Application of Probiotics in Cats and Dogs: Benefits and Mechanisms
Abstract
Simple Summary
Abstract
1. Introduction
2. Benefits of Probiotics on Pets
2.1. Gut Health
| Probiotics | Animals | Intestinal Benefits | References |
|---|---|---|---|
| Both Saccharomyces boulardii and Pediococcus acidilactici | Cat, n = 10 | Promoted beneficial bacterial colonization, elevated fecal antioxidants, and reduced inflammatory markers. | [5] |
| E. coli Nissle 1917 | Dog, n = 38 | Improved stool consistency and reduced duration of diarrhea. | [7] |
| Lactobacillus sakei | Dog, n = 16 | Regulated the gut microbiota balance and enhanced metabolic function. | [8] |
| B. longum KACC 91563 | Dog, n = 12 | Enhanced the fecal microbiota and immune response. | [9] |
| Bifidobacterium longum CECT-7347 (heat-treated) combined with Fibersol-2 | Cat, n = 12 | Anti-inflammatory and antioxidant. | [10] |
| Saccharomyces cerevisiae | Dog, n = 16 | Improved the dysbiosis index, significantly increased the abundances of Bifidobacterium and Turicibacter, and decreased the abundance of Escherichia coli in feces. | [11] |
| Enterococcus faecium Strain SF68 | Cat, n = 25 | Reduced clinical symptoms of vomiting and diarrhea. | [12] |
2.2. Obesity and Nutrient Metabolism
2.3. Nutrient Digestibility
| Probiotics | Animals | Effects | Reference |
|---|---|---|---|
| Lactobacillus Johnson CPN23 and Lactobacillus acidophilus NCDC15 | dogs | Improving the digestibility of fiber. | [33] |
| Lactobacillus | cats | Increasing crude protein digestibility, improving nutrient digestion, and reducing fecal odors. | [34] |
| Bacillus subtilis C-3102 | dogs | An upward trend in the apparent digestibility of crude fat and nitrogen-free extracts was observed. | [36] |
| Bacillus coagulans | dogs | Increasing the apparent digestibility of organic matter, crude protein, crude fat, and total energy in dogs. | [37] |
| Bacillus amyloidis SC06 and Bacillus subtilis B10 | cats | Improving the apparent digestibility of nutrients. | [39] |
2.4. Clinical Diseases
2.5. Limitations of Probiotic Application
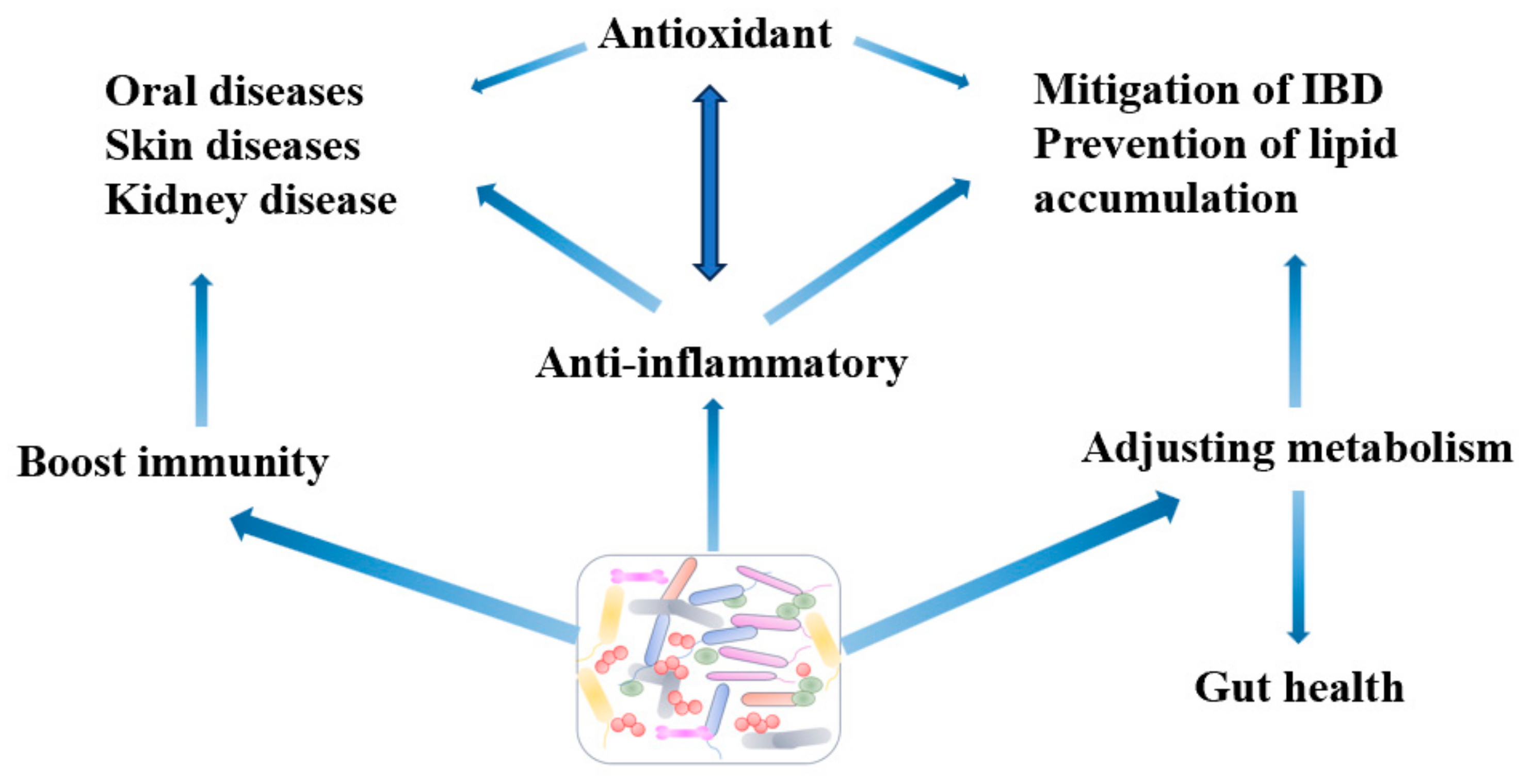
3. Mechanism
3.1. Antivirus
3.2. Anti-Inflammation
3.3. Immunity
3.4. Antioxidant
3.5. Adjusting Metabolism and Gut Microbiota Balance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paßlack, N.; Kohn, B.; Vahjen, W.; Zentek, J. Effects of dietary cellobiose on the intestinal microbiota and excretion of nitrogen metabolites in healthy adult dogs. J. Anim. Physiol. Anim. Nutr. 2021, 105, 569–578. [Google Scholar] [CrossRef]
- Halloran, K.; Underwood, M.A. Probiotic mechanisms of action. Early Hum. Dev. 2019, 135, 58–65. [Google Scholar] [CrossRef]
- Xu, H.; Wu, Y.P.; Mei, X.Q.; Xu, B.C.; Zhao, F.K.; Li, W. Research progress of probiotics effects on animal digestion, absorption and liver metabolism of nutrients. Chin. J. Anim. Nutr. 2018, 30, 3850–3856. (In Chinese) [Google Scholar]
- Kiepś, J.; Dembczyński, R. Current Trends in the Production of Probiotic Formulations. Foods 2022, 11, 2330. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ali, I.; Lei, Z.; Li, Y.; Yang, M.; Yang, C.; Li, L. Effect of a Multistrain Probiotic on Feline Gut Health through the Fecal Microbiota and Its Metabolite SCFAs. Metabolites 2023, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, Y.; Wang, H.; Zhang, H.; Chen, W.; Lu, W. Lactic acid bacteria-derived exopolysaccharide: Formation, immunomodulatory ability, health effects, and structure-function relationship. Microbiol. Res. 2023, 274, 127432. [Google Scholar] [CrossRef] [PubMed]
