-Like Porcine Reproductive and Respiratory Disorder Syndrome Virus Strain Affecting Sow Production. Front. Vet. Sci. 2023, 10, 1207189. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, C.; Hu, Y.; Fang, S.; Li, X.; Zhang, C.; Huang, L.; Qian, J.; Wang, G.; Fan, A.; et al. Protective Evaluation of the Commercialized Porcine Reproductive and Respiratory Syndrome Virus Vaccines in Piglets Challenged by Nadc34-Like Strain. Front. Microbiol. 2024, 15, 1422335. [Google Scholar] [CrossRef]
- Shi, M.; Lam, T.T.; Hon, C.C.; Hui, R.K.; Faaberg, K.S.; Wennblom, T.; Murtaugh, M.P.; Stadejek, T.; Leung, F.C. Molecular Epidemiology of Prrsv: A Phylogenetic Perspective. Virus Res. 2010, 154, 7–17. [Google Scholar] [CrossRef]
- Li, C.; Fan, A.; Liu, Z.; Wang, G.; Zhou, L.; Zhang, H.; Huang, L.; Zhang, J.; Zhang, Z.; Zhang, Y. Prevalence, Time of Infection, and Diversity of Porcine Reproductive and Respiratory Syndrome Virus in China. Viruses 2024, 16, 774. [Google Scholar] [CrossRef]
- Paiva, R.C.; Rademacher, C.; Peterson, T.; Silva, A.; Silva, G.S.; Linhares, D.C.; Trevisan, G. Description of Practices Adopted in Response to Porcine Reproductive and Respiratory Syndrome Outbreaks among Breeding Herds in the United States from 2019–2021. J. Swine Health Prod. 2024, 32, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, D.J.; Kliebenstein, J.B.; Neumann, E.J. Assessment of the Economic Impact of Porcine Reproductive and Respiratory Syndrome Virus on United States Pork Producers. J. Swine Health Prod. 2013, 21, 72–84. [Google Scholar]
- Schelkopf, A.; Nerem, J.; Cowles, B.; Amodie, D.; Swalla, R.; Dee, S. Reproductive, Productivity, and Mortality Outcomes in Late-Gestation Gilts and Their Litters Following Simulation of Inadvertent Exposure to a Modified-Live Vaccine Strain of Porcine Reproductive and Respiratory Syndrome (Prrs) Virus. Vaccine 2014, 32, 4639–4643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Z.; Li, H.; Yang, S.; Ren, F.; Bian, T.; Sun, L.; Zhou, B.; Zhou, L.; Qu, X. The Economic Impact of Porcine Reproductive and Respiratory Syndrome Outbreak in Four Chinese Farms: Based on Cost and Revenue Analysis. Front. Vet. Sci. 2022, 9, 1024720. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, T.; Lai, R.; Ding, Z.; Zhuang, Y.; Liu, H.; Cao, H.; Gao, X.; Luo, J.; Chen, Z.; et al. Prrsv Elimination in a Farrow-to-Finish Pig Herd Using Herd Closure and Rollover Approach. Viruses 2023, 15, 1239. [Google Scholar] [CrossRef]
- Morrison Swine Health Monitoring Project. Available online: https://mshmp.umn.edu/reports#Charts (accessed on 31 October 2024).