- Rudinsky, A.J.; Harrison, A.; Shi, B.; Hardison, R.; Prinster, T.; Huang, S.; Lee, S.; Byron, J.K.; Lucas, E.; Mason, K.M.; et al. The use of Escherichia coli strain Nissle 1917 shows promise for improving gastrointestinal and urinary health in dogs. Am. J. Vet. Res. 2023, 84, ajvr.23.03.0055. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Zhu, X.; Zhao, Y.; Iqbal, M.; Lin, Z.; Nawaz, S.; Xu, M.; Hu, M.; Bhutto, Z.A.; et al. The Effect of Lactobacillus sakei on Growth Performance and Intestinal Health in Dogs: Gut Microbiota and Metabolism Study. Probiot. Antimicrob. Proteins 2024, 16, 2116–2131. [Google Scholar] [CrossRef]
- Park, H.E.; Kim, Y.J.; Kim, M.; Kim, H.; Do, K.H.; Kim, J.K.; Ham, J.S.; Lee, W.K. Effects of Queso Blanco cheese containing Bifidobacterium longum KACC 91563 on fecal microbiota, metabolite and serum cytokine in healthy beagle dogs. Anaerobe 2020, 64, 102234. [Google Scholar] [CrossRef]
- Wang, F.; Gao, S.; Peng, Q.; Tan, L.; Chen, S.; Xia, Z. Effects of Heat-Treated Bifidobacterium longum CECT-7384 Combined with Fibersol-2 on the Intestinal Health of Cats Submitted to an Abrupt Dietary Change: A Randomized Controlled Study. Animals 2024, 14, 2179. [Google Scholar] [CrossRef]
- Bastos, T.S.; Souza, C.M.M.; Legendre, H.; Richard, N.; Pilla, R.; Suchodolski, J.S.; de Oliveira, S.G.; Lesaux, A.A.; Félix, A.P. Effect of Yeast Saccharomyces cerevisiae as a Probiotic on Diet Digestibility, Fermentative Metabolites, and Composition and Functional Potential of the Fecal Microbiota of Dogs Submitted to an Abrupt Dietary Change. Microorganisms 2023, 11, 506. [Google Scholar] [CrossRef]
- Torres-Henderson, C.; Summers, S.; Suchodolski, J.; Lappin, M.R. Effect of Enterococcus Faecium Strain SF68 on Gastrointestinal Signs and Fecal Microbiome in Cats Administered Amoxicillin-Clavulanate. Top. Companion Anim. Med. 2017, 32, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Osto, M.; Lutz, T.A. Translational value of animal models of obesity-Focus on dogs and cats. Eur. J. Pharmacol. 2015, 759, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Salas-Mani, A.; Jeusette, I.; Castillo, I.; Manuelian, C.L.; Lionnet, C.; Iraculis, N.; Sanchez, N.; Fernández, S.; Vilaseca, L. Fecal microbiota composition changes after a BW loss diet in Beagle dogs. J. Anim. Sci. 2018, 96, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Yeon, K.D.; Kim, S.M.; Kim, J.H. Association between Gut Microbiota and Metabolic Health and Obesity Status in Cats. Animals 2024, 14, 2524. [Google Scholar] [CrossRef]
- Kim, H.; Seo, J.; Park, T.; Seo, K.; Cho, H.W.; Chun, J.L.; Kim, K.H. Obese dogs exhibit different fecal microbiome and specific microbial networks compared with normal weight dogs. Sci. Rep. 2023, 13, 723. [Google Scholar] [CrossRef]
- Kang, A.; Kwak, M.-J.; Lee, D.J.; Lee, J.J.; Kim, M.K.; Song, M.; Lee, M.; Yang, J.; Oh, S.; Kim, Y. Dietary supplementation with probiotics promotes weight loss by reshaping the gut microbiome and energy metabolism in obese dogs. Microbiol. Spectr. 2024, 12, e0255223. [Google Scholar] [CrossRef]
- Kathrani, A.; Larsen, J.A.; Kass, P.H.; Fascetti, A.J. Effect of short-term probiotic Enterococcus faecium SF68 dietary supplementation in overweight and obese cats without comorbidities. Vet. Rec. Open 2016, 3, e000164. [Google Scholar] [CrossRef]
- Lee, H.-J.; Choi, W.-J.; Cho, J.H.; Park, S.-H.; Jung, B.-J.; Kim, H.B.; Gu, S.-H.; Song, K.H. Effects of synbiotic preparation containing Lactobacillus gasseri bnr17 on body fat in obese dogs: A pilot study. Animals 2022, 12, 642. [Google Scholar] [CrossRef]
- Strompfová, V.; Laukova, A.; Gancarcikova, S.; Marcinakova, M.; Simonova, M.; Bogovic-Matijasic, B. Application of potential probiotic Lactobacillus fermentum AD1 strain in healthy dogs. Anaerobe 2006, 12, 75–79. [Google Scholar] [CrossRef]
- Marcinakova, M.; Simonova, M.; Strompfova, V.; Lauková, A. Oral application of Enterococcus faecium strain EE3 in healthy dogs. Folia Microbiol. 2006, 51, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.; Li, Y.; Wang, J.; Liu, S.; Zhang, Y.; Liu, G.; Bao, Y.; Cheng, Y.; Yang, X. Bacterial Community Influences the Effects of Lactobacillus acidophilus on Lipid Metabolism, Immune Response, and Antioxidant Capacity in Dogs. Animals 2024, 14, 1257. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Liang, S.; Sun, J.; Tao, H.; Wang, Z.; Liu, B.; Wang, X.; Liu, J.; Wang, J. The Effect of Lactobacillus plantarum on the Fecal Microbiota, Short Chain Fatty Acids, Odorous Substances, and Blood Biochemical Indices of Cats. Microorganisms 2024, 12, 91. [Google Scholar] [CrossRef]
- Smeets-Peeters, M.J.; Minekus, M.; Havenaar, R.; Schaafsma, G.; Verstegen, M.W.A. Description of a dynamic in vitro model of the dog gastrointestinal tract and an evaluation of various transit times for protein and calcium. Altern. Lab. Anim. 1999, 27, 935–949. [Google Scholar] [CrossRef]
- Clauss, M.; Kleffner, H.; Kienzle, E. Carnivorous mammals: Nutrient digestibility and energy evaluation. Zoo Biol. 2010, 29, 687–704. [Google Scholar] [CrossRef]
- Case, L.P.; Daristotle, L.; Hayek, M.G.; Raasch, M.F. Canine and Feline Nutrition—A Resource for Companion Animal Professionals; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Golder, C.; Weemhoff, J.L.; Jewell, D.E. Cats Have Increased Protein Digestibility as Compared to Dogs and Improve Their Ability to Absorb Protein as Dietary Protein Intake Shifts from Animal to Plant Sources. J. Feline Med. Surg. 2020, 10, 541. [Google Scholar]
- Kim, H.S.; Hong, J.S.; Park, C.W. Evaluation of grooming behaviour and apparent digestibility method in cats. J. Feline Med. Surg. 2018, 21, 373–378. [Google Scholar] [CrossRef]
- Bajagai, Y.S.; Klieve, A.V.; Dart, P.J.; Bryden, W.L. Probiotics in Animal Nutrition: Production, Impact And Regulation; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Yu, H.F.; Wang, A.N.; Li, X.J.; Qiao, S.Y. Effect of viable Lactobacillus fermentum on the growth performance, nutrient digestibility and immunity of weaned pigs. J. Anim. Sci. 2008, 17, 61–69. [Google Scholar] [CrossRef]
- Jugan, M.C.; Rudinsky, A.J.; Parker, V.J.; Gilor, C. Use of probiotics in small animal veterinary medicine. J. Am. Vet. Med. Assoc. 2017, 250, 519–528. [Google Scholar] [CrossRef]
- Sun, H.Y.; Kim, I.J. Effect of Lactobacillus acidophilus based probiotic product supplementation on the blood profile, fecal noxious gas emission, and fecal shedding of lactic acid bacteria and coliform bacteria in healthy adult Beagle dogs. J. Anim. Sci. 2020, 47, 437–443. [Google Scholar] [CrossRef]