- Kikuti, M.; Picasso-Risso, C.; Melini, C.M.; Corzo, C.A. Time Farms Stay Naive for Porcine Reproductive and Respiratory Syndrome. Animals 2023, 13, 310. [Google Scholar] [CrossRef]
- Linhares, D.; Johnson, C.; Morrison, R.B. Correction: Economic Analysis of Vaccination Strategies for Prrs Control. PLoS ONE 2016, 11, e0150444. [Google Scholar]
- Corzo, C.A.; Mondaca, E.; Wayne, S.; Torremorell, M.; Dee, S.; Davies, P.; Morrison, R.B. Control and Elimination of Porcine Reproductive and Respiratory Syndrome Virus. Virus Res. 2010, 154, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Nathues, H.; Alarcon, P.; Rushton, J.; Jolie, R.; Fiebig, K.; Jimenez, M.; Geurts, V.; Nathues, C. Modelling the Economic Efficiency of Using Different Strategies to Control Porcine Reproductive & Respiratory Syndrome at Herd Level. Prev. Vet. Med. 2018, 152, 89–102. [Google Scholar] [PubMed]
- Dee, S.A.; Molitor, T.W. Elimination of Porcine Reproductive and Respiratory Syndrome Virus Using a Test and Removal Process. Vet. Rec. 1998, 143, 474–476. [Google Scholar] [CrossRef]
- Rathkjen, P.H.; Dall, J. Control and Eradication of Porcine Reproductive and Respiratory Syndrome Virus Type 2 Using a Modified-Live Type 2 Vaccine in Combination with a Load, Close, Homogenise Model: An Area Elimination Study. Acta Vet. Scand. 2017, 59, 4. [Google Scholar] [CrossRef]
- Trevisan, G.; Johnson, C.; Benjamin, N.; Bradner, L.; Linhares, D.C.L. Description of Changes of Key Performance Indicators and Prrsv Shedding over Time in a Naive Breeding Herd Following a Prrs Mlv Exposure. Transbound. Emerg. Dis. 2021, 68, 3230–3235. [Google Scholar] [CrossRef]
- Linhares, D.C.; Cano, J.P.; Torremorell, M.; Morrison, R.B. Comparison of Time to Prrsv-Stability and Production Losses between Two Exposure Programs to Control Prrsv in Sow Herds. Prev. Vet. Med. 2014, 116, 111–119. [Google Scholar] [CrossRef]
- Holtkamp, D.; Torremorell, M.; Corzo, C.A.; Linhares, D.C.L.; Almeida, M.N.; Yeske, P.; Polson, D.D.; Becton, L.; Snelson, H.; Donovan, T.; et al. Proposed Modifications to Porcine Reproductive and Respiratory Syndrome Virus Herd Classification. J. Swine Health Prod. 2021, 29, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Google Map. Available online: https://www.google.com.hk/maps/ (accessed on 15 May 2022).
- Weather Report. Available online: https://tianqi.2345.com/ (accessed on 20 May 2022).
- Kikuti, M.; Vilalta, C.; Sanhueza, J.; Pamornchainavakul, N.; Kevill, J.; Yang, M.; Paploski, I.A.D.; Lenskaia, T.; Odogwu, N.M.; Kiehne, R.; et al. Porcine Reproductive and Respiratory Syndrome (Prrsv2) Viral Diversity within a Farrow-to-Wean Farm Cohort Study. Viruses 2023, 15, 1837. [Google Scholar] [CrossRef]
- Khaled Fahim, N.; Negida, A. Sample Size Calculation Guide—Part 1: How to Calculate the Sample Size Based on the Prevalence Rate. Adv. J. Emerg. Med. 2018, 2, e50. [Google Scholar]
- Moura, C.A.A.; Philips, R.; Silva, G.S.; Holtkamp, D.J.; Linhares, D.C.L. Comparison of Virus Detection, Productivity, and Economic Performance between Lots of Growing Pigs Vaccinated with Two Doses or One Dose of Prrs Mlv Vaccine, under Field Conditions. Prev. Vet. Med. 2022, 204, 105669. [Google Scholar] [CrossRef]
- Henao-Diaz, A.; Gimenez-Lirola, L.; Baum, D.H.; Zimmerman, J. Guidelines for Oral Fluid-Based Surveillance of Viral Pathogens in Swine. Porcine Health Manag. 2020, 6, 28. [Google Scholar] [CrossRef]
- Opriessnig, T.; Baker, R.B.; Halbur, P.G. Use of an Experimental Model to Test the Efficacy of Planned Exposure to Live Porcine Reproductive and Respiratory Syndrome Virus. Clin. Vaccine Immunol. 2007, 14, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.F.; Magalhaes, E.S.; Silva, A.P.S.P.; Moraes, D.C.A.; Cezar, G.; Mil-Homens, M.P.; Osemeke, O.H.; Paiva, R.; Moura, C.A.A.; Gauger, P.; et al. Porcine Reproductive and Respiratory Syndrome Virus Rna Detection in Tongue Tips from Dead Animals. Front. Vet. Sci. 2022, 9, 993442. [Google Scholar] [CrossRef] [PubMed]
- Houben, S.; van Reeth, K.; Pensaert, M.B. Pattern of Infection with the Porcine Reproductive and Respiratory Syndrome Virus on Swine Farms in Belgium. J. Vet. Med. Ser. B 1995, 42, 209–215. [Google Scholar] [CrossRef]
- Yang, J.S.; Moon, H.J.; Lee, C.S.; Park, S.J.; Song, D.S.; Kang, B.K.; Choi, J.U.; Park, B.K. Elimination of Porcine Reproductive and Respiratory Syndrome Virus from a Seedstock Breeding Farm and a Supplying Boar Stud by a Modified Test and Removal Method. Vet. Rec. 2008, 162, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Pertich, A.; Barna, Z.; Makai, O.; Farkas, J.; Molnar, T.; Balint, A.; Szabo, I.; Albert, M. Elimination of Porcine Reproductive and Respiratory Syndrome Virus Infection Using an Inactivated Vaccine in Combination with a Roll-over Method in a Hungarian Large-Scale Pig Herd. Acta Vet. Scand. 2022, 64, 12. [Google Scholar] [CrossRef]
- Rowland, R.R.; Morrison, R.B. Challenges and Opportunities for the Control and Elimination of Porcine Reproductive and Respiratory Syndrome Virus. Transbound. Emerg. Dis. 2012, 59 (Suppl. S1), 55–59. [Google Scholar] [CrossRef]
- China’s Pig Inventory to Grow Marginally Due to Recovery in Sow Numbers. Available online: https://www.pig333.com/latest_swine_news/chinas-pig-inventory-to-grow-marginally-due-to-recovery-in-sow-herd_20747/ (accessed on 10 June 2022).
- Sanchez, F.; Galvis, J.A.; Cardenas, N.C.; Corzo, C.; Jones, C.; Machado, G. Spatiotemporal Relative Risk Distribution of Porcine Reproductive and Respiratory Syndrome Virus in the United States. Front. Vet. Sci. 2023, 10, 1158306. [Google Scholar] [CrossRef] [PubMed]
- Moeller, J.; Mount, J.; Geary, E.; Campler, M.R.; Corzo, C.A.; Morrison, R.B.; Arruda, A.G. Investigation of the Distance to Slaughterhouses and Weather Parameters in the Occurrence of Porcine Reproductive and Respiratory Syndrome Outbreaks in U.S. Swine Breeding Herds. Can. Vet. J. 2022, 63, 528–534. [Google Scholar]
- Arruda, A.G.; Vilalta, C.; Perez, A.; Morrison, R. Land Altitude, Slope, and Coverage as Risk Factors for Porcine Reproductive and Respiratory Syndrome (Prrs) Outbreaks in the United States. PLoS ONE 2017, 12, e0172638. [Google Scholar] [CrossRef]