- Kumar, S.; Pattanaik, A.K.; Sharma, S.; Gupta, R.; Jadhav, S.E.; Dutta, N. Comparative assessment of canine-origin Lactobacillus johnsonii CPN23 and dairy-origin Lactobacillus acidophilus NCDC 15 for nutrient digestibility, faecal fermentative metabolites and selected gut health indices in dogs. J. Nutr. Sci. 2017, 6, e38. [Google Scholar] [CrossRef]
- Zha, M.; Zhu, S.; Chen, Y. Probiotics and Cat Health: A Review of Progress and Prospects. Animals 2024, 12, 1080. [Google Scholar] [CrossRef]
- Elshagabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus As Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- Schauf, S.; Nakamura, N.; Castrillo, C. Effect of Calsporin® (Bacillus subtilis C-3102) addition to the diet on faecal quality and nutrient digestibility in healthy adult dogs. J. Appl. Anim. Nutr. 2019, 7, e3. [Google Scholar] [CrossRef]
- Acuff, H.L.; Aldrich, C.G. PSVI-23 Evaluation of graded levels of Bacillus coagulans (GBI-30, 6086) on apparent nutrient digestibility, stool quality, and intestinal health indicators in healthy adult dogs. J. Anim. Sci. 2020, 98, 314–315. [Google Scholar] [CrossRef]
- Acuff, H.L.; Aldrich, C.G. Evaluation of graded levels of Bacillus coagulans GBI-30, 6086 on apparent nutrient digestibility, stool quality, and intestinal health indicators in healthy adult dogs. J. Anim. Sci. 2021, 99, skab137. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, X.; Xu, S.J.; Xu, C.; Mei, X.; Li, W. Effects of compound Bacillus on growth, nutrient apparent digestibility and health of pet cats. Anim. Nutr. 2022, 34, 2596–2605. [Google Scholar]
- You, I.; Mahiddine, F.Y.; Park, H.; Kim, M.J. Lactobacillus acidophilus novel strain, MJCD175, as a potential probiotic for oral health in dogs. Front. Vet. Sci. 2022, 9, 946890. [Google Scholar] [CrossRef]
- Marsella, R. Evaluation of Lactobacillus rhamnosus strain GG for the prevention of atopic dermatitis in dogs. Am. J. Vet. Res. 2009, 70, 735–740. [Google Scholar] [CrossRef]
- Rishniw, M.; Wynn, S.G. Azodyl, a synbiotic, fails to alter azotemia in cats with chronic kidney disease when sprinkled onto food. J. Feline Med. Surg. 2011, 13, 405–409. [Google Scholar] [CrossRef]
- Palmquist, D.R. A Preliminary Clinical Evaluation of Kibow Biotics, a Probiotic Agent, on Feline Azotemia. J. Am. Holist. Vet. Med. Assoc. 2006, 24, 23–27. [Google Scholar]
- Simpson, K.W.; Rishniw, M.; Bellosa, M.; Liotta, J.; Lucio, A.; Baumgart, M.; Czarnecki-Maulden, G.; Benyacoub, J.; Bowman, D. Influence of Enterococcus faecium SF68 probiotic on giardiasis in dogs. J. Vet. Intern. Med. 2009, 23, 476–481. [Google Scholar] [CrossRef]
- Deng, P.; Swanson, K.S. Gut microbiota of humans, dogs and cats: Current knowledge and future opportunities and challenges. Br. J. Nutr. 2015, 113, S6–S17. [Google Scholar] [CrossRef] [PubMed]
- Grześkowiak, Ł.; Endo, A.; Beasley, S.; Salminen, S. Microbiota and probiotics in canine and feline welfare. Anaerobe 2015, 34, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, H.A.; Mahiddine, F.Y.; Park, H.; Kim, M.J. Antiviral Effects of Lactococcus lactis on Feline Calicivirus, A Human Norovirus Surrogate. Food Environ. Virol. 2014, 6, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Lappin, M.R.; Veir, J.K.; Satyaraj, E.; Czarnecki-Maulden, G.L. Pilot study to evaluate the effect of oral supplementation of Enterococcus faecium SF68 on cats with latent feline herpesvirus 1. J. Feline Med. Surg. 2009, 11, 650–654. [Google Scholar] [CrossRef]