- Yoon, I.J.; Soojoo, H.; Christianson, W.T.; Morrison, R.B.; Dial, G.D. Persistent and Contact Infection in Nursery Pigs Experimentally Infected with Porcine Reproductive and Respiratory Syndrome Virus. Swine Health Prod. 1993, 1, 5–8. [Google Scholar]
- Christianson, W.T.; Choi, C.S.; Collins, J.E.; Molitor, T.W.; Morrison, R.B.; Joo, H.S. Pathogenesis of Porcine Reproductive and Respiratory Syndrome Virus Infection in Mid-Gestation Sows and Fetuses. Can. J. Vet. Res. 1993, 57, 262–268. [Google Scholar]
- Galvis, J.A.; Corzo, C.A.; Machado, G. Modelling and Assessing Additional Transmission Routes for Porcine Reproductive and Respiratory Syndrome Virus: Vehicle Movements and Feed Ingredients. Transbound. Emerg. Dis. 2022, 69, e1549–e1560. [Google Scholar] [CrossRef]
- Sanz-Fernandez, S.; Diaz-Gaona, C.; Simoes, J.; Casas-Rosal, J.C.; Alos, N.; Tusell, L.; Quintanilla, R.; Rodriguez-Estevez, V. The Impact of Herd Age Structure on the Performance of Commercial Sow-Breeding Farms. Porcine Health Manag. 2024, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.M.; Medley, G.F.; Creasey, S.J.; Green, L.E. A Stochastic Mathematical Model of the within-Herd Transmission Dynamics of Porcine Reproductive and Respiratory Syndrome Virus (Prrsv): Fade-out and Persistence. Prev. Vet. Med. 2010, 93, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Torrents, D.; Miranda, J.; Gauger, P.C.; Ramirez, A.; Linhares, D. Effect of Prrsv Stability on Productive Parameters in Breeding Herds of a Swine Large Integrated Group in Spain. Porcine Health Manag. 2021, 7, 21. [Google Scholar] [CrossRef]
- Trevisan, G.; Zeller, M.; Li, G.; Zhang, J.; Gauger, P.; Linhares, D.C.L. Implementing a User-Friendly Format to Analyze Prrsv Next-Generation Sequencing Results and Associating Breeding Herd Production Performance with Number of Prrsv Strains and Recombination Events. Transbound. Emerg. Dis. 2022, 69, e2214–e2229. [Google Scholar] [CrossRef]
- Wu, Z.; Chang, T.; Wang, D.; Zhang, H.; Liu, H.; Huang, X.; Tian, Z.; Tian, X.; Liu, D.; An, T.; et al. Genomic Surveillance and Evolutionary Dynamics of Type 2 Porcine Reproductive and Respiratory Syndrome Virus in China Spanning the African Swine Fever Outbreak. Virus Evol. 2024, 10, veae016. [Google Scholar] [CrossRef]
- Hodges, A.L.; Walker, L.R.; Everding, T.; Mote, B.E.; Vu, H.L.X.; Ciobanu, D.C. Metagenomic Detection and Genome Assembly of Novel Prrsv-2 Strain Using Oxford Nanopore Flongle Flow Cell. J. Anim. Sci. 2025, 103, skae395. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Chen, K.; Qian, J.; Hu, Y.; Fang, S.; Sun, Z.; Zhang, C.; Huang, L.; Zhang, J.; et al. Efficacy of the Synergy between Live-Attenuated and Inactivated Prrsv Vaccines against a Nadc30-Like Strain of Porcine Reproductive and Respiratory Syndrome Virus in 4-Week Piglets. Front. Vet. Sci. 2022, 9, 812040. [Google Scholar] [CrossRef]
- Sartwell, P.E. Memoir on the Reed-Frost Epidemic Theory. Am. J. Epidemiol. 1976, 103, 138–140. [Google Scholar] [CrossRef]
- Vaccination Against Prrsv: A Practical Approach. Available online: https://www.pig333.com/articles/vaccination-against-porcine-reproductive-and-respiratory-syndrome-viru_7485/ (accessed on 20 July 2022).
- Oh, T.; Kim, H.; Park, K.H.; Jeong, J.; Yang, S.; Kang, I.; Chae, C. Comparison of Four Commercial Prrsv Mlv Vaccines in Herds with Co-Circulation of Prrsv-1 and Prrsv-2. Comp. Immunol. Microbiol. Infect. Dis. 2019, 63, 66–73. [Google Scholar] [CrossRef]
- Moura, C.A.A.; Philips, R.; Silva, G.S.; Ramirez, A.; Gauger, P.C.; Holtkamp, D.J.; Linhares, D.C.L. Association of Wild-Type Prrsv Detection Patterns with Mortality of Mlv-Vaccinated Growing Pig Groups. Prev. Vet. Med. 2021, 189, 105270. [Google Scholar] [CrossRef] [PubMed]
- Prickett, J.; Simer, R.; Christopher-Hennings, J.; Yoon, K.J.; Evans, R.B.; Zimmerman, J.J. Detection of Porcine Reproductive and Respiratory Syndrome Virus Infection in Porcine Oral Fluid Samples: A Longitudinal Study under Experimental Conditions. J. Vet. Diagn. Investig. 2008, 20, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Benfield, D.; Nelsona, J.; Rossow, K.; Nelsona, C.; Steffen, M.; Rowland, R. Diagnosis of Persistent or Prolonged Porcine Reproductive and Respiratory Syndrome Virus Infections. Vet. Res. 2000, 31, 71. [Google Scholar] [CrossRef]
- Wilta, F.; Chong, A.L.C.; Selvachandran, G.; Kotecha, K.; Ding, W. Generalized Susceptible-Exposed-Infectious-Recovered Model and Its Contributing Factors for Analysing the Death and Recovery Rates of the COVID-19 Pandemic. Appl. Soft Comput. 2022, 123, 108973. [Google Scholar] [CrossRef]
- Angulo, J.; Yang, M.; Rovira, A.; Davies, P.R.; Torremorell, M. Infection Dynamics and Incidence of Wild-Type Porcine Reproductive and Respiratory Syndrome Virus in Growing Pig Herds in the U.S. Midwest. Prev. Vet. Med. 2023, 217, 105976. [Google Scholar] [CrossRef]
- Batista, L.; Dee, S.A.; Rossow, K.D.; Deen, J.; Pijoan, C. Assessing the Duration of Persistence and Shedding of Porcine Reproductive and Respiratory Syndrome Virus in a Large Population of Breeding-Age Gilts. Can. J. Vet. Res. 2002, 66, 196–200. [Google Scholar] [PubMed]
- Karniychuk, U.U.; Nauwynck, H.J. Pathogenesis and Prevention of Placental and Transplacental Porcine Reproductive and Respiratory Syndrome Virus Infection. Vet. Res. 2013, 44, 95. [Google Scholar] [CrossRef] [PubMed]
- Klinge, K.L.; Vaughn, E.M.; Roof, M.B.; Bautista, E.M.; Murtaugh, M.P. Age-Dependent Resistance to Porcine Reproductive and Respiratory Syndrome Virus Replication in Swine. Virol. J. 2009, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Dee, S.A.; Bierk, M.D.; Deen, J.; Molitor, T.W. An Evaluation of Test and Removal for the Elimination of Porcine Reproductive and Respiratory Syndrome Virus from 5 Swine Farms. Can. J. Vet. Res. 2001, 65, 22–27. [Google Scholar]




| Sample Type | Pig Age (Days) | No. of Samples | Pooling Strategies |
|---|---|---|---|
| PF | 3–5 | 3–6 | Pooling of samples from every 30–50 L |
| Serum | 21–25 | 30 | Pooling of five samples |
| OF | 42–46 | 6 | - |
| Sample Type | No. of Samples | ELISA or PCR | Sampling Timepoint | Objective |
|---|---|---|---|---|
| OF | All pens | PCR | 12–24 h after challenge | Evaluating intervention efficiency |