- Luo, L.; Gu, Z.; Pu, J.; Chen, D.; Tian, G.; He, J.; Zheng, P.; Mao, X.; Yu, B. Synbiotics improve growth performance and nutrient digestibility, inhibit PEDV infection, and prevent intestinal barrier dysfunction by mediating innate antivirus immune response in weaned piglets. J. Anim. Sci. 2024, 102. [Google Scholar] [CrossRef]
- Wang, F.; Pan, B.; Xu, S.; Xu, Z.; Zhang, T.; Zhang, Q.; Bao, Y.; Wang, Y.; Zhang, J.; Xu, C.; et al. A meta-analysis reveals the effectiveness of probiotics and prebiotics against respiratory viral infection. Biosci. Rep. 2021, 41, BSR20203638. [Google Scholar] [CrossRef]
- Delucchi, L.; Fraga, M.; Zunino, P. Effect of the probiotic Lactobacillus murinus LbP2 on clinical parameters of dogs with distemper-associated diarrhea. Can. J. Vet. Res. 2017, 81, 118–121. [Google Scholar]
- Higueras, C.; Sainz, Á.; García-Sancho, M.; Rodríguez-Franco, F.; Rey, A.I. Faecal Short-Chain, Long-Chain, and Branched-Chain Fatty Acids as Markers of Different Chronic Inflammatory Enteropathies in Dogs. Animals 2024, 14, 1825. [Google Scholar] [CrossRef]
- Minamoto, Y.; Minamoto, T.; Isaiah, A.; Sattasathuchana, P.; Buono, A.; Rangachari, V.R.; Mcneely, I.H.; Lidbury, J.; Steiner, J.M.; Suchodolski, J.S. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 2019, 33, 1608–1618. [Google Scholar] [CrossRef]
- Ziese, A.L.; Suchodolski, J.S. Impact of Changes in Gastrointestinal Microbiota in Canine and Feline Digestive Diseases. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 155–169. [Google Scholar] [CrossRef]
- Guard, B.C.; Honneffer, J.B.; Jergens, A.E.; Jonika, M.M.; Torresson, L.; Lawrence, Y.A.; Webb, C.B.; Hill, S.; Lidbury, J.A.; Steiner, J.M. Longitudinal assessment of microbial dysbiosis, fecal unconjugated bile acid concentrations, and disease activity in dogs with steroid-responsive chronic inflammatory enteropathy. J. Vet. Intern. Med. 2019, 33, 1295–1305. [Google Scholar] [CrossRef]
- Li, M.; Van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garrsen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, H.; Cheng, T.; Wang, L.; Wang, G.; Zhang, H.; Chen, W. Bifidobacterium longum S3 alleviates loperamide-induced constipation by modulating intestinal acetic acid and stearic acid levels in mice. Food Funct. 2024, 15, 6118–6133. [Google Scholar] [CrossRef]
- Rossi, G.; Cerquetella, M.; Scarponi, S.; Peng, G.; Fettucini, K.; Bassotti, G.; Jergens, A.E.; Suchodolski, J.S. Effects of probiotic bacteria on mucosal polyamines levels in dogs with IBD and colonic polyps: A preliminary study. Benef. Microbes 2018, 9, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Allenspach, K.; Mochel, J.P.; Parker, V.; Rudinsky, A.J.; Winston, J.A.; Bourgois-Mochel, A.; Ackermann, M.; Heilmann, R.M.; Köller, G.; et al. Synbiotic-IgY Therapy Modulates the Mucosal Microbiome and Inflammatory Indices in Dogs with Chronic Inflammatory Enteropathy: A Randomized, Double-Blind, Placebo-Controlled Study. Vet. Sci. 2022, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Cherbut, C. Motor effects of short-chain fatty acids and lactate in the gastrointestinal tract. Proc. Nutr. Soc. 2003, 62, 95–99. [Google Scholar] [CrossRef]
- Elce, A.; Amato, F.; Zarrilli, F.; Calignano, A.; Troncone, R.; Castaldo, G.; Canani, R.B. Butyrate modulating effects on pro-inflammatory pathways in human intestinal epithelial cells. Benef. Microbes 2017, 8, 841–847. [Google Scholar] [CrossRef]