| Serum | ≥30 | ELISA | 4 weeks after challenge | Assessing infection rate |
| OF | All pens | PCR | Every 4 weeks until all pens became qPCR negative | Verifying shedding termination in the herd |
| Serum | ≥30 | ELISA | Every 3 weeks until T&R end (excluding some exceptions) | Monitoring exposure status dynamics |
| Sample Type | Piglet Age (Days) | No. of Samples | Pooling Strategy | PCR or ELISA |
|---|---|---|---|---|
| TTF | Stillborn | 2 | 20–100 tongue tips per sample | PCR |
| PF | 3–5 | 7–8 | 30–50 L per sample | PCR |
| Serum | 21–25 | 120–240 | 5 pigs per pool | PCR |
| Serum | 56 | 120 | - | ELISA |
| Serum | Perinatal sows | 60 | 5 sows per pool | PCR |
| Herd | Sample Type | No. of Samples | ELISA or PCR | Sampling Timepoint | Objective |
|---|---|---|---|---|---|
| Weaned sows | Serum | All weaned sows in a batch | ELISA | After weaning | Screening antibody positivity in sows for replacement with naïve gilts |
| Sentinel gilts | Serum | 30 | ELISA | 0, 30, and 60 days after commingling with sow herd | Ensuring achievement of herd status 3, according to AASV PRRS classification |
| Sows | Serum | 60 | ELISA | Accomplishment of whole-herd R&T; randomly selection of 60 sows for ELISA testing 30 and 90 days after T&R and rollover | Verifying achievement of herd status 4 |
| Batch No. | Sample Type | No. of Positive qPCR Results | No. of qPCR Results | Percentage of Positive qPCR Results | PRRSV Lineage |
|---|---|---|---|---|---|
| 20220514 | PF | 0 | 4 | 0% | - |
| Weaning serum | 5 | 6 | 83% | 5 | |
| Nursery serum | 6 | 6 | 100% | 5 | |
| 20220612 | PF | 0 | 6 | 0% | - |
| Weaning serum | 7 | 12 | 58% | 5 | |
| Nursery serum | 6 | 6 | 100% | 3, 8.7 | |
| 20220626 | PF | 0 | 4 | 0% | - |
| Weaning serum | 3 | 6 | 50% | 8.7 | |
| Nursery serum | 6 | 6 | 100% | 5 |
| Batch No. | Sample Type | No. of Positive qPCR Results | No. of qPCR Results | Percentage of Positive qPCR Results | PRRSV Lineage |
|---|---|---|---|---|---|
| 20220514 | PF | 0 | 3 | 0% | - |
| Weaning serum | 6 | 6 | 100% | 8.7 | |
| Nursery serum | 6 | 6 | 100% | 5 | |
| 20220612 | PF | 1 | 6 | 17% | FALSE |
| Weaning serum | 3 | 12 | 25% | 8.7 | |
| Nursery serum | 5 | 6 | 83% | 5 | |
| 20220626 | PF | 0 | 6 | 0% | - |
| Weaning serum | 1 | 6 | 17% | 8.7 | |
| Nursery serum | 6 | 6 | 100% | 8.7 |
| 4 WPC 1 | 16 WPC | 19 WPC | 22 WPC | 25 WPC | 28 WPC | 31 WPC | 44 WPC | 57 WPC | 60 WPC | 63 WPC | 66 WPC | 69 WPC | 72 WPC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of positive samples | 366 | 29 | 43 | 53 | 51 | 49 | 45 | 31 | 10 | 12 | 10 | 7 | 8 | 4 |
| No. of samples | 369 | 30 | 60 | 60 | 61 | 61 | 61 | 120 | 90 | 119 | 158 | 84 | 100 | 122 |
| Percentage of positive samples | 99% a | 97% a | 72% b | 88% b | 84% b | 80% b | 74% b | 26% c | 11.1% d | 10.1% d | 6.3% d | 8.3% d | 8.0% d | 3.3% e |
| Batch | Birthdate | Sample Type | No. of Positive qPCR Results | No. of qPCR Results | Percentage of Positive qPCR Results |
|---|---|---|---|---|---|
| Batch 1 | 4 July 2023 | TTF | 0 | 2 | 0% |