- Kuczyńska, B.; Wasilewska, A.; Biczysko, M.; Banasiewicz, T.; Drews, M. Krótkołańcuchowe kwasy tłuszczowe-mechanizm działania, potencjalne zastosowanie kliniczne oraz zalecenia dietetyczne. Now. Lek. 2011, 80, 299–304. [Google Scholar]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [PubMed]
- Wang, F.; Mei, X.; Wang, Q.; Zhao, P.; Zhou, Y.; Tang, L.; Wang, B.; Xu, S.; Li, X.; Jin, Q.; et al. Compound Bacillus alleviates diarrhea by regulating gut microbes, metabolites, and inflammatory responses in pet cats. Anim. Microbiome 2023, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, H.; Wu, Y.; Lu, Z.; Zhou, X.; Tan, F.; Zhao, X. Lactobacillus fermentum HFY06 attenuates D-galactose-induced oxidative stress and inflammation in male Kunming mice. Food Funct. 2021, 12, 12479–12489. [Google Scholar] [CrossRef]
- Kato, S.; Hamouda, N.; Kano, Y.; Oikawa, Y.; Tanaka, Y.; Matsumoto, K.; Amagase, K.; Shimakawa, M. Probiotic Bifidobacterium bifidum G9-1 attenuates 5-fluorouracil-induced intestinal mucositis in mice via suppression of dysbiosis-related secondary inflammatory responses. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1017–1025. [Google Scholar] [CrossRef]
- López-Gómez, L.; Alcorta, A.; Abalo, R. Probiotics and Probiotic-like Agents against Chemotherapy-Induced Intestinal Mucositis: A Narrative Review. J. Pers. Med. 2023, 13, 1487. [Google Scholar] [CrossRef]
- Benyacoub, J.; Czarnecki-Maulden, G.L.; Cavadini, C.; Sauthier, T.; Anderson, R.E.; Schiffrin, E.J.; von der Weid, T. Supplementation of food with Enterococcus faecium (SF68) stimulates immune functions in young dogs. J. Nutr. 2003, 133, 1158–1162. [Google Scholar] [CrossRef]
- Alonge, S.; Aiudi, G.G.; Lacalandra, G.M.; Leoci, R.; Melandri, M. Pre- and Probiotics to Increase the Immune Power of Colostrum in Dogs. Front. Vet. Sci. 2020, 7, 570414. [Google Scholar] [CrossRef]
- Melandri, M.; Aiudi, G.G.; Caira, M.; Alonge, S. A Biotic Support During Pregnancy to Strengthen the Gastrointestinal Performance in Puppies. Front. Vet. Sci. 2020, 7, 417. [Google Scholar] [CrossRef]
- Delucchi, L.; Fraga, M.; Perelmuter, K.; Cella, C.D.; Zunino, P. Effect of native Lactobacillus murinus LbP2 administration on total fecal IgA in healthy dogs. Can. J. Vet. Res. 2014, 78, 153–155. [Google Scholar]
- Kol, A.; Foutouhi, S.; Walker, N.J.; Kong, N.T.; Weimer, B.C.; Borjesson, D.L. Gastrointestinal microbes interact with canine adipose-derived mesenchymal stem cells in vitro and enhance immunomodulatory functions. Stem Cells Dev. 2014, 23, 1831–1843. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Pengo, G.; Caldin, M.; Palumbo Piccionello, A.; Steiner, J.M.; Cohen, N.D.; Jergens, A.E.; Suchodolski, J.S. Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS ONE 2014, 9, e94699. [Google Scholar] [CrossRef]
- Mikawa, S.; Matsuda, A.; Kamemori, Y.; Asanuma, S.; Kitagawa, H. Enhancement of natural killer cell activity by oral administration of a fermented soybean product in dogs. Open Vet. J. 2021, 11, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Quilodrán-Vega, S.R.; Muñoz-Flores, C.; Pino, A.; Buldres, P.; Sandoval, F.; Aguirre, A.; Portillo, B.; Parra, N.; Altamirano, C.; Albarracín, L.; et al. Isolation, characterization, and immunomodulatory activity evaluation of probiotic strains from colostrum and canine milk. Front. Vet. Sci. 2023, 10, 1266064. [Google Scholar] [CrossRef]