| PF | 0 | 7 | 0% | ||
| Serum | 0 | 48 | 0% | ||
| Batch 2 | 25 July 2023 | TTF | 0 | 2 | 0% |
| PF | 0 | 7 | 0% | ||
| Serum | 0 | 24 | 0% | ||
| Batch 3 | 15 August 2023 | TTF | 0 | 2 | 0% |
| PF | 0 | 7 | 0% | ||
| Serum | 0 | 24 | 0% | ||
| Batch 4 | 5 September 2023 | TTF | 0 | 2 | 0% |
| PF | 0 | 8 | 0% | ||
| Serum | - | - | - |
| Batch | No. of Positive qPCR Results | No. of qPCR Results | Percentage of Positive qPCR Results |
|---|---|---|---|
| Batch 1 | 0 | 6 | 0% |
| Batch 2 | 0 | 12 | 0% |
| Batch 3 | 0 | 12 | 0% |
| Batch 4 | 0 | 12 | 0% |
| Batch 5 | 0 | 12 | 0% |
| Batch 6 | 0 | 12 | 0% |
| Batch 7 | 0 | 12 | 0% |
| Batch | Mean S/P Value | No. of Positive Samples | No. of Samples | Percentage of Positive Samples |
|---|---|---|---|---|
| Batch 1 | 0.11 | 1 | 120 | 1.67% |
| Batch 2 | −0.14 | 0 | 120 | 0% |
| Batch 3 | −0.12 | 0 | 120 | 0% |
| Batch 4 | 0.03 | 0 | 120 | 0% |
| 0 DPI 1 | 30 DPI | 60 DPI | |
|---|---|---|---|
| Mean S/P | −0.08 | −0.08 | −0.07 |
| CV | 0.11 | 0.07 | 0.11 |
| Batch | Mean S/P Value | No. of Positive Samples | No. of Samples | Percentage of Positive Samples |
|---|---|---|---|---|
| Batch 1 | 0.077 | 11 | 90 | 12.2% a |
| Batch 2 | 0.003 | 12 | 119 | 10.1% a |
| Batch 3 | 0.015 | 10 | 108 | 9.3% ab |
| Batch 4 | 0.023 | 10 | 158 | 6.3% ab |
| Batch 5 | 0.055 | 7 | 84 | 8.3% ab |
| Batch 6 | 0.013 | 8 | 100 | 8.0% ab |
| Batch 7 | −0.013 | 4 | 122 | 3.3% b |
| 30 Days After T&R and Rollover | 90 Days After T&R and Rollover | |
|---|---|---|
| Mean S/P value | −0.01 | 0.02 |
| CV | 0.14 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Zhao, K.; Wu, G.; Hong, H.; Xia, T.; Liu, Z.; Wang, Y.; Sun, C.; Li, C.; Zhang, Z.; et al. Combining Load–Close–Homogenize with Testing, Removal, and Rollover Strategies to Repopulate PRRSV Elimination Breeding Herds Using PRRSV-Positive Weaned Gilts. Vet. Sci. 2025, 12, 1012. https://doi.org/10.3390/vetsci12101012
Hu Y, Zhao K, Wu G, Hong H, Xia T, Liu Z, Wang Y, Sun C, Li C, Zhang Z, et al. Combining Load–Close–Homogenize with Testing, Removal, and Rollover Strategies to Repopulate PRRSV Elimination Breeding Herds Using PRRSV-Positive Weaned Gilts. Veterinary Sciences. 2025; 12(10):1012. https://doi.org/10.3390/vetsci12101012
Chicago/Turabian StyleHu, Yulong, Kangning Zhao, Guangqiang Wu, Haozhou Hong, Tian Xia, Zhicheng Liu, Yijuan Wang, Chunqing Sun, Chaosi Li, Zhendong Zhang, and et al. 2025. "Combining Load–Close–Homogenize with Testing, Removal, and Rollover Strategies to Repopulate PRRSV Elimination Breeding Herds Using PRRSV-Positive Weaned Gilts" Veterinary Sciences 12, no. 10: 1012. https://doi.org/10.3390/vetsci12101012
APA StyleHu, Y., Zhao, K., Wu, G., Hong, H., Xia, T., Liu, Z., Wang, Y., Sun, C., Li, C., Zhang, Z., & Zhang, J. (2025). Combining Load–Close–Homogenize with Testing, Removal, and Rollover Strategies to Repopulate PRRSV Elimination Breeding Herds Using PRRSV-Positive Weaned Gilts. Veterinary Sciences, 12(10), 1012. https://doi.org/10.3390/vetsci12101012