- Wang, R.; Yan, S.; Ma, X.; Zhao, J.; Han, Y.; Pan, Y.; Zhao, H. The pivotal role of Bifida Ferment Lysate on reinforcing the skin barrier function and maintaining homeostasis of skin defenses in vitro. J. Cosmet. Dermatol. 2023, 22, 3427–3435. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. J. Funct. Foods 2022, 11, 2388. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, T.; Ding, H.; Chen, J.; Li, B.; Zhang, Q.; Yang, S.; Zhang, S.; Guan, W. Exploring the Benefits of Probiotics in Gut Inflammation and Diarrhea—From an Antioxidant Perspective. Antioxidants 2023, 12, 1342. [Google Scholar] [CrossRef]
- Zolotukhin, P.V.; Prazdnova, E.V.; Chistyakov, V.A. Methods to Assess the Antioxidative Properties of Probiotics. Probiotics Antimicrob. Proteins 2018, 10, 589–599. [Google Scholar] [CrossRef]
- Li, K.; Gu, Q.; Yang, W.; Yu, X. In vitro screening and probiotic evaluation of anti-obesity and antioxidant lactic acid bacteria. Food Biosci. 2023, 54, 102844. [Google Scholar] [CrossRef]
- Cruchet, S.; Furnes, R.; Maruy, A.; Hebel, E.; Palacios, J.; Medina, F.; Ramirez, N.; Orsi, M.; Rondon, L.; Sdepanian, V.; et al. The use of probiotics in pediatric gastroenterology: A review of the literature and recommendations by Latin-American experts. Paediatr. Drugs 2015, 17, 199–216. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Fu, A.; Gong, L.; Li, W.; Li, Y. Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Appl. Microbiol. Biotechnol. 2017, 101, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. J. Funct. Foods 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- De Souza, S.M.; He, F.; De Godoy, M.R.; Davenport, G.M. Effects of Probiotic and Heat-Killed Probiotic Supplementation on Systemic Biomarkers of Oxidative Stress and Inflammation and Fermentative end Products of Adult Cats. J. Anim. Sci. 2022, 100, 54. [Google Scholar] [CrossRef]
- Jang, H.J.; Son, S.; Kim, J.A.; Jung, M.Y.; Choi, Y.J.; Kim, D.H.; Lee, H.K.; Shin, D.; Kim, Y. Characterization and Functional Test of Canine Probiotics. Front. Microbiol. 2021, 12, 625562. [Google Scholar] [CrossRef]
- Meinieri, G.; Saettone, V.; Radice, E.; Bruni, N.; Martello, E.; Bergero, D. The synergistic effect of prebiotics, probiotics and antioxidants on dogs with chronic kidney disease. Ital. J. Anim. Sci. 2021, 20, 1079–1084. [Google Scholar] [CrossRef]
- Kim, K.T.; Kim, J.W.; Kim, S.I.; Kim, S.; Nguyen, T.H.; Kang, C.H. Antioxidant and Anti-Inflammatory Effect and Probiotic Properties of Lactic Acid Bacteria Isolated from Canine and Feline Feces. Front. Microbiol. 2021, 9, 1971. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, F.; Hou, Q.; Huang, W.; Liu, Y.; Zhang, H.; Sun, Z. Metagenomic analysis revealed beneficial effects of probiotics in improving the composition and function of the gut microbiota in dogs with diarrhea. Food Funct. 2019, 10, 2618–2629. [Google Scholar] [CrossRef]
- Tsai, C.W.; Huang, H.W.; Lee, Y.J.; Chen, M.J. Investigating the Efficacy of Kidney-Protective Lactobacillus Mixture-Containing Pet Treats in Feline Chronic Kidney Disease and Its Possible Mechanism. Animals 2024, 14, 630. [Google Scholar] [CrossRef]
- Crawford, C.K.; Beltran, A.; Castillo, D.; Matloob, M.S.; Uehara, M.E.; Quilici, M.L.; Cervantes, V.L.; Kol, A. Fenofibrate reduces glucose-induced barrier dysfunction in feline enteroids. Sci. Rep. 2023, 13, 22558. [Google Scholar] [CrossRef]
- Mao, A.; Zhao, W.; Zhu, Y.; Kong, F.; Chen, D.; Si, H.; Xu, C. Gut bacterial community determines the therapeutic effect of ginsenoside on canine inflammatory bowel disease by modulating the colonic mucosal barrier. Microorganisms 2023, 11, 2616. [Google Scholar] [CrossRef]
- Wells, J.M.; Rossi, O.; Meijerink, M.; van Baarlen, P. Epithelial crosstalk at the microbiota-mucosal interface. Proc. Natl. Acad. Sci. USA 2011, 108, 4607–4614. [Google Scholar] [CrossRef]
- Kumar, M.; Nagpal, R.; Verma, V.; Kumar, A.; Kaur, N.; Hemalatha, R.; Gautam, S.K.; Singh, B. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr. Rev. 2013, 71, 23–34. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef]
| Probiotics (Dose) | Animals and Duration | Effects | Reference |
|---|---|---|---|
| Lactobacillus gasseri BNR17 (2 × 109 CFU/g), Lactobacillus plantarum (109 CFU/g) | Five dogs for 10 weeks | Body weight and subcutaneous fat mass were decreased significantly, and microbial diversity was increased. | [19] |
| Lactobacillus fermentum AD1 (109 CFU/g) | Fifteen dogs for 7 days | Faecal Lactobacilli and Enterococci abundance were increased significantly. Total protein and total lipid were increased. | [20] |
| Enterococcus faecium EE3 (109 CFU/mL) | Eleven dogs for 1 week | Increased fecal Lactic acid bacteria abundance and decreased total lipid and protein levels. | [21] |
| Enterococcus faecium IDCC 2102 (1010 CFU/g) and Bifidobacterium lactis IDCC 4301 (1010 CFU/g) | Twenty dogs for 9 weeks | By restoring fecal microbiota stability, these probiotics enhanced systemic energy utilization and prevented lipid accumulation. | [16] |
| Lactobacillus acidophilus | Twelve dogs for 4 weeks | Serum cholesterol was apparently reduced. | [22] |
| Lactobacillus plantarum L11 | Twelve cats for 4 weeks | Serum total cholesterol was decreased, and the abundance of Bifidobacterium was improved. | [23] |
| Enterococcus faecium strain SF68 | Twenty cats for 8 weeks | No significant effects. | [18] |
| Probiotics | Animals | Clinical Diseases | Effects | Reference |
|---|---|---|---|---|
| Lactobacillus acidophilus | 13 dogs | Oral health | Porphyromonas gingivalis was inhibited. | [40] |
| Lactobacillus rhamnosus strain GG | 2 adult Beagles with severe AD and 16 puppies | Atopic Dermatitis (AD) | Immunologic indicators were reduced. | [41] |
| Probiotics and prebiotics | 10 cats with CKD | Chronic kidney disease (CKD) | No significance. | [42] |
| Kibow Biotics | Small number of cats with azotemia | Feline azotemia | BUN was decreased. | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Gu, X.; Zhang, H.; Zhao, L.; Wang, J.; Wang, X.; Tao, H.; Wang, Z.; Han, B. Application of Probiotics in Cats and Dogs: Benefits and Mechanisms. Vet. Sci. 2025, 12, 1008. https://doi.org/10.3390/vetsci12101008
Sun J, Gu X, Zhang H, Zhao L, Wang J, Wang X, Tao H, Wang Z, Han B. Application of Probiotics in Cats and Dogs: Benefits and Mechanisms. Veterinary Sciences. 2025; 12(10):1008. https://doi.org/10.3390/vetsci12101008
Chicago/Turabian StyleSun, Jintao, Xinshu Gu, Huaiyu Zhang, Lihong Zhao, Jinquan Wang, Xiumin Wang, Hui Tao, Zhenlong Wang, and Bing Han. 2025. "Application of Probiotics in Cats and Dogs: Benefits and Mechanisms" Veterinary Sciences 12, no. 10: 1008. https://doi.org/10.3390/vetsci12101008
APA StyleSun, J., Gu, X., Zhang, H., Zhao, L., Wang, J., Wang, X., Tao, H., Wang, Z., & Han, B. (2025). Application of Probiotics in Cats and Dogs: Benefits and Mechanisms. Veterinary Sciences, 12(10), 1008. https://doi.org/10.3390/vetsci12101008









